The pandemic of 2009 H1N1 influenza A emerged in February 2009, with high morbidity and mortality, and rapidly spread globally. São Paulo was among the most affected areas in Brazil. This study compares the clinical and epidemiological characteristics of influenza-like illness between outpatients and hospitalized patients and evaluates the impact of oseltamivir therapy on the outcome of 2009 H1N1 influenza A patients.
METHODSThis is a case series study comparing the clinical and epidemiological characteristics of influenza-like illness between outpatients attended at Hospital São Paulo in August 2009 (the peak of the first pandemic wave) and those patients hospitalized between May and September 2009 (the entire first pandemic wave).
RESULTSThe 1651 patients evaluated were predominantly female (927×686, p<0.001) and aged 31.71±16.42 years, with 148 reporting chronic pulmonary disease. Dyspnea was presented by 381 (23.4%) patients and was more frequent among those aged 30 years or more (p<0.001). Hospitalization occurred at 3.73±2.85 days, and antiviral treatment started 2.27±2.97 days after the onset of first symptoms. A delay of more than 5 days in starting oseltamivir therapy was independently associated with hospitalization (p<0.001), a stay in the ICU (p<0.001) and a higher risk of dying (OR = 28.1, 95% CI 2.81-280.2, p = 0.007).
CONCLUSIONThe 2009 pandemic of H1N1 influenza A affected young adults, presented a significant disease burden and produced severe cases with a significant fatality rate. However, promptly starting specific therapy improved the outcome.
The 2009 pandemic of H1N1 influenza A rapidly spread globally, provoking significant morbidity and mortality in specific groups, although it is now acknowledged not to be a highly virulent strain (1,2).
Despite initial uncertainty about the effectiveness of oseltamivir therapy, in the post-pandemic evaluation, it became clear that the therapy should be indicated for all patients with an influenza-like syndrome, presenting with lower respiratory tract involvement and/or high-risk conditions for complicated influenza disease. The therapy should preferably be initiated within the first 48-72 hours after symptom onset, although the treatment could be beneficial even if started later (3).
The 2009 H1N1 morbidity and mortality were high in Brazil during the first pandemic wave, and the State of São Paulo was one of the most affected areas (4). As of May 2009, the first cases of H1N1 influenza A infection were referred to Hospital São Paulo, a tertiary university health center designated as one of the reference centers for treating suspected H1N1 cases by the State of São Paulo health authorities. A special unit for outpatient attendance in suspected H1N1 cases was opened, and more than 4000 patients were evaluated and treated throughout the first pandemic wave.
The Ministry of Health authorities established a case definition for severe respiratory disease and set conditions for hospitalization (5). Patients received antiviral treatment if hospitalized, but oseltamivir prescription was not recommended for non-hospitalized patients.
This study describes the epidemiological and clinical aspects of ambulatory and hospitalized patients attended at Hospital São Paulo between May and September 2009 and evaluates oseltamivir therapy in the outcome of 2009 H1N1 influenza A-infected patients.
METHODSStudy DesignThis is a case series study comparing the clinical and epidemiological characteristics of influenza-like illness between outpatients attended at Hospital São Paulo in August 2009 (the peak of the first pandemic wave) and patients hospitalized between May and September 2009 (the entire first pandemic wave). The study was approved by the local Ethics Research Committee.
In agreement with official recommendations (5), patients with acute respiratory disease, characterized by fever (axillary temperature >38°C), cough and dyspnea, and patients with acknowledged risk factors for complicated influenza (such as pregnancy and cardiorespiratory or immune-compromising diseases), were hospitalized. The use of oseltamivir was recommended only for those patients and was preferably started within the first 48 hours after the onset of symptoms in suspected H1N1 cases, obeying the above criteria.
Epidemiological and Clinical DataClinical and epidemiological data were obtained from patients using a standardized questionnaire. Age, gender, occupation, the presence of comorbidities and/or risk factors (5), clinical findings at first attendance (for outpatients) or clinical, radiological and laboratory findings during the first 24 hours (for hospitalized patients), the need for supplemental oxygen, antibiotic or antiviral use and the clinical outcome (only for those hospitalized) were recorded for analysis.
Sample Collection and H1N1 DiagnosisA diagnosis of 2009 H1N1 influenza A infection was confirmed in nasopharyngeal swab specimens following the Centers for Disease Control and Prevention (CDC) protocol (6), with primers and probes for influenza A, swine flu A, swine H1 and RNaseP (SuperScript III, Invitrogen).
Statistical AnalysisData were recorded and analyzed using Statistica® 5.5 for Windows®. Descriptive statistics and comparisons between outpatients' and inpatients' clinical and epidemiological findings are presented. Continuous variables were compared using the Mann-Whitney test, a t-test for independent samples or ANOVA, as appropriate. Categorical variables were compared using a Yates-corrected chi-square test or Fisher's exact test. Odds ratios (ORs) with the respective 95% confidence intervals (CIs) were calculated whenever indicated. Multivariate regression analysis was performed to verify the variables independently associated with a stay in the intensive care unit (ICU) and death. The significance level chosen was 5%.
RESULTSWe assessed data from 1579 outpatients evaluated as suspected cases of 2009 pandemic H1N1 in August 2009, which is considered the peak of the first influenza pandemic wave in São Paulo city. This sample represents approximately 1/3 of the patients with influenza-like illness attended at our sentinel hospital from May to September 2009. In addition, data from all 72 adolescent and adult patients hospitalized between May and September 2009 were compared with the data from the outpatients described above. The demographic and epidemiological characteristics of the sample are shown in Table 1.
Demographic and epidemiological characteristics of the patients.
| Characteristics | Outpatients n (%) | Inpatients | ||||
|---|---|---|---|---|---|---|
| n (%) | p∗) | H1N1 | Non-H1N1 | p#) | ||
| Gender | ||||||
| Male | 663 (43) | 23 (32) | 0.082 | 9 (28) | 14 (35) | 0.534 |
| Female | 878 (57) | 49 (68) | 23 (72) | 26 (65) | ||
| Age (years) | ||||||
| 0-9 | 91 (06) | — | — | — | ||
| 10-19 | 205 (14) | 8 (11) | 3 (09) | 5 (13) | ||
| 20-29 | 537 (36) | 24 (33) | 9 (28) | 15 (38) | ||
| 30-39 | 246 (17) | 14 (19) | 0.035 | 7 (22) | 7 (18) | 0.138 |
| 40-49 | 179 (12) | 8 (11) | 5 (16) | 3 (08) | ||
| 50-59 | 133 (09) | 10 (09) | 7 (22) | 3 (08) | ||
| ≥60 | 88 (06) | 8 (11) | 1 (03) | 7 (18) | ||
| Comorbidities | ||||||
| None | 1309 (95) | 24 (08) | 11 (24) | 13 (25) | ||
| Cardiovascular disease | 15 (01) | 44 (14) | 5 (11) | 10 (20) | ||
| Chronic pulmonary disease | 21 (02) | 127 (42) | <0.001 | 13 (29) | 8 (16) | 0.065 |
| Immunopathies | 12 (01) | 24 (08) | 5 (11) | 7 (14) | ||
| HIV infection | 10 (01) | 46 (15) | 4 (09) | 6 (12) | ||
| Other conditions | 14 (01) | 39 (13) | 7 (16) | 7 (14) | ||
| Risk Factors | ||||||
| None | 1427 (92) | 44 (50) | 15 (54) | 23 (74) | ||
| Healthcare workers | 27 (02) | 14 (16) | 5 (18) | 0 (00) | ||
| Pregnancy | 41 (03) | 15 (17) | <0.001 | 3 (11) | 5 (16) | 0.286 |
| Tobaccoism | 45 (03) | 10 (11) | 5 (18) | 3 (10) | ||
| Obesity | 16 (01) | 5 (06) | 0 (00) | 0 (00) | ||
The patients were predominantly female (927 females and 686 males, p<0.001), without any difference in gender distribution between outpatients and inpatients. The average age was 31.7 years (95% CI: 30.9-32.5 years), and the hospitalized patients were older than the outpatients (37.2 years [95% CI: 32.9-41.4 years] x 31.44 years [95% CI: 30.6-32.3 years], p = 0.004). In addition, patients aged 20-29 years were significantly more affected than the other age classes (p<0.001) among the outpatients, inpatients and confirmed H1N1 cases (see Table 1).
A preexisting comorbidity was reported by 298 (18%) patients, of which the most frequent was chronic lung disease, in nearly 10% (148/1651). In addition, 228 (14%) patients had at least one acknowledged risk factor, as defined by the Brazilian health authorities (MS2009b), as 57 (25%) were pregnant women and 42 (18%) were healthcare workers.
Dyspnea was reported by 381 (23%) patients and was more frequently observed among patients aged 30-39 (30%), 50-59 (32%) and >60 (30%) years (p = 0.001). Notably, the majority of patients presenting with dyspnea (289/381, 76%) did not report a previous lung disease. Nevertheless, dyspnea was present in 62% of patients with chronic lung disease, who had a nearly sevenfold greater risk of presenting with dyspnea compared to patients without chronic lung disease (OR = 6.8, 95% CI: 4.8-9.7, p<0.001). Table 2 shows other clinical, radiological and laboratory findings for both outpatients and inpatients.
Clinical findings of patients attended during the first H1N1 epidemic wave in São Paulo.
| Characteristics | Outpatients n (%) | Inpatients | ||||
|---|---|---|---|---|---|---|
| n (%) | p∗) | H1N1 n (%) | Non-H1N1 n (%) | p#) | ||
| Symptoms | ||||||
| Fever | 1011 (81) | 67 (93) | 0.016 | 32 (100) | 35 (88) | 0.047 |
| Cough | 1040 (89) | 68 (95) | <0.001 | 29 (91) | 39 (98) | 0.442 |
| Dyspnea | 322 (21) | 59 (82) | <0.001 | 26 (81) | 33 (83) | 0.986 |
| Sore throat | 581 (95) | 23 (32) | <0.001 | 9 (28) | 14 (35) | 0.616 |
| Rhinorrhea | 511 (95) | 22 (31) | <0.001 | 11 (34) | 11 (28) | 0.611 |
| Myalgia | 895 (98) | 33 (46) | <0.001 | 23 (70) | 12 (30) | 0.001 |
| Headache | 459 (96) | 20 (28) | <0.001 | 11 (34) | 9 (23) | 0.299 |
| Diarrhea | 53 (79) | 7 (10) | <0.001 | 6 (19) | 1 (3) | 0.039 |
| Clinical Findings | ||||||
| Altered oroscopy | — | 60 (86) | 28 (88) | 32 (84) | 0.745 | |
| Altered pulmonary auscultation | — | 35 (49) | 16 (50) | 19 (54) | 0.833 | |
| Respiratory frequency | — | 27.1±5.1 | 29.3±16.4 | 0.487 | ||
| Chest Radiography | ||||||
| Normal (or not performed) | — | 43 (60) | 17 (53) | 26 (61) | ||
| Interstitial infiltrate | — | 22 (31) | 13 (41) | 9 (23) | 0.218 | |
| Focal condensation | — | 7 (10) | 2 (6) | 5 (13) | ||
| Laboratory Findings | ||||||
| SaO2 | 90.3±6.7 | 89.4±12 | 0.752 | |||
| PaO2 | 63.8±16.7 | 79.8±42.8 | 0.085 | |||
| PaCO2 | 33.6±7.7 | 34.3±16.3 | 0.841 | |||
| DHL | 401±430 | 271±191 | 0.149 | |||
| ALT | 51±36 | 27±37 | 0.041 | |||
| AST | 69±62 | 33±34 | 0.019 | |||
| CPK | 465±811 | 201±448 | 0.185 | |||
Hospitalized patients had more fever, cough and dyspnea than outpatients, whereas the outpatients had more sore throat, rhinorrhea, headache, myalgia and diarrhea. With the exceptions of fever, myalgia and diarrhea (more frequent in H1N1-positive patients), symptoms did not differ between hospitalized H1N1-positive and H1N1-negative patients. Additionally, H1N1-positive patients had higher levels of AST and ALT than hospitalized H1N1-negative patients and tended to have higher CPK levels (see Table 2).
Chest radiography showed that confirmed H1N1 cases had more interstitial infiltrates, whereas unconfirmed H1N1 presented more parenchymal condensation, although these differences were not significant (see Table 2).
A laboratory evaluation of the patients in the first 24 hours of hospitalization revealed that hypoxemia tended to be more pronounced among confirmed H1N1 patients than unconfirmed H1N1 patients (PaO2: 63.8×79.8, p = 0.085; see Table 2). Hypoxemia also tended to be more frequent among confirmed H1N1 patients when considering either the proportion of patients with PaO2<80 (20/24, 83% x 17/28, 61%, p = 0.067) or the proportion of patients with SaO2<95% (18/24, 75% x 14/28, 50%, p = 0.058).
The majority of hospitalized patients (91.7%) had a good outcome, but 4 (5.5%) patients died. Three of the fatal cases were H1N1 patients, and 2 had a pulmonary comorbidity. However, only 21 (29%) of the 72 hospitalized patients had previous pulmonary disease, of which 62% (13/21) had a confirmed H1N1 infection. In contrast, 32/72 (44%) of the hospitalized patients with an influenza-like syndrome were confirmed H1N1 cases, of which 19/32 (60%) had no pulmonary comorbidity. In addition, confirmed H1N1 patients tended to remain in the ICU longer (25×9.8 days, p = 0.116) than unconfirmed patients.
Overall, the average time from the onset of symptoms to the first attendance was 2.1 days (95% CI: 1.9-2.4 days), with a range of 0-15 days, whereas the average time between symptom onset and hospitalization was 3.7 days (95% CI: 3-4.4 days), with no difference between confirmed and unconfirmed H1N1 patients (p = 0.836).
Oseltamivir was prescribed for 25% (405/1651) of patients, and nearly 84% (340/405) of the prescriptions were for outpatients. Nevertheless, hospitalized patients were 33-fold more likely to receive oseltamivir than outpatients (65/72×340/1579; OR = 33.3, 95% CI: 15.1-73.3, p<0.001), which reflected the MS recommendations regarding H1N1 therapy at that time. In contrast, antibiotics were prescribed for 15% (245/1651) of patients, with no difference between outpatients and hospitalized patients (p = 0.915).
Oseltamivir was initiated 2.3 days (95% CI: 1.9-2.6 days) after symptom onset, but approximately 13% of patients only received antiviral therapy 5 or more days after symptom onset.
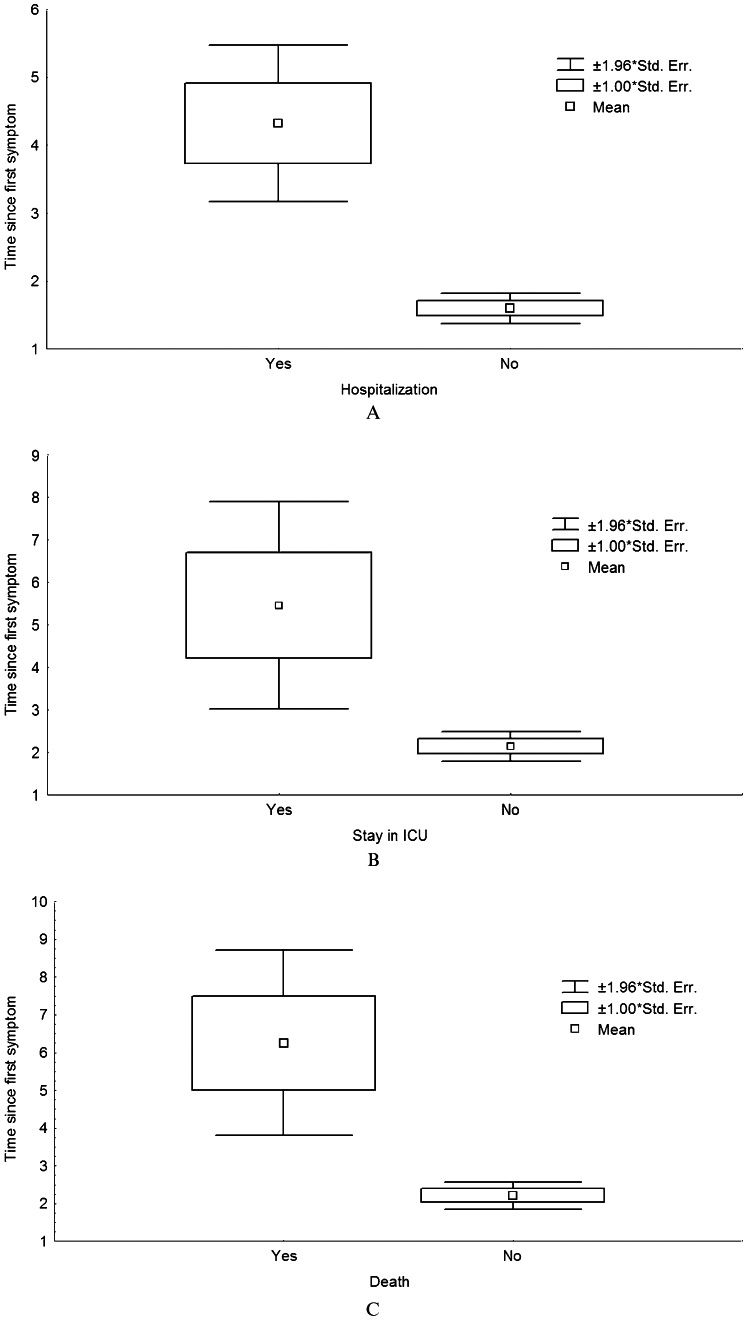
As observed in Figure 1, the duration between the onset of symptoms and the beginning of oseltamivir therapy was consistently longer for patients who were hospitalized (p<0.001), entered the ICU (p<0.001) or died (p = 0.006) than for patients without these complications. In addition, a delay of 5 or more days in starting oseltamivir therapy was associated with hospitalization (p<0.001), a stay in the ICU (p<0.001) and a higher risk of death (OR = 28.1, 95% CI: 2.8-280.2, p = 0.005) in the univariate analysis. The delay was also independently associated with a need to stay in the ICU and death in the multivariate analysis, as shown in Table 3.
Results of the multivariate analysis, considering death (A) and a stay in the ICU (B) as the outcome variables of interest (n = 69 hospitalized patients).
| A | β (SE) | B (SE) | p | |
|---|---|---|---|---|
| Intercept | -0.116 (0.088) | 0.192 | ||
| Gender | 0.104 (0.108) | 0.052 (0.054) | 0.340 | |
| Age (in years) | -0.067 (0.110) | -0.001 (0.001) | 0.542 | |
| Confirmed H1N1 | 0.180 (0.109) | 0.084 (0.051) | 0.095 | |
| Stay in ICU | 0.341 (0.112) | 0.218 (0.071) | 0.003 | |
| Pulmonary disease | 0.140 (0.111) | 0.073 (0.058) | 0.210 | |
| Delay ≥5 days∗) | 0.296 (0.109) | 0.152 (0.056) | 0.009 | |
| B | Intercept | 0.207 (0.153) | 0.182 | |
| Gender | -0.094 (0.122) | -0.074 (0.096) | 0.445 | |
| Age (in years) | 0.022 (0.124) | 0.000 (0.003) | 0.861 | |
| Confirmed H1N1 | -0.189 (0.121) | -0.139 (0.089) | 0.123 | |
| Pulmonary disease | 0.190 (0.123) | 0.156 (0.100) | 0.126 | |
| Delay ≥5 days∗) | 0.226 (0.120) | 0.182 (0.097) | 0.064 |
β stands for the non-adjusted regression coefficients, B for the adjusted regression coefficients and SE for the standard error of the coefficients.
Table 3 shows the multivariate analysis results, correlating gender (male or female), age (in years), confirmed H1N1 infection (yes or no), previous lung disease (yes or no) and the time between symptom onset and the initiation of oseltamivir therapy (<5 or ≥5 days) to a stay in the ICU or death, which were the outcome variables of interest. In the analysis focusing on death, having stayed in the ICU (yes or no) was also included in the multivariate panel.
As shown in Table 3, starting oseltamivir therapy 5 or more days after the onset of first symptoms and having stayed in the ICU were independently associated with death (Panel A). As shown in Panel B, a time interval equal to or longer than 5 days was also associated with having been admitted to the ICU, although the chosen significance level was not achieved (p = 0.064).
DISCUSSIONThe 2009 pandemic of H1N1 influenza virus emerged in February 2009 and rapidly spread globally, with lethality averaging 0.6% (0.0004-1.47%) (1). By that time, the majority of Brazilian cases were still imported, mainly from Argentina, the USA and Chile (7,8), and local transmission was acknowledged by the Brazilian public health authorities only on July 16, 2009 (4).
The 2009 H1N1 virus has joined the mix of seasonal influenza viruses but still affects young adults and children more severely than the seasonal flu, which kills more elderly people (9). Our data confirmed the great burden of H1N1 infection among young adults (see Table 1).
The majority of patients attended during the pandemic peak in São Paulo presented with typical influenza-like illness but had an unexpectedly high frequency of dyspnea. Dyspnea was observed in 21% of outpatients and 82% of inpatients (see Table 2), with the majority (76%) not reporting any chronic respiratory disease. In addition, patients with confirmed 2009 H1N1 infection had more pronounced hypoxemia than non-H1N1 cases. Experimentally, it has been shown that lung replication of the 2009 H1N1 virus is higher than that of seasonal influenza A viruses (10,11).
In this study, the most frequent risk factor for complicated 2009 H1N1 infection was chronic lung disease, but most attended patients had no reported comorbidity. This finding is in agreement with worldwide clinical data on 2009 H1N1 infection, which show that 25-50% of 2009 H1N1 patients who were hospitalized or died had no underlying comorbidities (12–16).
Although the efficacy of oseltamivir therapy is now widely acknowledged (17), the therapy's efficacy in preventing hospitalizations and deaths related to mild infections caused by the 2009 H1N1 influenza virus remains controversial (8),. However, there is now a consensus that treatment should be offered to 2009 H1N1-infected individuals with a high risk of complicated influenza or with signs of lower respiratory tract involvement. Moreover, therapy should start as soon as possible after symptom onset, regardless of the clinical severity at presentation (3),. This recommendation is due to difficulties in recognizing patients who present with mild infection that will evolve clinical complications within the first 48 hours after presentation.
An important result of this study was that delaying oseltamivir for 5 or more days after symptom onset was independently related to higher risks of hospitalization, staying in the ICU and death. Regarding death, despite the large CI observed in the univariate analysis (95% CI: 2.8-280.2), which could raise suspicions of the results being unreliable, the average risk of death was increased nearly 30-fold (OR = 28.1) when treatment was delayed for 5 or more days. Furthermore, the minimum of the 95% CI (2.8) showed that the risk of death after delayed treatment increased at least threefold, which was not only statistically significant but also of utmost biological and medical importance. In addition, this finding was confirmed in the multivariate analysis. In fact, obtaining such an expressive result, considering the low number of samples analyzed (4 deaths among 72 hospitalized patients, of whom only 69 had recorded data complete enough to remain in the final multivariate analysis), and the result's medical relevance seem to us important enough to deserve publication. Yu et al. (22) also noted that delaying oseltamivir therapy for more than 5 days increased the risk of severe disease, defined as dying or having stayed in the ICU. However, the researchers did not analyze each endpoint independently.
This study has certain limitations that should be discussed. First, this was a retrospective study describing the clinical and epidemiological profiles of H1N1 patients attended during the first pandemic wave in São Paulo. Second, it was not possible to confirm H1N1 infection in the majority of outpatients due to national public health police limitations. Therefore, several different influenza subtypes and other respiratory viruses could have circulated at same time, as previously shown (24,25). However, during the first pandemic wave in Brazil, approximately 70% of the cases of influenza-like syndromes with laboratory-confirmed etiologies were due to 2009 pandemic influenza A of the H1N1 strain (26).
Another limitation was that the outcome of antiviral-treated outpatients could not be systematically assessed for comparison due to lack of control of the medical reevaluations. However, national regulations stated that, during the pandemic, influenza outpatients with complications or worsening of the initial clinical status should be reevaluated at the same site of the first attendance, at least preventing the loss of available complicated cases.
Therefore, despite the above-mentioned limitations, our results are robust and indicate a truly harmful effect of delaying the initiation of antiviral therapy in certain 2009 H1N1-infected patients, which is in accordance with current medical knowledge. In addition, to the best of our knowledge, this work is the first to be published that shows an independent increase in the risk of death that is simply related to delaying the beginning of antiviral therapy.
In conclusion, it is now clear that delaying oseltamivir therapy for 5 or more days is related to an increased risk of severe infection and death, primarily for patients who develop lower respiratory tract involvement, such as dyspnea and cough. Accordingly, oseltamivir therapy should be started immediately for individuals at risk of complications or presenting with lower respiratory tract symptoms, regardless of the severity of the initial clinical presentation, as previously stated in the new policies regarding the treatment of H1N1-infected patients in Brazil (23).
AUTHOR CONTRIBUTIONSBellei NC planned the study and was responsible for the data collection, laboratory analysis, data analysis and manuscript writing. Cabeça TK was responsible for the laboratory analysis, data collection, data analysis and manuscript writing. Carraro E was responsible for the laboratory analysis, data analysis and manuscript writing. Goto JM, Cuba GT and Hidalgo SR were responsible for the data collection, data analysis and manuscript writing. Burattini MN planned the study and was responsible for the data collection, data analysis and manuscript writing.
We would like to acknowledge the members of the Influenza Working Group of Hospital São Paul, Eduardo Medeiros, Odair Marson (in memory), Celso Granato, Suely Yashiro, Katsumi Osiro, Enfermeira Nazaré, Ana Luiza Fernandes, Luis Eduardo Nery and Nivia Pissaya and the medical staff of the emergency ward for their contribution to the preparedness of the hospital and to the treatment of the patients attended at Hospital São Paulo during the first 2009 H1N1 pandemic wave. We also acknowledge the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for partially supporting this work.
No potential conflict of interest was reported.