Studies comparing high frequency oscillatory and conventional ventilation in acute respiratory distress syndrome have used low values of positive end-expiratory pressure and identified a need for better recruitment and pulmonary stability with high frequency.
OBJECTIVETo compare conventional and high frequency ventilation using the lower inflection point of the pressure-volume curve as the determinant of positive end-expiratory pressure to obtain similar levels of recruitment and alveolar stability.
METHODSAfter lung lavage of adult rabbits and lower inflection point determination, two groups were randomized: conventional (positive end-expiratory pressure = lower inflection point; tidal volume=6 ml/kg) and high frequency ventilation (mean airway pressures= lower inflection point +4 cmH2O). Blood gas and hemodynamic data were recorded over 4 h. After sacrifice, protein analysis from lung lavage and histologic evaluation were performed.
RESULTSThe oxygenation parameters, protein and histological data were similar, except for the fact that significantly more normal alveoli were observed upon protective ventilation. High frequency ventilation led to lower PaCO2 levels.
DISCUSSIONDetermination of the lower inflection point of the pressure-volume curve is important for setting the minimum end expiratory pressure needed to keep the airways opened. This is useful when comparing different strategies to treat severe respiratory insufficiency, optimizing conventional ventilation, improving oxygenation and reducing lung injury.
CONCLUSIONSUtilization of the lower inflection point of the pressure-volume curve in the ventilation strategies considered in this study resulted in comparable efficacy with regards to oxygenation and hemodynamics, a high PaCO2 level and a lower pH. In addition, a greater number of normal alveoli were found after protective conventional ventilation in an animal model of acute respiratory distress syndrome.
Since its initial description, acute respiratory distress syndrome (ARDS) has been associated with high mortality rates.1 More recently, however, it has been demonstrated that the choice of ventilation strategy used to treat patients with ARDS can influence the mortality risk.2–4 The same applies to other forms of acute and severe pulmonary distress.5 This can be explained by the mechanisms underlying the lesions generated by mechanical ventilation. The lungs suffer a heterogeneous overall reduction in compliance, such that collapsed areas may be close to zones of hyperdistension, both of which are associated with aggravation of hypoxemia and lung injury.4 Lesions associated with mechanical ventilation include: barotrauma, which is generated by application of an excess of pressure during mechanical ventilation;6 volutrauma, which is secondary to an excessive volume of air that penetrates the areas of normal compliance;7 atelectrauma, which is secondary to maintenance in the area of the collapsed lung at end expiration during mechanical ventilation;8 and biotrauma, which describes effects on the lung and other organs caused by inflammatory mediators released by damaged pulmonary tissue.9,10
In order to minimize these effects, the use of high-frequency oscillatory ventilation (HFOV) with higher mean airway pressures (MAP) has been proposed,11,12 and new approaches to conventional ventilation have been developed such as permissive hypercapnia,13 open-lung ventilation14 and protective conventional ventilation (PCV), which is based on a combination of the first two methods.2
HFOV has demonstrated consistently better recruitment and pulmonary stability in the treatment of ARDS than conventional mechanical ventilation, which is typically used at low values of positive end-expiratory pressure (PEEP), resulting in higher alveolar instability.11,12,15,16 This comparison could be erroneous if one assumes that HFOV is superior to conventional ventilation for the treatment and prognosis of ARDS.
The objective of this study is to compare, in an ARDS animal model, the efficacy of HFOV to conventional mechanical ventilation using a PEEP based on the lower inflection point (LIP) of the pressure-volume (PV) curve, associated with the use of low tidal volumes. Recorded outcomes are oxygenation, hemodynamic effects and degree of damage to the pulmonary parenchyma.
MATERIALS AND METHODSThis study was conducted in the laboratory of the Experimental Research Unit of the Department of Pediatrics, and the protocol was approved by the Commission of Ethics for Analysis of Research Projects. The established protocol for management of laboratory animals was followed.
Preparation of the animals and establishment of the ARDS modelAdult New Zealand White rabbits were sedated (10 mg/kg ketamine and 0.1 mg/kg acepromazine, IM) and the anterior region of the neck was anesthetized (2% xylocaine), followed by dissection and insertion of 4 French catheters into the carotid artery (for blood pressure and heart rate monitoring and blood gas measurements) and the jugular vein (for continuous infusion of a 5% dextrose solution at 4 ml/kg/h). The trachea was exposed and an endotracheal tube (3.5 mm I.D. or 4.0 mm I.D.) was passed into it and secured. Blood pressure, heart rate and body temperature were continuously monitored. The latter was maintained between 38 oC and 39.5 oC using warming pads. During the entire study, the animals remained in the dorsal decubitus position.
Generation of the ARDS experimental model was performed according to the technique described by Lachmann et al.17 Briefly, successive pulmonary lavages were carried out with saline at 37 oC for approximately 60 seconds (20 s for infusion and 40 s for withdrawal, using external thoracic compression movements) in 30 ml/kg aliquots and at a maximum infusion pressure of 40 cm H2O. The procedure was repeated at 3 to 5 minute intervals, until the partial pressure of O2 (PaO2)/fractional inspired oxygen concentration (FiO2) ratio dropped to less than 100. At this moment, the LIP of the PV curve was determined. During the entire lavage procedure, the animals were ventilated as follows: PEEP, 5 cm H2O; respiratory rate, 50 breaths/min; inspiratory time, 0.5 s; FiO2, 100%; and the peak inspiratory pressure was continuously adjusted to achieve a desired and fixed tidal volume (Vt) of 10 ml/kg. In the case of systemic arterial hypotension (defined as a mean systemic blood pressure <50 mm Hg), noradrenaline was used at a starting concentration of 0.2 μg/kg.min.
Determination of the lower inflection point of the inspiratory PV curve (LIP)To determine the LIP, a 60 ml syringe containing pure oxygen was connected to the endotracheal tube and to a closed computerized system for data acquisition (LabView 5.1, National Instruments), developed specifically for this purpose (R.A. Eletro Sistemas Ltda, Campinas, Brazil). The pressure was read through a pressure sensor (model DP45-24, Validyne Corp., Northridge, CA), and the volume was measured using a pneumotachometer (model 3700 series, Hans Rudolph Inc., Kansas City, MO).
After aspiration of the endotracheal tube, each animal received pancuronium (0.1 mg/kg, IV) and was disconnected from the ventilator for 2 sec, followed by progressive inflation of the lungs with 100% O2 in regular aliquots of 2 ml up to a total volume of 60 ml, and at regular intervals over approximately 40 seconds and a maximum pressure of 25 cmH2O. Using the same data acquisition system, the inspiratory limb of the PV curve was constructed and the LIP was identified by visual analysis.18 The exact point was defined as the mean of the values found by two separate investigators. When these values differed by more than 2 cm H2O, the entire process was repeated. After the LIP determination, the animal was disconnected from the system used for the PV curve, left for a few seconds without any sort of pressure support, and then reconnected to the ventilator, as explained below.
Study groups and ventilation strategiesAfter LIP determination, the animals were randomized into two study groups according to the type of ventilation used: protective conventional ventilation (PCV) or high-frequency oscillatory ventilation (HFOV). Animals submitted to PCV were ventilated with a time-cycled pressure-limited infant ventilator (Inter 3®, Intermed, Sao Paulo, Brazil). PEEP was set equal to LIP; Vt, 6 ml/kg; inspiratory time, 0.5 s; respiratory rate, 50 breaths/min.; and FiO2, 100%.
The HFOV group was ventilated with a SensorMedics 3100B ventilator (SensorMedics Critical Care Corp., Yorba Linda, CA) set to deliver a MAP = LIP + 4 cm H2O; respiratory rate, 15 Hz; initial amplitude, 15 cm H2O; and FiO2, 100%. The amplitude was modified as necessary in order to obtain a target PaCO2 between 50 and 55 mm Hg.
Arterial blood samples were collected at 30 minute intervals (I-Stat®, Abbott Laboratories Inc, East Windsor, NJ, USA) for blood gas analysis. The indexes and parameters used for oxygenation evaluation were: PaO2/FiO2 ratio, oxygenation index (O.I. = [(FiO2 x MAP)/PaO2]x100), arterial-alveolar PaO2 ratio (a/A O2) and alveolar-arterial oxygen difference (A-a O2).
Sacrifice of animals and lung processingAfter 4 hours of ventilation, animals were deeply sedated with sodium pentobarbital (25 mg/kg, IV), followed by sacrifice via severing the abdominal aorta. The lungs were removed, weighed and thoroughly lavaged five times with cold saline (4 oC) via the endotracheal tube. The total alveolar lavage volume was recorded, and aliquots (200 μl) were used for protein content determination according to the method of Lowry.19
Lung histologyTissue samples from the left lung were formalin-fixed and paraffin-embedded. Five micron (5 μm) sections were H&E stained. Morphometric study of the lung architecture was performed by optical microscopy at 100x magnification, using a 100-point and 50-line grid of known area in one of the eyepieces. The analysis was blinded and conducted by a single investigator. Areas of normal and abnormal alveolar architecture, alveolar collapse and hyperdistension were quantified.20 Fifteen microscopic fields of alveolar parenchyma were read per slide. Values are expressed as percentage.
Data analysisAll values are given as mean ± SD. Differences over time and between ventilation groups were evaluated by two-way analysis of variance (two-way ANOVA) for repeated measurements, where time was the within-subjects factor and treatment was the between-subjects factor. The Student-Neuman-Keuls test was used for post-hoc analysis. When individual time points were compared, differences were analyzed by t-test. Histopathologic data was assessed by the Chi-square or Fisher’s exact test, as indicated. Statistical significance for all analyses was reported at p < 0.05.
RESULTSDescription of the animals:Eight animals were studied in each group. The variables PaO2/FiO2 ratio and the LIP were comparable in both group (Table 1).
Characteristics of the animals in each study group. Values are mean ± SD
| Group | N | Weight (g) | Number of lavages | PaO2/FiO2 | LIP |
|---|---|---|---|---|---|
| PCV | 8 | 2625 ± 160* | 4.9 ± 1.8 | 65.5 ± 22.7 | 14.1 ± 2.5 |
| HFOV | 8 | 3015 ± 320 | 5.1 ± 0.8 | 84.3 ± 15.6 | 14.0 ± 2.0 |
PCV: Protective conventional ventilation; HFOV: High frequency oscillatory ventilation; LIP: Lower inflexion point of PV-curve.
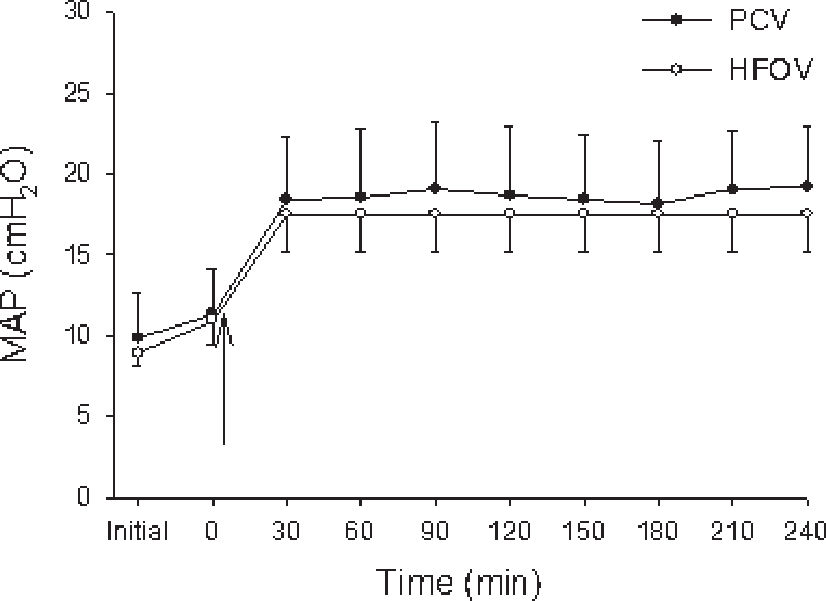
The two ventilation strategies (i.e., choice of PEEP or MAP based on LIP) resulted in similar MAPs of approximately 18 cm H2O (Figure 1).
Animals from PCV group (closed circles) and HFOV group (open circles) were ventilated with MAP around 18 cm H2O. Values are expressed in mean± SD. Both ventilation strategies resulted in similar mean airway pressures (MAP). “Initial” time are values before lung lavage; “zero” time corresponds to values immediately after the installation of the experimental model. The arrow indicates the moment of LIP determination and beginning of both ventilatory strategies
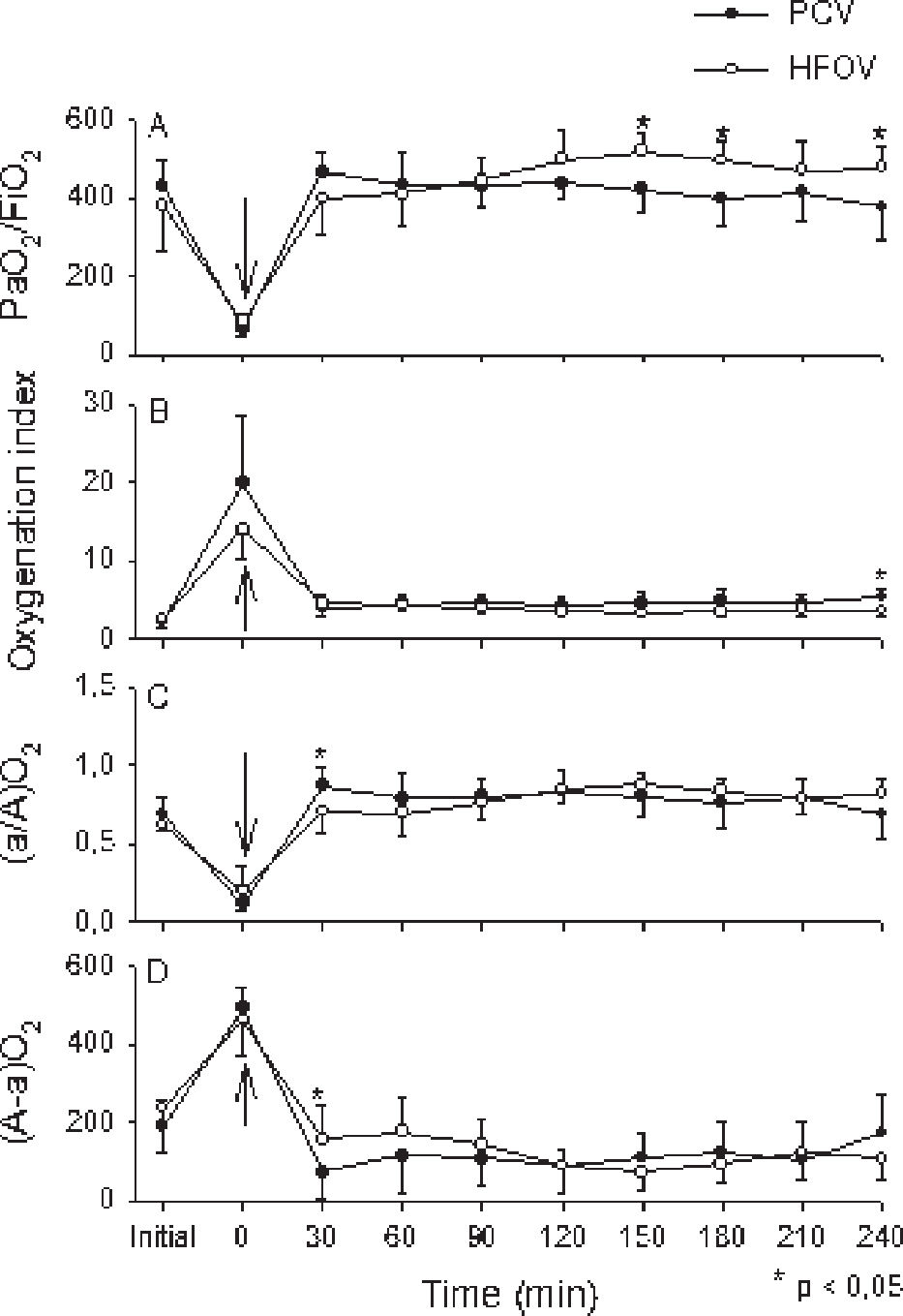
The indexes and parameters used for oxygenation evaluation during the 4 hour ventilation period are shown in Fig. 2, according to the gasometric values obtained at 30 minute intervals. The PaO2/FiO2 ratio was better in HFOV when compared to PCV at 150, 180 and 240 minutes (Figure 2A). The oxygenation index was also similar with both ventilatory strategies, except for a small but significant difference at 240 min, with a higher oxygenation index among animals from the PCV group (Figure 2B). When oxygenation was evaluated via the arterial-alveolar PaO2 ratio (a/A O2) and alveolar-arterial oxygen difference (A-a O2), we found a small and transient but significantly higher oxygenation at 30 min. with PCV vs. HFOV (Figures 2C and 2D).
Group mean±SD are shown for PCV group (closed circles) and HFOV group (open circles). “Initial” time are values before lung lavage; “zero” time corresponds to values immediately after the installation of the experimental model. The arrow indicates the moment of LIP determination and beginning of both ventilatory strategies. Panel A : PaO2/FiO2. The HFOV group presented better oxygenation at 150, 180 and 240 min; Panel B: Oxygenation index; Panel C : (a/A)O2and Panel D (A-a)O2. Similar increase in oxygenation with the beginning of ventilatory strategies was seen in both groups, with small but significant difference favoring the HFOV group at 240 min (Panel B) and 30 min (Panel C and Panel D)
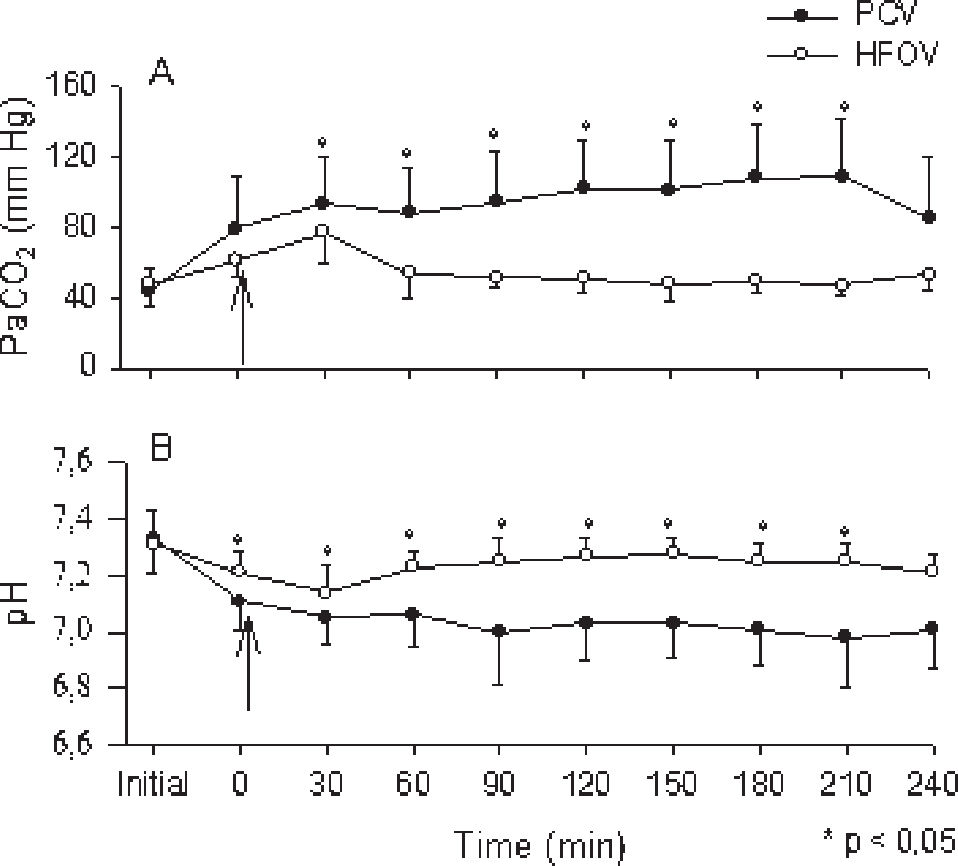
The protective ventilatory approach used for PCV resulted in different PaCO2 levels, with lower values in the HFOV group (Figure 3A). This also resulted in lower pH values among animals from the PCV group, which remained between 7.0 and 7.2 throughout the ventilation time (Figure 3B).
Group mean±SD are shown for PCV group (close circles) and HFOV group (open circles). “Initial” time are values before lung lavage; “zero” time corresponds to values immediately after the installation of the experimental model. The arrow indicates the moment of LIP determination and beginning of both ventilatory strategies. Panel A : PaCO2. Due to the protective ventilatory approach adopted, the PaCO2 values were higher among animals from PCV group; Panel B: pH. Significant decrease was seen among animals from PCV group
During the entire study period, no differences were found between the groups with regards to arterial blood pressure and heart rate (Table 2). Noradrenaline was administered to 3 animals in the HFOV group and 4 animals in the PCV group to maintain a systemic blood pressure higher than 50 mm Hg.
Characterization of the hemodynamic repercussion (mean arterial systemic pressure, and heart rate), lung weight (normalized to body weight) and total protein present in alveolar lavage (normalized to body weight) in both study groups. Values given as mean ± SD
| Group | N | MASP | Heart rate | Lung weight (g/kg) | Protein (mg/kg) |
|---|---|---|---|---|---|
| PCV | 8 | 79.2±4.0 | 199±4 | 5.9 ± 2.0 | 38.5 ± 18.6 |
| HFOV | 8 | 98.1±3.9 | 193±4 | 6.4 ± 2.2 | 47.4 ±28.1 |
MASP: mean arterial systemic pressure; PCV: Protective conventional ventilation; HFOV: High frequency oscillatory ventilation.
No differences were found between the groups with regards to lung weight and proteins present in the alveolar lavage, indicating that both ventilation strategies resulted in similar lung damage (Table 2). For the histologic evaluation of lung injury, hyperinflated and collapsed alveoli were grouped together and the architecture redefined as abnormal alveoli. The findings from normal and abnormal alveoli were then compared. There were more normal alveoli in the PCV group than in the HFOV group (p < 0.01). Values are presented in Table 3. In both groups, a large number of alveolar neutrophils were identified.
Alveolar architecture changes according to the type of ventilatory strategy. Abnormal alveoli were collapsed or hyperinflated. Values expressed as percentages
| Group | Abnormal alveoli | Normal alveoli | Total |
|---|---|---|---|
| PCV | 43.2% | 56.8% | 100% |
| HFOV | 51.5% | 48.5% | 100% |
PCV: Protective conventional ventilation; HFOV: High frequency oscillatory ventilation. p<0.01
The use of experimental models to study ARDS and the impact of mechanical ventilation has been covered by various publications in the last few years. Several experimental models have been used, including rabbits,12,21–25 pigs,26 rats27,28 and sheep,18 with different techniques for the induction of ARDS. The most frequent technique used for ARDS induction is the saline lung lavage model, which has the advantage of easy reproducibility and none of the disadvantages of the other techniques.17,22 However, most of the studies that compared protective conventional ventilation with HFOV did not use a strategy to avoid alveolar collapse, meaning that it was impossible to accurately compare the two ventilatory techniques.
This study explores the use of the LIP of the PV curve to tailor the ventilator settings such that the comparison between CPV and HFOV is not influenced by the use of PEEP below the opening pressure. Our results demonstrate that in this experimental model of ARDS, the use of conventional mechanical ventilation using the LIP of the PV curve to determine the PEEP associated with a protective strategy is comparable to HFOV. Oxygenation was comparable; neither the hemodynamic status nor the markers of lung injury were altered, suggesting that the use of PEEP equal to the LIP value can result in similar oxygenation and aggravation of the pulmonary parenchyma, as compared with HFOV.
Unlike the strategy used in this study, the adoption of low PEEP values in previous reports might have contributed to the demonstration of advantages with HFOV when compared to conventional ventilation, due to the impossibility of obtaining an appropriate alveolar volume at the end of expiration with conventional ventilation.11,23,29
We considered PaO2/FiO2 values around 500 mm Hg25–27 or PaO2 + PaCO2 levels higher than 400 mm Hg30 as “open lung” indicators, with the selection of PEEP in PCV and MAP in HFOV guided by the LIP of the inspiratory limb of the PV curve. We obtained these values with both ventilatory strategies at an early stage without requiring alveolar recruitment maneuvers.
A potential bias in this type of study is the method of LIP determination. The software developed for data acquisition and LIP determination is very precise, and the researchers came up with similar values for the LIP for all animals studied. We found LIP values similar to those described in other studies with this animal species22,24,25 and, more importantly, the values were much higher than the PEEP used in many studies that compared strategies of conventional mechanical ventilation with HFOV.
The adoption of MAP values in HFOV of 4 cm H2O above LIP was based on the description of Luecke, who obtained better results with this ventilation strategy when compared to a MAP equal to LIP.26
There is a still a significant debate about the ultimately different pulmonary response in pulmonary and extrapulmonary ARDS. There are different data that have arisen from animal and clinical studies.31,32 Even the initial approach to a patient with respiratory distress can have an important influence on the diagnosis of ARDS; thus, it is difficult to determine whether ARDS begins in the lungs or if it is a result of an inflammatory process that starts elsewhere.33–35 Recently, aggressive recruitment maneuvers were able to open very sick lungs in patients with both pulmonary and extrapulmonary early ARDS, suggesting that the aim of the respiratory assistance should be to reverse the airspace collapse with a pulmonary shunt.36
The ventilatory strategy adopted to minimize ventilation-induced lung injury through the use of low Vt for animals from the PCV group resulted in higher PaCO2 values, compared to the more appropriate values observed in the HFOV group, due to the possibility of adjusting the HFOV to a target PaCO2 around 50 to 55 mm Hg. The pulmonary shunt estimated by (A-a)O2 and (a/A)O2, reflected an appropriate lung response to both ventilatory strategies, with similar results during all ventilation time. Recently, new evidence has suggested a protective and therapeutic role of CO2, including an improvement in surfactant function due to a reduction of inflammatory mediators in the alveolar lavage and a reduction of the intrapulmonary shunt.37,38
In spite of the use of higher PEEP values, no hemodynamic repercussions were observed in either ventilation method. In fact, these are typically associated with the use of high plateau pressures rather than elevated PEEP values.39 Hyperdynamic states have been described as being associated with permissive hypercapnia;39 however, this was not observed in the present study.
The lung weight, as with the total amount of protein in the alveolar lavage, indirectly reflects the intensity of the inflammatory process generated by ARDS and the method of ventilation. This, in the final analysis, indicates the dramatic modifications present in the alveolocapillary membrane in ARDS, with a corresponding loss of selectivity.40 The finding of similar values in both groups supports the observations that both ventilation methods resulted in comparable effects on the lung tissue.
Although there are reports of more frequent lung lesions in animals under conventional mechanical ventilation when compared to those under HFOV,12,23 in the present study, a comparison of normal and abnormal alveoli suggested that the lungs were better preserved in the PCV group than the HFOV group. This difference, however, was not translated into more favorable gasometric alterations in the PCV group. This finding reinforces the concept that the approach to PCV used in this study attenuates lung lesions.
This experiment yielded two important clinical considerations with regards to the determination of the LIP of the PV curve in conventional mechanical ventilation. The first is the importance of determining the adequate alveolar volume at end expiration when comparing different ventilatory strategies to treat ARDS. Second is the utility of bedside monitoring of respiratory mechanics and using the LIP to adjust ventilator settings in the treatment of ARDS. Our results reinforce the idea that the development of methods for precise LIP determination during severe respiratory insufficiency can improve upon conventional mechanical ventilation by improving oxygenation and reducing ventilator induced lung injury.
Based on our data, we conclude that the use of conventional mechanical ventilation based on PEEP as determined by the LIP of the PV curve, associated with low tidal-volume as a protective strategy, is comparable to HFOV with regards to oxygenation, hemodynamic status and lung injury in this experimental ARDS model.
The authors thank Dr. Marcelo B. Passos Amato and Susimeire Gomes from the Laboratory of Experimental Pneumology for their assistance during the study.