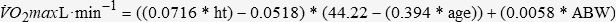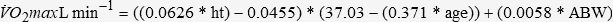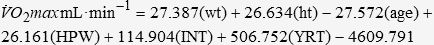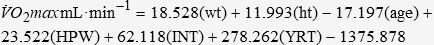The purpose of this paper is to provide strategies for cardiopulmonary exercise testing of pectus excavatum patients. Currently, there are no standardized methods for assessing cardiovascular and pulmonary responses in this population; therefore, making comparisons across studies is difficult if not impossible. These strategies are intended for physicians, pulmonary technicians, exercise physiologists, and other healthcare professionals who conduct cardiopulmonary exercise testing on pectus excavatum patients. By using the strategies outlined in this report, comparisons across studies can be made, and the effects of pectus excavatum on cardiopulmonary function can be assessed with greater detail.
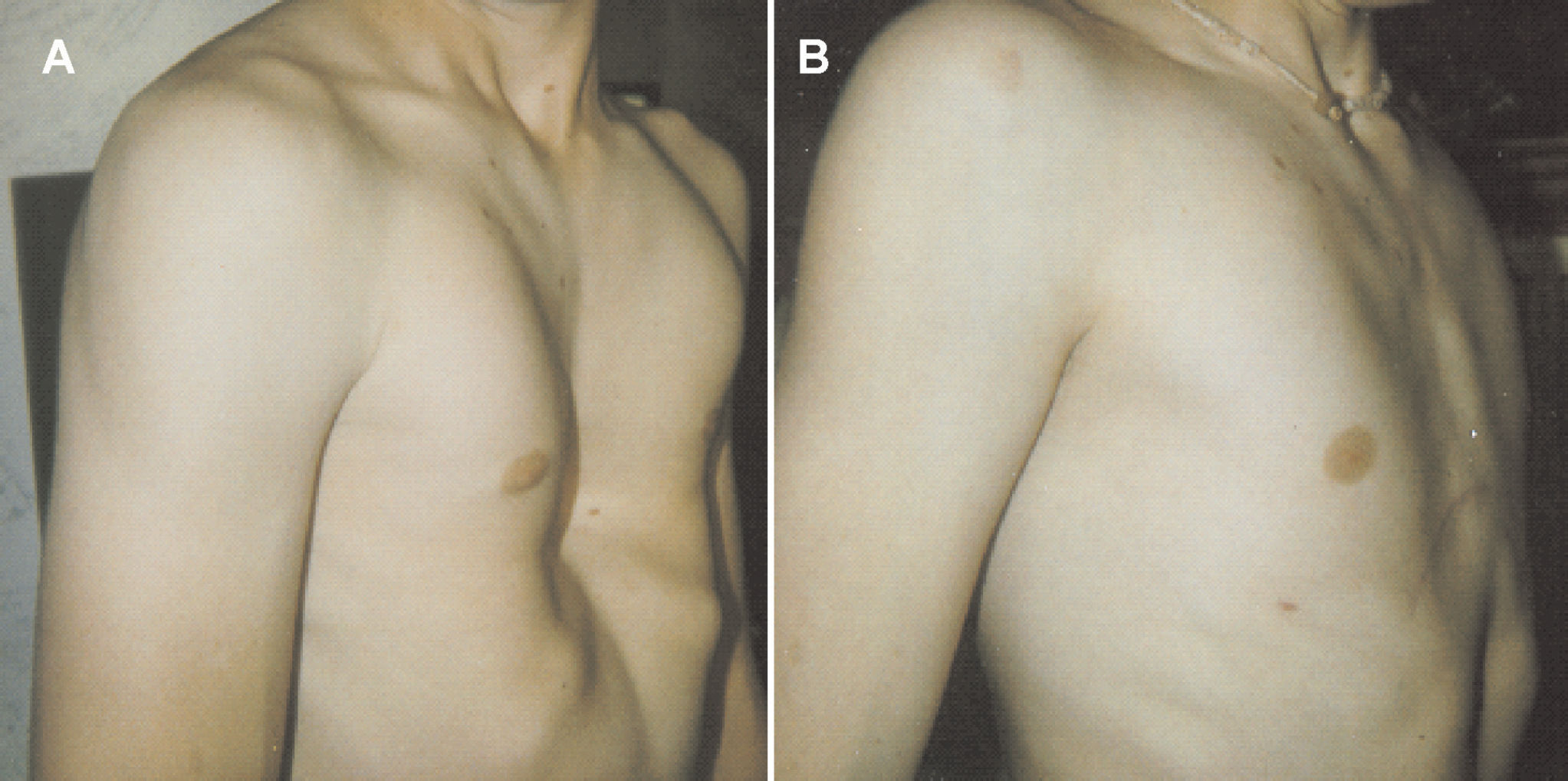
Pectus excavatum (Figure 1) is a relatively common congenital deformity of the chest wall, with an incidence of approximately one in every 300 to 400 Caucasian male births.1 This condition is more common than Down syndrome, which occurs one in every 600 to 1,000 births.2 Although the pathogenesis of pectus excavatum remains unclear, investigators have hypothesized that the deformity results from unbalanced overgrowth in the costochondral regions. As a result, the chest appears concave, and a displaced heart is often palpable on the left mid-axillary line slightly below the armpit. Pectus excavatum occurs more often in males than females (6:1) and accounts for 90% of congenital chest wall deformities.3,4 Approximately 40% of pectus excavatum patients are aware of one or more members of their family who have pectus deformities; however, a genetic link has not been established.4
Depiction of patient with pectus excavatum before (A) and after (B) surgical repair using the Highly Modified Ravitch Repair (HMRR)59
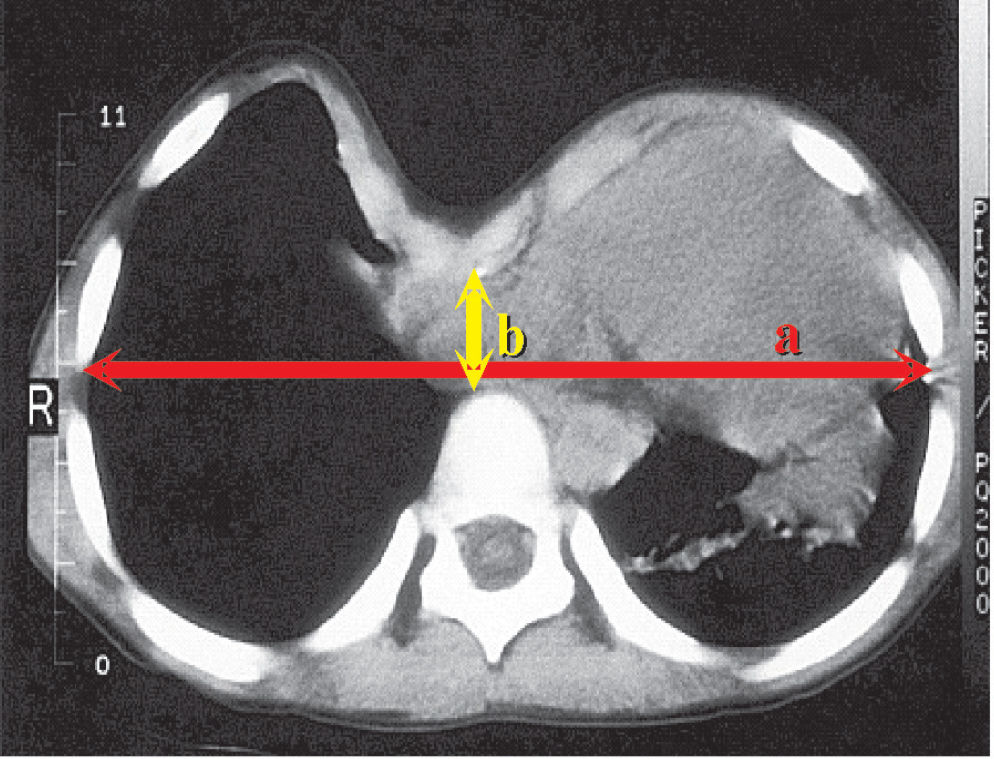
The severity of pectus excavatum can be calculated by dividing the inner width of the chest at the widest point (a) by the distance between the posterior surface of the sternum and anterior surface of the spine (b) as determined by computed tomography (CT) scans or chest radiographs (Figure 2).5 The normal chest has an index of 2.5, however, in our experience we have observed symptomatic pectus excavatum patients with severity indices ranging from 3.2 to 8.0.6,7 It should be noted that older patients often experience more severe symptoms with a lower index than do adolescents.4
Recently, Malek and colleagues8–10 conducted two meta-analyses examining the effects of surgical repair on cardiovascular and pulmonary function in pectus excavatum patients. The investigators reported that cardiovascular function significantly and clinically improved after surgical repair (ES = 0.59; P < 0.05),9 whereas pulmonary function did not significantly improve after surgical repair (ES = 0.08; P > 0.05).10 A salient finding in the meta-analyses of Malek et al.9,10 was the fact that many studies did not utilize a standardized method of determining cardiovascular function, pectus severity index, or control for potentially confounding variables, such as the subject’s habitual physical activity level preoperatively and postoperatively. Based on the results of Malek et al.9,10 and our experience6,7 with pectus excavatum patients, it is our position that specific strategies related to assessing cardiopulmonary function in pectus excavatum patients is warranted in order to more effectively compare results between studies without employing a meta-analytic approach. Although there are two textbooks11,12 and one paper13 on exercise testing and interpretation, they are not specific to pectus excavatum patients and do not provide step-by-step strategies for performing cardiopulmonary exercise testing (CPET). Therefore, the purpose of this paper is to provide specific strategies for examining cardiopulmonary function in pectus excavatum patients.
Introduction to Cardiopulmonary Testing (CPET)There are various invasive and noninvasive methods of assessing cardiovascular and ventilatory function, however, the use of CPET allows examination of the integrative responses of the cardiovascular and ventilatory response to maximal incremental exercise.11–13 CPET is an invaluable assessment tool used to 1) classify individuals for health risk, 2) quantify training intensity for aerobic exercise prescription, and 3) monitor the effects of aerobic training programs in healthy and clinical populations13–15. Although other assessment techniques such as electrocardiograph, questionnaires, and/or submaximal exercise protocols have been used to estimate exercise tolerance, Meyers16 stated, “… measurements of ventilation and gas exchange responses… [are] the only modality that provides an accurate and objective expression of exercise capacity.” (p.S49). CPET can be performed using different modes of exercise that include the treadmill,17 arm ergometry,18 single-leg knee extension ergometry,19,20 or cycle ergometry.7 Although each mode has associated advantages and disadvantages, it is our position that studies examining cardiopulmonary function in pectus excavatum patients should use cycle ergometry. Cycle ergometry provides a number of advantages over the other modes of exercise testing: 1) subject comfort, 2) reduction of potential joint injuries associated with weight-bearing exercise, 3) control of cadence, and 4) researcher control of the external work rate.12,13 This last feature is particularly important since small increments in work rate over the duration of the test will allow for detection of subtle changes in cardiovascular and ventilatory function, which may provide insight into the patient’s exercise tolerance.
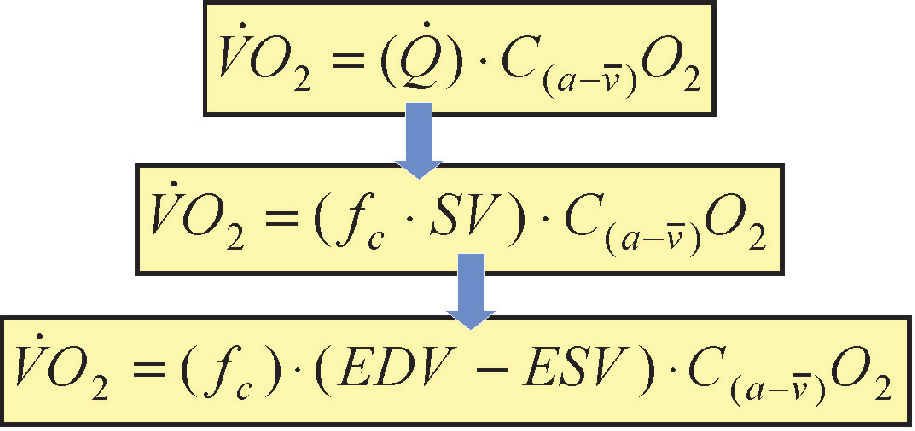
Exercise Physiology of CPETAs shown in Figure 3, one of the primary components used to describe cardiopulmonary function is oxygen uptake (V̇O2 ) which is represented by the Fick equation where Q̇ is cardiac output and C(a-v̄)O2 is the difference between the oxygen content of the arterial (≈ 20 ml of O2 ml O2·100 ml−1 of blood) and venous sides (varies with exercise intensity). Cardiac output, however, is a product of fc (cardiac frequency) and stroke volume (SV). Stroke volume can be further decomposed to end-diastolic volume (EDV) and end-systolic volume (ESV). During incremental cycle ergometry, SV increases to a percentage of V̇O2 max and plateaus,21 whereas fc continues to increase and may eventually plateau at V̇O2 max The C(a-v̄)O2 gradient during rest is approximately 4 ml O2·100 ml−1 of blood, whereas at near-maximal exercise capacity this gradient increases to approximately 16 ml O2·100 ml−1. Thus, the maximal volume of oxygen uptake (V̇O2 max) represents the maximal amount of oxygen transported and utilized during aerobic metabolism.11,13 Related to pectus excavatum patients, the impairment of one or more components in this equation results in decreased exercise tolerance. It should be noted, however, that V̇O2 max can be influenced by various other factors, such as age, gender, and habitual exercise history.7,22–25 Also, direct and indirect measurements of SV, EDV, ESV, and C(a-v̄)O2 are possible; however, these procedures often require specialized equipment, are impractical, and do not offer the same advantages as CPET. Therefore, the examination of gas exchange indices measured at the mouth via a two-way breathing valve and metabolic cart provides a more practical alternative that is easily tolerable with healthy and clinical populations.11
The Fick equation for calculating oxygen uptake (V̇O2). Refer to text for detailed discussion. Q̇ is cardiac output; C(a-v̄)O2 is the difference between the oxygen content of the arterial (≈ 20 ml of O2 mL O2·100 mL−1 of blood) and venous sides; fc is cardiac frequency; SV is stroke volume; EDV is end-diastolic volume; and ESV is end-systolic volume
The following sections are guidelines for assessing pectus excavatum patients during CPET. The preparation phase is described, followed by the testing phase and then an interpretation of various physiological indices.
Cardiopulmonary Test (Preparation Phase)Documenting Patient’s Exercise HistoryIn order to assess the severity of pectus excavatum, investigators need to document the patient’s habitual exercise history. Studies have found that in untrained individuals, V̇O2 max can increase as much as 20% following 12 weeks of endurance training.11,26 The adaptations to endurance training, however, will not be retained (i.e., deconditioning) if a minimal level of exercise is not maintained. Studies27–29 have shown that seven to eight weeks of deconditioning, in moderately training individuals, resulted in a complete reversal of V̇O2 max values. Related to components of the Fick equation, Perhonen et al.30 found that after two weeks of bed rest, Q̇ and SV decreased by 17% and 25%, respectively, from baseline values. Furthermore, the investigators30 reported an 8.0% decrease in left ventricular volume after six weeks of bed rest, whereas the right ventricular volume decreased by 10% during the same amount of time. Coyle et al.31 hypothesized that the decrease in SV may be linked to the reduction in blood volume and not necessarily decreases in cardiac dimensions. Perhonen et al.32 reported, however, that reductions in SV measured in an upright position were “… greater after bed rest than after acute hypovolemia alone…” (p.1856). With regard to C(a-v̄)O2 , studies have reported a reduction in the oxygen extraction capabilities of the skeletal muscle, which may be mediated by factors such as capillary density, myoglobin concentration, mitochondria size and density, and oxidative enzymes.27,33
When evaluating the pectus excavatum patient preoperatively and postoperatively, investigators need to document the patient’s habitual exercise history in order to minimize the confounding effects of deconditioning. This is a critical component of the evaluation process, because in the months following the surgical repair, patients may either reduce their level of physical activity or adopt a sedentary lifestyle in order to prevent displacement of the Adkins strut1 or Lorenz metal bar.34 Therefore, it is recommended that the mode (i.e., type of exercise performed), frequency (i.e., sessions per week), duration (hours per week), length of time the exercise regimen has been consistently maintained, and intensity of exercise be documented for each patient. Malek and colleagues24,25 used the following series of questions to document the habitual exercise history of individuals tested in their laboratory: “What type of exercise do you perform?”; “How many sessions per week do you exercise?”; “How many hours per week do you exercise?; “How long have you consistently, no more than one month without exercise, been exercising?; and “Indicate [using the Borg Rating of Perceived Exertion (RPE) 6–20 scale], in general, the intensity at which you perform your exercise regimen.
Subject PreparationBefore the CPET can begin, a number of procedures must be performed. In their meta-analyses, Malek and colleagues9,10 reported that studies varied in their approach to measuring pectus severity. Therefore, in order to compare findings across studies in the future, pectus severity should be assessed using the procedures of Haller et al.5 This approach uses the CT scan and provides a more objective method of estimating pectus severity than do other approaches such as the Welch index,35 which utilizes lateral chest x-ray.5 It should be noted, however, that CT scans are not part of standard assessment procedures for evaluating pectus severity; they may be cost-prohibitive. Nevertheless, investigators should justify this cost in their grant or department budget. In addition, if a complete pulmonary function test has not been conducted, then an abbreviated version should be performed to determine forced expiratory volume in the first second (FEV1), forced vital capacity (FVC), and maximum voluntary ventilation (MVV) values (see ventilatory response variables section for details). The 12-lead ECG electrodes should be placed in accordance with standard guidelines.15 Although there is little information in the literature regarding the patterns of heart rhythm in pectus excavatum patients, we recommend placement of ECG electrodes such that any abnormal rhythms may be documented. Depending on the severity of the pectus excavatum, however, electrode placement may have to be modified (particularly leads v1, v2 and v3) at the discretion of the attending physician and/or exercise physiologist.
Cardiopulmonary Test (Exercise Phase)Prior to beginning the CPET, the seat height of the cycle ergometer should be adjusted so that the patient has a slight bend (≈ 5°) in their knees. The handle bars should be adjusted so the patient is not leaning forward but rather has an erect posture. Furthermore, the mouthpiece and breathing valve should be adjusted so that the patient is not struggling to maintain the apparatus in their mouth. The nose clip should be properly placed over the nostrils so that no air is escaping as the patient exhales. Also, the metabolic cart should be calibrated prior to each testing session. The CPET protocol that is recommended includes three minutes of rest (i.e., baseline), followed by three minutes of warm-up at unloaded pedaling (i.e., 0 W), and then an incremental (i.e., ramp) increase in power output every minute (15 to 20 W·min1) thereafter.6,7,11,12 The determination of the ramp rate often depends on the patient’s level of physical conditioning and, therefore, should be individualized. For example, a faster ramp rate (i.e., 20 or 25 W·min1) is appropriate for those individuals who engage in regular endurance exercise, whereas a slower ramp rate (i.e., 10 or 15 W·min−1) may be more appropriate for those individuals who are disabled or sedentary.13 The ramp protocol is recommended over a step protocol (i.e., increase in power output every two or three minutes), because the physiological responses are more uniform.36 The preferred cycling cadence, in a clinical setting, may range between 60 and 70 rev·min−1. In our experience with pectus excavatum patients, we have found that 60 rev·min1 is well tolerated across different degrees of severity.6,7
The criteria for terminating the CPET may range from absolute (i.e., acute myocardial infarction, sustained ventricular tachycardia, or request to stop by the subject) to relative (i.e., hypertensive response, changes in ST segment depression or elevation, or exercise-induced bundle branch block not distinguishable from ventricular tachycardia) indicators.12,13 If, however, none of the above indicators are present, patients should receive strong verbal encouragement in order to achieve the upper limits of their physiological capacity. In our laboratory,7,17,23,24,37,38V̇O2 max is considered achieved if two of the following three criteria during the test are met:13,39,40 a) 90% of age-predicted maximum heart rate (i.e., 220–age), b) respiratory exchange ratio > 1.20, and c) a plateauing of oxygen uptake (≤ 150 mL·min−1 in V̇O2 over the last 30 seconds of the test). In addition, V̇O2 max is determined by taking the highest V̇O2 value in the last 30 seconds of the CPET. It is important to note that the patient be allowed to cool-down following the CPET for as long as they want, with fc, blood pressure, and ECG being continually monitored until these indices return to those values observed during warm-up.
Physiological Response Variables and InterpretationDue to the lack of consistent physiological variables that are reported in the pectus excavatum literature resulting from CPETs,9,10 the following section will focus on aerobic capacity, cardiovascular, ventilatory, and gas exchange responses that should be reported in all future articles when CPET is performed on pectus excavatum patients preoperatively and/or postoperatively. Although each of the four categories can be classified by numerous indices, the physiological variables recommended below are easily obtained from the metabolic cart and require no additional equipment (i.e., blood lactate analyzer). This approach was intentionally designed so that each laboratory can provide the same information in their manuscripts without incurring additional cost for specialized equipment and/or personnel. In addition, data from the metabolic cart should be reported using moving averages. Most metabolic carts have a range of sampling options from breath-by-breath to 2-minute averages. We recommend that researchers use a moving average (5- or 8-breath). Myers et al.41 reported that 5- and 10-second averages of breath-by-breath data resulted in high variability (≈ 4.5 and 3.5 mL·kg−1·min−1, respectively), whereas moving averages (i.e., 5- or 8-breath) resulted in lower variability (≈ 1.7 and 1.6 mL·kg−1·min−1). The investigators41 concluded that the “…gain in accuracy is attained by using larger samples or averaging breaths. Although 60-s sampling offers the least variability (SD = 0.08 mL·kg−1·min−1), it could be argued that samples this large may be too imprecise for evaluating certain interventions.” (p.409).
Aerobic Capacity Response VariablesThe response to exercise is a function of numerous physiological mechanisms. The ability to sustain high-intensity exercise is contingent on four aerobic parameters: 1) V̇O2 max 2) the gas exchange threshold (GET), above which there is a sustained increase in blood lactic acid concentration; and 3) work efficiency represented as the slope of ΔV̇O2 /ΔẆ42. It should be noted that the fourth aerobic parameter, the time constant for oxygen uptake kinetics (τV̇O2 max), requires multiple constant-power output exercises, which are performed over a number of visits to the laboratory;43 therefore, it is not a practical assessment tool for this population. Thus, we focus our discussion on the first three aerobic parameters.
As discussed earlier, V̇O2 max is a measure of aerobic power and should be reported in both absolute (L·min−1) and relative (mL·kg−1·min1) terms. The normalization of V̇O2 max to body weight in kilograms allows the researcher to compare the patient’s V̇O2 max to normative data as well as other pectus excavatum patients within a sample and across studies. In addition, the patient’s V̇O2 max value should be reported as a percentage of their predicted V̇O2 max. Although there are numerous V̇O2 max prediction equations in the literature, we recommend that investigators use the equations by Cooper and Storer,12 because these were derived from a composite of five to six prediction equations that included a wide range of clinical populations as well as sedentary to mildly active individuals:
where ht is the patient’s height in meters, age is measured in years, and ABW is the actual body weight of the patient in kilograms. The equations of Cooper and Storer,12 however, are not appropriate to predict V̇O2 max for aerobically trained individuals/patients, which Malek et al.24,37 operationally defined as “…[individuals] who had participated in continuous aerobic exercise three or more sessions per week for a minimum of one hour per session, for at least the past 18 months.” Therefore, in such cases, the following equations by Malek et al.24,37 should be used to predict V̇O2 max in aerobically trained pectus excavatum patients:
where wt is the patient’s weight in kilograms, ht is the patient’s height in meters, age is measured in years, HPW is the hours per week of training, INT is the overall intensity of each training session as measured by the Borg RPE (6–20) scale, and YRT is the natural log of years of training. Regardless of which prediction equation is used, the researcher should also report the pectus excavatum patient’s V̇O2 max relative to the percentage of the predicted value (i.e., %predict. V̇O2 max = [observed V̇O2 max / predicted V̇O2 max] x 100).
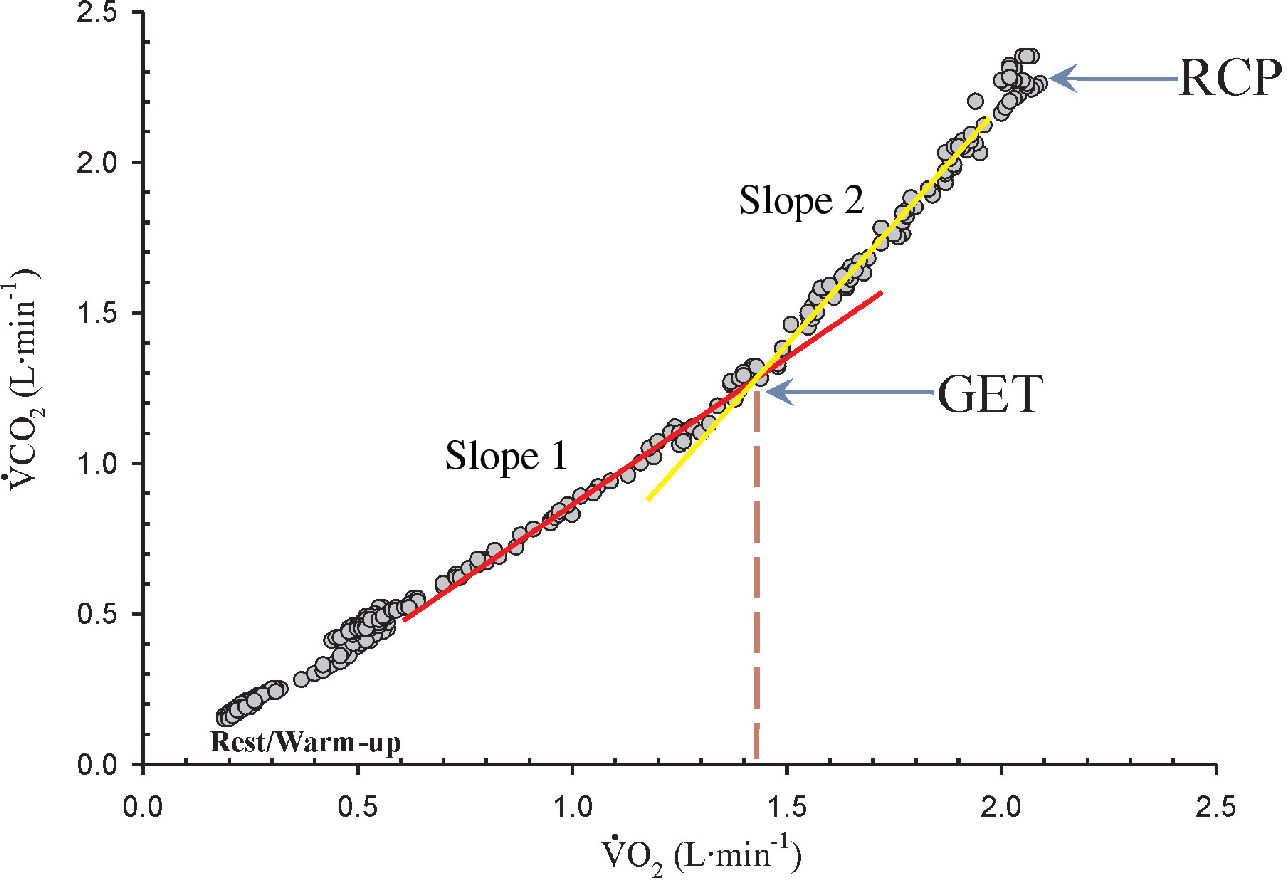
The GET is also known as the lactate acidosis threshold, or ventilatory threshold, but these terms are essentially used to describe the disproportionate increase in CO2 output relative to oxygen uptake resulting from an accelerated reliance on glycolysis for energy production during incremental exercise11,12. The GET has been used to identify an individual’s level of aerobic fitness in clinical44,45 and sports46,47 related settings, as well as for monitoring training adaptations.22 The GET is determined through the V-slope method48 by using either regression analysis or visual inspection by trained personnel. Briefly, as shown in Figure 4, V̇O2 and VCO2 increase proportionately at the beginning of incremental exercise, thus yielding a linear slope. As the exercise bout continues, a second slope develops due to the disproportionate increase in CO2 output relative to oxygen uptake.11,13 However, because of the potential of acute hyperventilation, it is recommended that the V-slope method be used in conjunction with analyses of the ventilatory equivalents (i.e., V̇E /V̇O2 and V̇E /V̇CO2) and end-tidal gas tensions (i.e., PETO2 and PETCO2) for oxygen and carbon di-oxide [for a detailed discussion refer to Wasserman et al.11]. Gaskill et al.49 recently reported that determining the GET using a combination of the V-slope, ventilatory equivalents, and end-tidal gas tensions methods was more reliable than using any of these methods separately. The GET values should be reported in terms of oxygen uptake similar to the V̇O2 max values (i.e., L·min−1 and mL·kg−1min−1) as well as a percentage of the predicted V̇O2 max (i.e., %pred V̇O2 max = [measured GET / predicted V̇O2 max] x 100).12 It is recommended that the predicted V̇O2 max value be used, and not the measured V̇O2 max value, because the “…measured V̇O2 max can produce serious errors of interpretation in the case of a suboptimal effort…” (p.103),12 which is more likely in clinical settings. Typically, in healthy individuals, the %pred V̇O2 max for GET occurs at 50% to 60%, whereas in diseased populations, this value is often equal to or less than 40%.12 For example, Malek et al.7 reported that physically active pectus excavatum patients with a severity index greater than 5.0 had GET values ≤39%. Thus, investigators can use % pred V̇O2 max to characterize the level of conditioning in their sample, which may provide insight into the patient’s muscle energetics.
The measure of work efficiency, ΔV̇O2/ΔẆO2 , is independent of gender, age, or height and has a consistency for apparently healthy subjects, being 10.3 mL·min−1·W−1 (SD = 1.0 mL·min−1·W−1 ) with a 95% confidence interval of 8.3 to 12.3 mL·min−1·W−1.12,13 The ΔV̇O2/ΔẆ examines the oxygen utilization by the skeletal muscles in their performance of work. Poole et al.50 found that the Δ V̇O2/ΔẆ calculated from pulmonary gas exchange measurements and that measured directly from the leg (i.e., constant-infusion thermodilution) had similar values (9.9 vs. 9.2 mL·min−1·W−1). Therefore, the investigators50 concluded that Δ V̇O2/ΔẆ represents the muscular efficiency of the exercising limb. A reduction in V̇O2/ΔẆ may indicate inadequacies with factors related to oxygen transport [for a detailed review, refer to citation 33].
Cardiovascular Response VariablesHeart Rate (fc) and Cardiac ReserveThe cardiovascular response to incremental exercise is one potential limitation to exercise tolerance.11 In pectus excavatum patients, the assessment of cardiovascular variables can provide valuable information related to the efficiency of surgical repair as well as the patient’s response to maximal exercise. Some researchers have used the predicted fc max as a marker for achieving the upper-limits of exercise tolerance, and thus terminate the CPET on this basis. It is well-agreed in the exercise physiology literature that this approach is inappropriate because of the variances associated with the available prediction equations for fc max16 Despite this drawback, however, the patient’s fc max relative to their predicted value should be reported. Typically, one of two prediction equations are used to estimate a fc max: 1) 220–age or 2) 210-(age x 0.65).11,12 Regardless of which equation is used to estimate fc max, investigators should report the mean and standard deviation of the actual and the percentage of predicted (i.e., [actual fc max/ predicted fc max] x 100) for their sample. In addition to reporting fc max, the resting fc value, which is recorded during the baseline phase of the CPET, should also be reported by the investigator.
Related to fc max is cardiac reserve, defined as the difference between the measured and predicted fc max values. This information can be used to determine whether the subject achieved cardiovascular limitation. As a general rule, a small cardiac reserve value and a high ventilatory reserve value (discussed below) indicates cardiovascular limitation to incremental exercise.11,12
Oxygen Pulse (V̇O2 max / fc max)Due to the close relationship between fc and V̇O2 (Figure 3), the slope of the fc / V̇O2 relationship can provide information related to the patient’s level of cardiovascular conditioning. Δfc / ΔV̇O2 is the slope of the relationship between heart rate and V̇O2 during incremental exercise. This slope is related both to stroke volume and to the difference in oxygen content between arterial and mixed venous blood11–13. Δfc / ΔV̇O2 s also the reciprocal of the asymptotic oxygen pulse (V̇O2 / fc), which is a measure of cardiovascular efficiency with units of milliliters per beat.11–13 Thus, V̇O2 / fc is closely related to SV and may be used to estimate SV at various stages of incremental exercise testing.11–13
Ventilatory Response VariablesThe examination of ventilatory indices during incremental CPET provides information related to the pectus excavatum patient’s pulmonary function. Previous studies have found that values for pulmonary function indices, such as FEV1, FVC, and MVV, are in the low normal range and that pectus excavatum does not influence the patient’s overall pulmonary function.6,7,10,51–53 (For a detailed review of the effects of pectus excavatum on pulmonary function, refer to citation 10). Because pulmonary function tests are effort-dependent, it is recommended that the patient perform several trials with strong verbal encouragement from the pulmonary technician. For detailed guidelines regarding pulmonary function testing, refer to citation.54 Consistent with these findings, Malek et al.10 found a small mean-weighted effect size (ES = 0.07, P > 0.05) for pulmonary function following surgical repair, but noted that many of the indices were measured at rest and not during exercise. It is well-established in the clinical exercise physiology literature that examination of ventilatory function during CPET is equally as important in patient assessment as cardiovascular function.11–13,55 Therefore, future studies should examine and report the following ventilatory responses to CPET in order to more accurately assess the potential impairment of pectus excavatum on pulmonary function.
Minute Ventilation (V̇E)At rest, breathing frequency (fR) in healthy individuals is approximately 12 to 15 beats·min−1, whereas tidal volume (VT) is 0.500 liters. Minute ventilation (V̇E) is thus the product of fR and VT and is typically 6.0 L·min−1 at rest. However, during vigorous exercise fR and VT increase to 50 beats·min−1 and 3.0 L·min−1, respectively, resulting in a V̇E of 150 L·min−1. It should be noted, however, that during low-intensity exercise, VT primarily increases, whereas during higher-intensity exercise (i.e., 80% of V̇O2max), both VT and fR increase, with only fR increasing as the individual approaches V̇O2max11–13V̇E is a critical component of regulating acid-base balance during increased metabolic demands by the exercising muscles.11–13 During the CPET, the relationship between CO2 output and V̇E is mostly linear, up to approximately 85 to 90% of V̇O2max.11–13 Thereafter, V̇E increases disproportionately to CO2 output. This breakpoint is called the respiratory compensation point (RCP). The underlying mechanism of the RCP is related to the stimulation of the peripheral chemoreceptors of the carotid bodies.
Ventilatory ReserveTo evaluate whether the patient exhibited ventilatory limitation to exercise, the researcher should compare the V̇Emax value attained at V̇O2max as a percentage of the patient’s MVV value (i.e., %MVV = [V̇Emax/MVV] x 100). In healthy individuals, the %MVV at V̇O2max is approximately 50–70%.,11–13 whereas in patients with respiratory disease (e.g., COPD), this value may be greater than 80%.11,56,57 Johnson et al.58 reported that, in healthy individuals, the V̇Emax achieved at maximal exercise capacity was highly correlated with the measured MVV. In order to better understand the physiological limitation of pectus excavatum to exercise, future studies should report the %MVV value for their sample in addition to the V̇Emax and MVV values.
CONCLUSIONIn summary, Table 1 provides a step-by-step approach that should be used when assessing cardiopulmonary responses in a pectus excavatum patient before and after surgical repair. Although investigators are encouraged to use other laboratory techniques to assess the effects of pectus excavatum on cardiovascular and pulmonary function, it is imperative that, at a minimum, they conduct a CPET as outlined above and report the corresponding indices.
Step-by-step approach for CPET of pectus excavatum patients
| Preparation Phase |
|
| Subject Preparation |
|
| Exercise Phase |
|
| Aerobic capacity response variables |
|
| Cardiovascular response variables |
|
| Ventilatory response variables |
|
Note: Refer to text for discussion of each variable and procedure.