In the present study, the peripheral mechanism that mediates the pressor effect of angiotensin-(1-7) in the rostral ventrolateral medulla was investigated.
METHOD:Angiotensin-(1-7) (25 pmol) was bilaterally microinjected in the rostral ventrolateral medulla near the ventral surface in urethane-anesthetized male Wistar rats that were untreated or treated (intravenously) with effective doses of selective autonomic receptor antagonists (atenolol, prazosin, methyl-atropine, and hexamethonium) or a vasopressin V1 receptor antagonist [d(CH2)5 -Tyr(Me)–AVP] given alone or in combination.
RESULTS:Unexpectedly, the pressor response produced by angiotensin-(1-7) (16±2 mmHg, n = 12), which was not associated with significant changes in heart rate, was not significantly altered by peripheral treatment with prazosin, the vasopressin V1 receptor antagonist, hexamethonium or methyl-atropine. Similar results were obtained in experiments that tested the association of prazosin and atenolol; methyl-atropine and the vasopressin V1 antagonist or methyl-atropine and prazosin. Peripheral treatment with the combination of prazosin, atenolol and the vasopressin V1 antagonist abolished the pressor effect of glutamate; however, this treatment produced only a small decrease in the pressor effect of angiotensin-(1-7) at the rostral ventrolateral medulla. The combination of hexamethonium with the vasopressin V1 receptor antagonist or the combination of prazosin, atenolol, the vasopressin V1 receptor antagonist and methyl-atropine was effective in blocking the effect of angiotensin-(1-7) at the rostral ventrolateral medulla.
CONCLUSION:These results indicate that angiotensin-(1-7) triggers a complex pressor response at the rostral ventrolateral medulla that involves an increase in sympathetic tonus, release of vasopressin and possibly the inhibition of a vasodilatory mechanism.
The rostral ventrolateral medulla (RVLM) is well known as a key region in the central nervous system (CNS) for the tonic and reflex control of circulation (1), especially because it is considered to be the main source of the excitatory activation of sympathetic pre-ganglionic neurons that innervate the cardiovascular system (2,3). In addition, it is well accepted that the pressor response evoked by stimulation of neurons in the RVLM is caused by an increase in the total peripheral resistance that results mainly from the widespread stimulation of sympathetic vasomotor activity (4-6).
The RVLM has been identified as a site in the CNS where angiotensin (Ang) peptides may modulate arterial pressure (7-9). Currently, Ang II and Ang-(1-7) are recognized as the main effectors of the renin-angiotensin system (RAS) in different tissues and in the brain (10-12). The Ang selective receptors AT1 and Mas (13) are widely distributed in the CNS, especially in the areas related to cardiovascular control, including the RVLM (7,14,15). Microinjection of Ang II or Ang-(1-7) into the RVLM produces increases in arterial pressure in different species, especially in anesthetized (16) or conscious rats (17,18), which suggests that these peptides function as excitatory modulators in this region. The pressor effects of these peptides are selectively blocked by losartan, which is an AT1 receptor antagonist, or A-779, which is a Mas receptor antagonist (19). Furthermore, bilateral microinjection of the selective Ang-(1-7) antagonist A-779 into the RVLM produces a sustained reduction in the mean arterial pressure and heart rate (17), whereas microinjection of losartan, which is an AT1 receptor antagonist, does not affect arterial pressure (18,20,21). The pressor effect produced by Ang II at the RVLM is attributed to an increase in sympathetic activity at the periphery (9). In the present study, we evaluated the peripheral mechanism responsible for mediating the pressor effect produced by bilateral microinjection of Ang-(1-7) at the RVLM by using selective autonomic receptor blockers and a vasopressin V1 receptor antagonist.
MATERIAL AND METHODSAll experiments were conducted in accordance to the National Institutes of Health guide for the care and use of laboratory animals (NIH Publications No. 85-23, revised 1996) and the Comite de Etica em Experimentação Animal/Universidade Federal de Minas Gerais (CETEA/UFMG; http://www.ufmg.br/bioetica/cetea/).
Surgical proceduresThe experiments were performed using 119 male Wistar rats (260-280 g) that were anesthetized with urethane (1.4 g/kg i.p, Sigma Chemical Co, St. Louis, MO, USA). A tracheostomy was performed, and catheters were inserted into the abdominal aorta through the femoral artery and into the inferior cava vein through the femoral vein for the measurement of arterial pressure and injection of drugs, respectively. The animals were then placed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA, USA) with the tooth bar 11 mm below the level of the interaural line. The dorsal surface of the brainstem was exposed by a limited occipital craniotomy and incision of the atlanto-occiptal membrane as previously described (16).
Arterial pressure measurementsArterial pressure and heart rate (HR) were continuously monitored using a solid-state strain gauge transducer coupled to a Nihon–Kohden polygraph (Tokyo, Japan) or a blood pressure signal amplifier (Stemtech, Inc.–Quintron, Milwaukee, WI, USA) connected to a data acquisition system (Codas Instruments, Akron, OH, USA) with a 600-Hz sampling rate.
Microinjection proceduresSimultaneous bilateral microinjections of Ang-(1-7) or sterile saline (vehicle; 0.9% NaCl) in a volume of 200 nl were performed over a 20-30-s period into the rostral ventrolateral medulla (RVLM: 2.0 mm anterior, 1.8 mm lateral to the obex) in the ventral surface just above the pia mater, as previously described (16). The microinjections were performed using Hamilton syringes fitted with metallic needles (injector needles, 30-gauge) and polyethylene tubing. The injector needle was introduced through a guide cannula (22-gauge) that was fixed to the stereotaxic manipulator. Only the injector needles were inserted into the brain tissue through the dorsal surface. The experiments were performed only at sites where the positioning of the injector needle produced a transient mechanical pressor response, as we previously found this to be an indication of the correct placement into the RVLM (16). In all animals, a minimum time interval of 20 minutes was given between the positioning the injector needles and the beginning of the experiments (peripheral treatment and/or RVLM microinjection).
Experimental Protocols1. Cardiovascular effects of Ang-(1-7) at the RVLMThe effects on arterial pressure and heart rate produced by RVLM bilateral microinjection of saline (200 nl, n = 5) or Ang-(1-7) in different animals at doses of 2.5 pmol (n = 4), 12.5 pmol (n = 7), 25 pmol (n = 12) or 50 pmol (n = 5) were evaluated.
2. Cardiovascular effects of Ang-(1-7) after peripheral blockade of the autonomic nervous system and/or vasopressin antagonismThe effects on arterial pressure and heart rate produced by bilateral microinjection of Ang-(1-7) (25 pmol) into the RVLM were evaluated after intravenous treatment with: 1) the β1-adrenergic receptor antagonist atenolol, 2.5 mg/kg (n = 6); 2) the α1-adrenergic receptor antagonist prazosin, 90 μg/kg (n = 7); 3) the muscarinic receptor antagonist methyl-atropine (M-atropine; 2.0 mg/kg, n = 5); 4) the ganglionic blocking agent hexamethonium, 20 mg/kg (n = 8); 5) the vasopressin V1 receptor antagonist [d(CH2)5-Tyr(Me)-AVP] (VPa), 10 μg/kg (n = 7); 6) 90 μg/kg prazosin combined with 2.5 mg/kg atenolol, (n = 6); 7) 90 μg/kg prazosin combined with 2.0 mg/kg M-atropine (n = 6); 8) 2.0 mg/kg M-atropine combined with 10 μg/kg VPa (n = 4); 9) 90 μg/kg prazosin combined with 2.5 mg/kg atenolol and 10 μg/kg VPa (n = 7); 10) 90 μg/kg prazosin combined with 2.5 mg/kg atenolol and 10 μg/kg VPa, and 2.0 mg/kg M-atropine (n = 5) or 11) 20 mg/kg hexamethonium combined with 10 μg/kg VPa (n = 7). In another group of animals, effects on arterial pressure and HR produced by bilateral microinjection of glutamate (20 nmoles) were observed before and 20 minutes after peripheral treatment with 90 μg/kg prazosin combined with 2.5 mg/kg atenolol, and 10 μg/kg VPa (n = 4).
To control for non-specific effects produced by the injection volume and/or tissue damage we evaluated the effects on arterial pressure and HR produced by bilateral microinjection of vehicle (saline; 0.9% NaCl, 200 nl) into the RVLM of additional animals, following peripheral treatment with: 1) 90 μg/kg prazosin (n = 6); 2) 10 μg/kg VPa (n = 2); 3) 90 μg/kg prazosin combined with 2.5 mg/kg atenolol and 10 μg/kg VPa (n = 3); 4) 90 μg/kg prazosin combined with 2.5 mg/kg atenolol, 10 μg/kg VPa and 2.0 mg/kg M-atropine (n = 3). Saline was microinjected into the RVLM 5-20 minutes after the intravenous treatment.
Histological verification of injection sites:
At the end of each experiment, in all animals, the injector needles were withdrawn and replaced by a pair of needles containing Alcian blue dye (5%). The ventromedullary sites were marked by the injection of 200 nl of the dye, bilaterally. The brain was then carefully removed and fixed in 10% phosphate-buffered formalin. Serial coronal sections (25-50 μm) of the medulla oblongata were obtained and stained with neutral red. The microinjection sites were identified by the deposition of Alcian blue dye using light microscopy and referred to standard anatomical structures of the brain stem according to the atlas of Paxinos & Watson (22).
DrugsAng-(1-7), glutamate, VPa and the autonomic blockers were dissolved in sterile isotonic saline (NaCl, 0.9%) immediately before use. Ang-(1-7) was purchased from Bachem (Torrance, CA, USA), and the autonomic receptor blockers, glutamate and VPa [d(CH2)5-Tyr(Me)-AVP] were purchased from Sigma Chemical Company (St. Louis, MO, USA). The doses of the autonomic blockers and VPa were chosen based on preliminary experiments that showed that the selected doses abolished the cardiovascular effects produced by each specific agonist for up to 60 min. In addition, the degree of blockade was evaluated in the majority of the animals at the end of each experiment.
Statistical analysisThe results are expressed as the means±SEM. The measured parameters before and after injection in the same animal were compared using Student's t-test for paired observations. Multiple groups were compared using ANOVA followed by the Newman-Keuls test. All data analysis was performed using the statistics program PRISM (Graphpad Prism Software, version 4.0; San Diego, CA, USA). The criterion for statistical significance was p<0.05.
RESULTSCardiovascular effects of Ang-(1-7) at the RVLMMicroinjection of Ang-(1-7) into the RVLM produced a shallow dose-dependent increase in MAP ranging from 11±2 mmHg with a dose of 2.5 pmol (duration: 9±2 minutes) to 19±4 mmHg with a dose of 50 pmol (duration: 24±9 minutes) (Table 1), as we have shown in previous studies (16,17). The changes in blood pressure were significantly different from the changes produced by the vehicle (change in MAP: 4±1 mmHg, duration: 6±2 minutes; n = 5, ANOVA followed by Newman-Keuls test). The pressor effect produced by Ang-(1-7) was not accompanied by consistent changes in HR (Table 1).
Baseline values for the mean arterial pressure (MAP, mmHg) and heart rate (HR, beats/min) as well as MAP and HR responses produced by RVLM bilateral microinjection of Ang-(1-7) or saline.
| Baseline Values | Changes in MAP (mmHg) | MAP Duration (min) | Changes in HR (beats/min) | |||
|---|---|---|---|---|---|---|
| n | MAP (mmHg) | HR (beats/min) | ||||
| Saline, 200 nl | 5 | 100±5 | 334±20 | 4±1 | 6±2 | -3±3.0 |
| Ang-(1-7) | ||||||
| 2.5 pmol | 4 | 109±6 | 375±20 | 11±2 | 9±2 | 1±2.5 |
| 12.5 pmol | 7 | 106±6 | 336±6 | 15±3∗) | 21±2∗) | -3±10 |
| 25.0 pmol | 12 | 108±5 | 339±8 | 14±1∗) | 14±3∗) | -5±12 |
| 50.0 pmol | 5 | 106±9 | 323±22 | 19±4∗) | 25±9∗) | -2±25 |
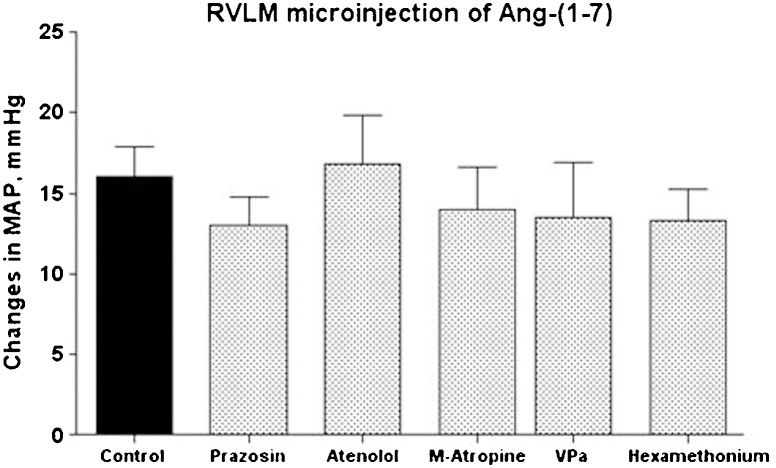
Table 2 shows the baseline values of the MAP and HR before and after the administration of different blockers or receptor antagonists. As expected, pre-treatment with the α1-adrenergic receptor antagonist prazosin or the ganglionic blocker hexamethonium produced a sustained decrease in MAP (Table 2). A significant decrease in HR was also observed after hexamethonium administration (Table 2). Atenolol or M-atropine produced significant changes in the baseline HR that were not accompanied by changes in the baseline values of MAP (Table 2). Surprisingly, none of these peripheral treatments alone significantly affected the magnitude or duration (data not shown) of the pressor response produced by the injection of 25 pmol of Ang-(1-7) into the RVLM (16±2 mmHg, control animals, n = 12; Figure 1.
Baseline levels for the mean arterial pressure (MAP, mm Hg) and heart rate (HR, beats/min) before and after peripheral treatment with autonomic and/or vasopressin receptor antagonists.
| Peripheral Treatment | Before treatment | After treatment | |||
|---|---|---|---|---|---|
| n | MAP (mmHg) | HR (beats/min) | MAP (mmHg) | HR (beats/min) | |
| Prazosin | 6 | 116±7 | 354±13 | 70±5∗ | 316±18 |
| Hexamethonium | 6 | 96±7 | 319±16 | 66±5∗ | 297±11∗ |
| Atenolol | 6 | 121±5 | 352±8 | 115±8 | 282±14∗ |
| Methyl-atropine | 5 | 105±8 | 339±8 | 96±7 | 423±14∗ |
| VP V1 antagonist | 6 | 106±4 | nd | 86±5 | nd |
| Prazosin+atenolol | 6 | 123±6 | 311±10 | 67±5∗ | 254±266∗ |
| Prazosin+methyl-atropine | 5 | 108±5 | 347±20 | 56±2∗ | 381±19∗ |
| Methyl-atropine+VP V1 antagonist | 4 | 107±9 | 320±6 | 84±4 | 345±17∗ |
| Prazosin+atenolol+VP V1 antagonist | 5 | 114±4 | 316±26 | 49±2∗ | 212±25∗ |
| Hexamethonium+VP V1 antagonist | 5 | 103±7 | 339±8 | 38±2∗ | 297±18∗ |
| Prazosin+atenolol+VP V1 antagonist+methyl-atropine | 5 | 111±9 | 371±6 | 43±5∗ | 282±11∗ |
Antagonist doses: prazosin, 90 μg/kg; atenolol, 2.5 mg/kg; methyl-atropine, 2 mg/kg; hexamethonium, 20 mg/kg; vasopressin (VP) V1 receptor antagonist, 10 μg/kg. The MAP and HR values before treatment were obtained 20 minutes after placement of the injector needle into the RVLM. nd = not determined. ∗p<0.05 in comparison to before treatment (Student's t-test).
Average change in the mean arterial pressure (MAP, mmHg) produced by bilateral microinjection of Ang-(1-7) (25 pmol) into the RVLM of control animals (n = 12) or rats treated with prazosin (90 μg/kg, n = 6); atenolol (2.5 mg/kg, n = 6); M-atropine (M-atropine, 2.0 mg/kg, n = 5); VP V1 receptor antagonist (VPa, 10 μg/kg, n = 6); or hexamethonium (20 mg/kg, n = 6).
Pre-treatment with VPa did not significantly change the baseline values of MAP (Table 2) or significantly modify the increase in MAP induced by the microinjection of Ang-(1-7) into the RVLM (14±3 mmHg, n = 6 in comparison to 16±2 mmHg of control rats, n = 12; Figure 1. Treatment with VPa produced a tendency to decrease the duration of the Ang-(1-7) MAP effect; however, the difference was not statistically significant (8.4±1.9 min in comparison to 14±3 min in control rats; p>0.05, Student's t-test; data not shown).
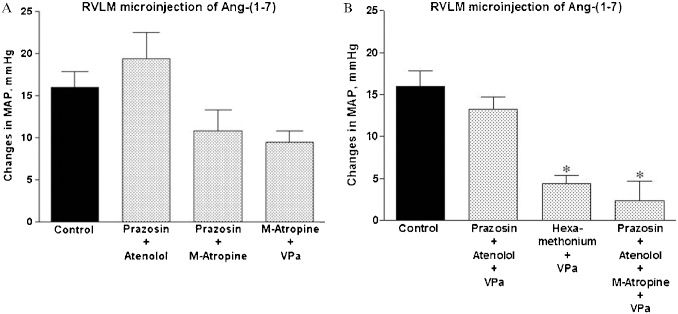
Cardiovascular effects of Ang-(1-7) in the RVLM after treatment with combinations of different autonomic blockers and a VP V1 antagonistThe combination of different blockers of the autonomic nervous system produced the expected changes in the baseline MAP and HR as shown in Table 2. The combination of α1 (prazosin) and β1 (atenolol) adrenergic blockade (Figures 2A and 3A) did not significantly affect the magnitude or duration of the pressor effect of Ang-(1-7). The combination of α1 adrenergic blockade (prazosin) and muscarinic receptor blockade (M-atropine) or the combination of M-atropine and the VP V1 receptor antagonist tended to reduce (∼30%) the magnitude (Figure 3A) of the pressor effect of Ang-(1-7) in the RVLM. However, the pressor effect was not significantly different from that observed after Ang-(1-7) microinjection in non-treated rats. The combination of α1- and β1-adrenergic blockade with VPa, which abolishes the pressor effect induced by the injection of 20 nmol of glutamate in the RVLM (1.2±1.1 mmHg in comparison to 25±2 mmHg before treatment, n = 4; data not shown) did not significantly influence the pressor effect of Ang-(1-7) in the RVLM (13±1 mmHg, n = 5 in comparison to 16±2 mmHg in untreated rats, n = 12; Figures 2B and 3B). Only the pre-treatment with the combination of α1 and β1 adrenergic blockade, M-atropine and VPa was able to effectively abolish the pressor action of Ang-(1-7) in the RVLM (Figures 2C and 3B). In addition, the pressor effect of Ang-(1-7) was significantly reduced by the combination of ganglionic blockade with hexamethonium and VPa (Figure 3B). Similarly to control rats, no significant changes in HR were observed after Ang-(1-7) microinjection in treated animals (data not shown).
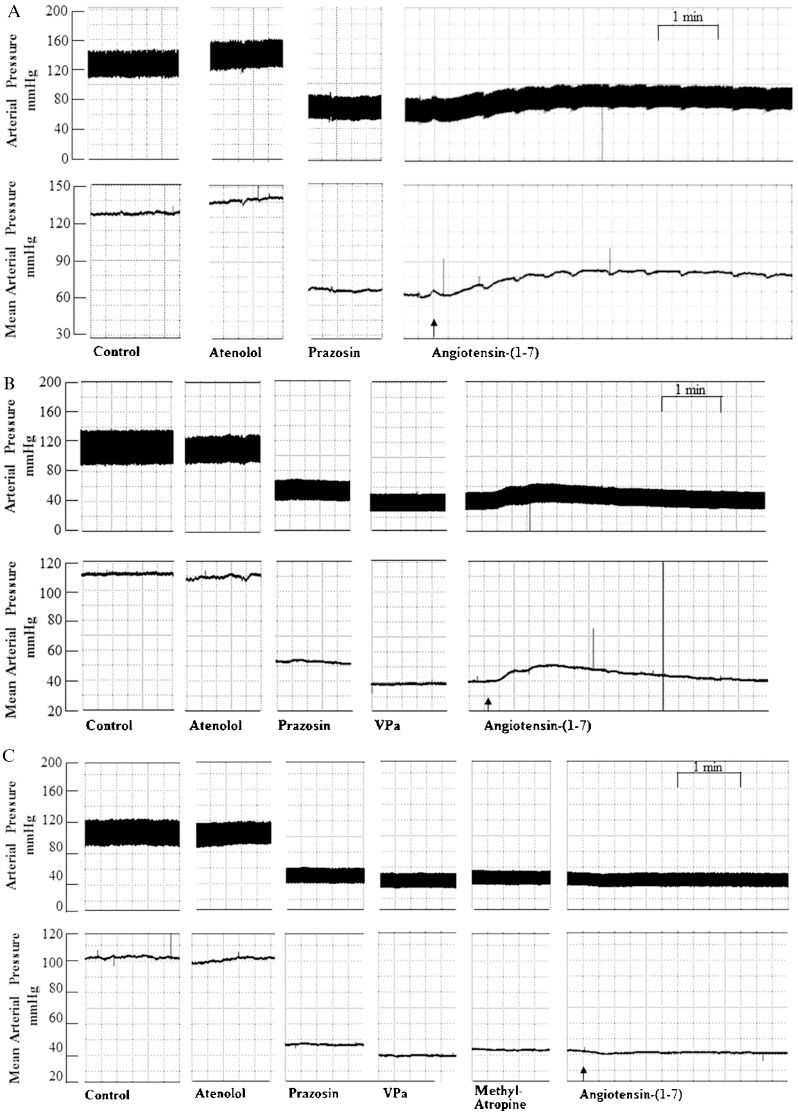
Pulsatile (mmHg; upper panels) and mean (mmHg; lower panels) arterial pressure tracings showing the typical effect of Ang-(1-7) (25 pmol) microinjection into the RVLM after peripheral treatment with (A) prazosin (90 μg/kg) followed by atenolol (2.5 mg/kg); (B) prazosin (90 μg/kg) combined with atenolol (2.5 mg/kg) and a VP V1 receptor antagonist (VPa, 10 μg/kg); and (C) prazosin (90 μg/kg) combined with atenolol (2.5 mg/kg), a VP V1 receptor antagonist (VPa, 10 μg/kg) and methyl-atropine (2.0 mg/kg). Arrows indicate the end of the microinjection into the RVLM.
Average changes in the mean arterial pressure (MAP, mmHg) produced by bilateral RVLM microinjection of Ang-(1-7) (25 pmol, n = 12) in control rats or rats treated with (A): prazosin, 90 μg/kg, combined with atenolol, 2.5 mg/kg (n = 5); prazosin, 90 μg/kg, combined with M-atropine, 2.0 mg/kg (n = 5); M-atropine, 2.5 mg/kg, combined with the VP V1 receptor antagonist (VPa, 10 μg/kg; n = 4) or (B): prazosin, 90 μg/kg, combined with atenolol, 2.5 mg/kg, and the VP V1 receptor antagonist (VPa), 10 μg/kg (n = 5); prazosin, 90 μg/kg, combined with atenolol, 2.5 mg/kg, VPa, 10 μg/kg and methyl-atropine, 2.0 mg/kg (n = 5); or hexamethonium, 20 mg/kg, combined with VPa, 10 μg/kg (n = 5). ∗p<0.05 compared with the effect produced by microinjection of Ang-(1-7) in control, untreated rats (ANOVA followed by Newman-Keuls test).
Microinjection of saline into the RVLM of animals treated with autonomic receptor antagonists and/or VPa produced similar increases in MAP that were averaged together (MAP changes = 5.5±0.8 mmHg, n = 14; data not shown). These changes were not significantly different from those observed in non-treated animals (4±1 mmHg, n = 5; Table 1).
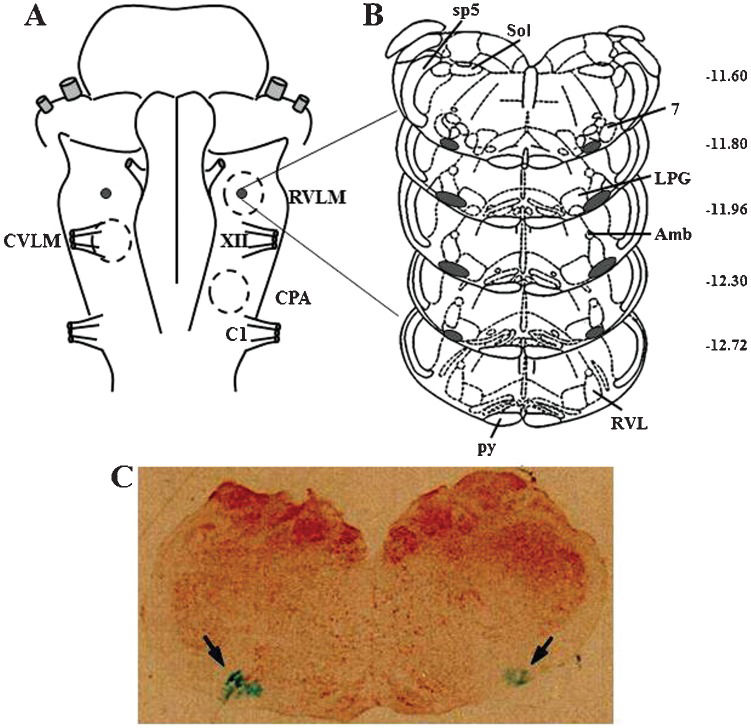
Histological examinationAs shown by the composite of the injection sites shown in Figure 4, the deposition of Alcian Blue dye (Sigma-Aldrich, St Louis, MO, USA) in all animals used in this study was confined to the ventral portion of the lateral paragigantocellular nucleus and the rostroventrolateral reticular nucleus.
(A) Diagram of the ventral surface of the medulla illustrating the localization of the injection sites (black dots) determined by macroscopic examination of the deposition of the dye in the brain stem of all animals. Dashed circles represent the areas of the ventral surface of the medulla involved in the control of blood pressure. (B) Diagrams of coronal sections of the medulla showing the localization of the injection sites (shaded area) determined microscopically by the spread of the dye. (C) Image of a histological section of the medulla showing the center of a bilateral microinjection into the RVLM marked by the deposition of the Alcian Blue dye (arrows). Maps and coordinates (in mm, right margin) are from the atlas of Paxinos and Watson (22). 7 = facial nucleus; Amb = ambiguus nucleus; C1 = root of the first cervical nerve; CPA = caudal pressor area; CVLM = caudal ventrolateral medulla; LPG = lateral paragigantocellular nucleus; py = pyramidal tract; RVL = rostroventrolateral reticular nucleus; RVLM = rostral ventrolateral medulla; Sol = solitary tract nucleus; sp5 = spinal trigeminal tract; XII = root of the hypoglossal nerve.
In the present study, we showed that the pressor effect induced by bilateral microinjection of Ang-(1-7) into the RVLM is not solely dependent on an increase in sympathetic drive; it also depends on the release of AVP and on the inhibition of the vasodilator mechanism. This conclusion was based on the following observations: 1) The effect of Ang-(1-7) was not affected by isolated peripheral treatment with α1 or β1 adrenergic blockade, VP V1 receptor antagonism, or even with a ganglionic inhibitor alone; 2) The combination of α1 and β1 adrenergic blockade with an AVP V1 receptor antagonist, which abolished the effect of glutamate, did not affect the pressor effect of Ang-(1-7); and 3) Only the combination of α1 and β1 adrenergic blockade with cholinergic blockade and a VP V1 receptor antagonist or the combination of ganglionic blockade with a VP V1 receptor antagonist completely abolished the pressor effect of Ang-(1-7).
The pressor effect produced by Ang-(1-7) at the RVLM is consistent with that described previously in anesthetized (16,17) or conscious rats (18). The lack of a clear dose-response curve is compatible with the well-known neuromodulatory effect of peptides (9,23). The finding that the Ang-(1-7) pressor effect was not accompanied by consistent changes in HR even in the presence of B1 receptor blockade indirectly suggests that a reduction in the vagal activity in the heart is not an important component for the pressor effect of Ang-(1-7). This finding agrees with our previous studies (16-18), and it is also a common observation after microinjection of Ang II in this region (7-9,24).
Surprisingly, the peripheral administration of the α1 adrenergic receptor antagonist prazosin or the β1 adrenergic receptor antagonist atenolol alone or in combination did not modify the pressor effect of Ang-(1-7). Moreover, the administration of hexamethonium, which is a ganglionic blocking drug, did not affect the activity of Ang-(1-7) at the RVLM. These results show that an isolated increase in sympathetic activity does not completely explain the pressor response induced by Ang-(1-7). A comparable observation was reported by Ross et al. (6), who studied the stimulatory effect of glutamate in the RVLM. These authors were able to significantly reduce, but not abolish, the pressor effect produced by glutamate by using adrenergic receptor blockade alone. Only the combination of adrenergic blockade and VP antagonism was sufficient to eliminate the cardiovascular effects of glutamate at the RVLM (6). The participation of VP in the pressor response of glutamate is consistent with the well-known projections of RVLM to the hypothalamic nuclei that are involved in the release of VP, such as projections to the paraventricular nucleus and supra-optic nucleus (20,25). Interestingly, our data show that the combination of adrenergic blockade and VP antagonism produced only a small reduction in the pressor effect of Ang-(1-7) at the RVLM. These data indicate that the stimulatory effect of glutamate depends on adrenergic and vasopressinergic mechanisms, but factors other than adrenergic and vasopressinergic mechanisms are involved in the effects of Ang-(1-7) at the RVLM.
Treatment with the VP V1 receptor antagonist alone did not alter the pressor effect of Ang-(1-7), as it did not affect the glutamate response (6). This finding suggests that in the intact animal, the release of VP stimulated through neurons projecting from the RVLM can be counterbalanced by arterial baroreceptor activity induced by the increased pressure elicited by sympathetic stimulation. This counterbalancing mechanism would be attenuated in the presence of a sympathetic blockade.
As a major observation in the present study, only the combination of ganglionic blockade with a VP V1 receptor antagonist or the combination of α1 and β1 sympathetic blockade, VP antagonism and muscarinic receptor blockade was effective in abolishing the effect of Ang-(1-7) in the RVLM. These results indicate that in addition to the increase in sympathetic output and the release of VP, Ang-(1-7) elicits a reduction of vasodilator tonus at the RVLM that is susceptible to blockade by M-atropine. At least part of this vasodilator tonus could be dependent upon hexamethonium-insensitive post-synaptic ganglionic receptors. Another possibility is the involvement of muscarinic receptors in the heart. Even though we cannot completely rule out this possibility, it is unlikely because the HR alterations induced by Ang-(1-7) microinjection into the RVLM were not consistent, even in animals treated with the combination of adrenergic blockade and AVP antagonism. Based on the present knowledge, it is difficult to conceive that an isolated increase in inotropism elicited by a decrease in vagal activity would result in an increase in cardiac output sufficient to explain part of the increase in arterial pressure.
We have previously shown that the peripheral mechanisms triggered by angiotensin peptides at the caudal ventrolateral medulla (CVLM) are also strikingly different (26). The hypotensive effect of Ang-(1-7) was not accompanied by significant changes in cardiac output, but it was completely abolished by peripheral treatment with M-atropine and the NOS inhibitors L-NAME and 7-NI (26).
It is well known that an active sympathetic neurogenic vasodilator system exists in the skeletal muscle vasculature of several species (27-31). Various neurotransmitters and neuromodulators are active in these neuro-vascular junctions. However, the relative contribution of these neurotransmitters is still poorly defined. In addition, there are no data in the literature that indicate a role of the RVLM in the modulation of a vasodilator tonus. It has been reported that centrally mediated activation of the lumbar sympathetic chain by air-jet stress produces pronounced hindlimb vasodilation that is mediated by a sympathetic neurogenic vasodilator system that uses nitric oxide (NO) and/or related nitrosyl factors (32). In addition, the selective inhibition of neuronal nitric oxide synthase has been shown to abolish the vasodilation of the hindlimb induced by the stimulation of the superior laryngeal nerve (33). Thus, taken together, these data provide evidence for a novel and intriguing possibility, i.e., the existence of a functional vasodilator cholinergic and/or nitrergic sympathetic vascular system that may be modulated by the CVLM/RVLM neuronal network.
In our study, peripheral treatment with M-atropine alone did not affect the effect of Ang-(1-7) in the RVLM, which indicates that the peripheral cholinergic system is not solely responsible for the effects of Ang-(1-7) in the RVLM. The participation of central cholinergic receptors in the hypotensive response of Ang-(1-7) is another possibility but seems unlikely given that M-atropine is a quaternary ammonium derivative of atropine that lacks a central effect because of its poor penetration in the brain (34). Another possibility is that the effect of M-atropine is related to the blockade of M1 ganglionic receptors. Although we cannot completely rule out this possibility, it seems unlikely because the dose of M-atropine used alone or in combination with other autonomic antagonists did not affect the baseline values for arterial pressure. In the present study, ganglionic blockade did not inhibit the RVLM pressor effect of Ang-(1-7). However, hexamethonium completely blocked the RVLM response induced by Ang II (7,9,35), which suggests that different pathways are stimulated by Ang II and Ang-(1-7) in the RVLM.
Finally, an important concern that is always raised in studies performed on anesthetized animals is the possibility that altered autonomic outflow interferes with central responses. In the present study, urethane was chosen on the basis of previous studies that showed that this anesthetic has less of an effect than other anesthetics on baroreflex control and autonomic function (36,37). In addition, we have previously shown that microinjection of angiotensin peptides into the RVLM induces similar pressor effects in anesthetized and conscious rats (17,18). Thus, although anesthesia may alter cardiovascular and respiratory functions, urethane may have had a minor impact on the pressor responses induced by RVLM microinjections of Ang II and Ang-(1-7).
In summary, the data presented in this study show that the pressor effect produced by bilateral microinjection of Ang-(1-7) into the RVLM of anesthetized rats cannot be exclusively attributed to an increase in sympathetic drive. Furthermore, our study opens an interesting possibility that Ang-(1-7) acts at the RVLM on neuronal cells that modulate the activity of a vasodilator tonus that is sensitive to atropine.
This work was supported by FAPEMIG (Fundação de Amparo à Pesquisa do Estado de Minas Gerais) and CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) through PRONEX (Programa de Grupos de Excelência) and INCT (Instituto Nacional de Ciência e Tecnologia – Nanobiofar) grants. This study is part of RC Oliveira's master dissertation in the Post-graduation Program in Biological Sciences: Physiology and Pharmacology. RC Oliveira was a recipient of a CNPq fellowship. We are thankful to Jose R. Silva and Soraia S. Silva for their skillful technical assistance.
SOURCES OF FINANCIAL SUPPORT: FAPEMIG (Fundação de Amparo à Pesquisa do Estado de Minas Gerais) and CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) through grants from PRONEX (Programa de Grupos de Excelência) and INCT (Instituto Nacional de Ciência e Tecnologia – Nanobiofar).
No potential conflict of interest was reported.
Oliveira RC performed the experiments, analyzed the data, prepared the figures, interpreted the results of the experiments, drafted the manuscript and approved the final version of the manuscript. Campagnole-Santos MJ analyzed the data, interpreted the results of the experiments, drafted the manuscript, edited and revised the manuscript and approved the final version of the manuscript. Santos RA contributed to the conception and design of the research, analyzed the data, interpreted the results of the experiments, drafted the manuscript, edited and revised the manuscript and approved the final version of the manuscript. All of the authors have participated sufficiently in the work and take public responsibility for appropriate portions of its content.