In addition to its roles in the stimulation of growth hormone secretion and the regulation of appetite and metabolism, ghrelin exerts immunomodulatory, anti-inflammatory and antioxidant actions in several organ systems. In this study, we investigated the effects of ghrelin on the healing of experimental colonic anastomoses.
METHODS:Wistar rats were randomly divided into two groups (n = 10 in each). A segment of colon was excised, and an end-to-end anastomosis was performed in the distal colon. The Ghrelin Group received 10 ng/kg/day IP ghrelin for seven days postoperatively, whereas the Control Group received an identical volume of saline. On the seventh postoperative day, the anastomotic bursting pressures and hydroxyproline levels were measured, and adhesion formation around the anastomoses was examined. Histopathological analyses were performed to evaluate inflammatory cell infiltration, fibroblast infiltration, collagen density and neovascularization.
RESULTS:In the Ghrelin Group, the bursting pressure and hydroxyproline levels were significantly higher than in the Control Group. The adhesion formation scores were lower in the Ghrelin Group than in the Control Group. Although the inflammatory cell infiltration was diminished in the Ghrelin Group, the degrees of fibroblast infiltration, collagen density and neovascularization were not significantly different between the groups.
CONCLUSION:Our results indicate that ghrelin improves the healing of colonic anastomoses in rats.
Intestinal resection and the subsequent creation of an anastomosis are common gastrointestinal operations. Anastomotic healing has been extensively investigated in many clinical and experimental studies over the past two centuries (1-3). The patient's age and condition, the blood supply and microbial flora of the colon, the presence of inflammation at the anastomotic site and the choice of surgical technique are the primary factors involved in the healing of intestinal anastomoses (3,4). The available techniques have improved considerably, and the potential adverse effects of multiple local and systemic factors (e.g. radiation, antineoplastic drugs) have been documented (1). Despite the amount of progress that has been made, anastomotic dehiscence remains a major complication of this procedure.
The process of colon anastomosis healing involves complex interactions between peptide growth factors and the collagen turnover process during the phases of inflammation, fibrosis and maturation (5,6). Ghrelin, an orexigenic hormone, was first identified in the rat stomach in 1999 as an endogenous ligand of the growth hormone secretagogue receptor type 1a (GHSR-1a, a.k.a. ghrelin receptor) (7). In addition to its roles in growth hormone (GH) secretion stimulation and appetite regulation, ghrelin exerts immunomodulatory, anti-inflammatory and antioxidant actions in several organ systems (8-10). In addition, ghrelin has protective effects against ischemia/reperfusion injury (11).
Because of the immunomodulatory and anti-inflammatory properties of ghrelin and its role in GH secretion, we tested the effects of ghrelin on the healing of colonic anastomoses in rats. We assessed bursting pressure (BP), hydroxyproline (HP) content, adhesion formation and histopathological changes to evaluate the effects of ghrelin on colonic anastomosis healing.
MATERIALS AND METHODSMale Wistar rats weighing 200-250 g were used in this study. All of the animals were maintained under conditions of controlled temperature (23.2°C) and humidity (55.5%) and a 12-hour light/dark cycle. The rats were fed regular rat chow and had ad libitum access to tap water. The rats were randomly divided into two groups.
In the Control Group, a segment of colon was excised, and an anastomosis was created. The animals received 1 ml of saline solution via intraperitoneal (IP) injection for seven days postoperatively.
In the Ghrelin Group, a segment of colon was excised, and an anastomosis was created. The animals received 10 ng/kg/day of rat ghrelin (Sigma-Aldrich Corp, St. Louis, MO, USA) dissolved in 1 ml of saline solution via IP injection for seven days postoperatively.
In both groups, a 1-cm-long colonic segment was excised prior to the creation of the anastomosis to measure the basal HP.
Surgical proceduresAll of the animals underwent an overnight fast before the surgery. The animals were anesthetized with 40 mg/kg of ketamine (Ketalar, Eczacıbaşı, İstanbul) and 8 mg/kg of xylazine (Rompun, Bayer AG, Leverkusen, Germany). A 3-cm midline incision was created in the abdomen. The sigmoid colon was divided 3 cm from the peritoneal reflection while the vascular arcades were preserved. Approximately 1 cm of colon was excised for basal HP measurements. A single-layer, end-to-end anastomosis was created using 6-8 sutures (6-0 polypropylene; Prolene®; Ethicon, Edinburgh, Scotland) placed 1 mm apart. The fascia and skin were closed separately with running 4-0 silk sutures (Mersilk®; Ethicon). All of the procedures were performed by the same surgeon using sterile surgical techniques.
Adhesion formation scoresAll of the rats were euthanized on postoperative day seven. Postmortem, the anastomoses were examined macroscopically, and the integrity of the anastomosis, the presence of perianastomotic abscesses or peritonitis and the formation of any adhesions were recorded.
The results were evaluated in a blind fashion according to the scoring scale of van der Hamm et al. (12): 0 = no adhesions; 1 = minimal adhesions occurring mainly between the anastomosis and the omentum; 2 = moderate adhesions occurring between the omentum and the anastomotic site and between the anastomosis and a loop of small bowel; and 3 = severe and extensive adhesions, including abscess formation.
Bursting Pressure MeasurementsAn anastomotic segment approximately 10 cm in length, with a centrally situated suture line, was maintained in vivo without adhesiolysis. Two feeding catheters (6 Fr) were placed into the lumen through each end of the colon. Both ends of the colon were occluded with 2-0 silk sutures. A saline solution infusion was begun at a rate of 2 ml/min using an IVAC 770 infusion pump (IVAC Corp., San Diego, California, USA), while the pressure within the lumen was continuously monitored via the transducer of a pressure monitoring system connected to the other catheter (Harvard Rodent Ventilator, Model 683) (13). Data were recorded on a computer using AcqKnowledge version 3.7.2. The pressure that caused anastomotic leakage was recorded as the BP.
Determination of HP contentAfter the bursting pressure was measured, a 5-mm-wide ring of tissue that included the anastomosis was removed. One-half of the removed tissue was wrapped in aluminum foil and was stored at −20°C for the subsequent measurement of HP content. The remaining half of the anastomotic site was stored in 10% formaldehyde for later histopathological evaluations. After the samples had been warmed to room temperature, their dry weights were recorded, and their HP content was determined using the method reported by Jamall et al. (14).
Histopathological assessmentAfter hematoxylin and eosin staining, the colonic tissues and anastomoses were examined under a light microscope and were scored in a blind fashion using the modified 0-to-4 Ehrlich and Hunt numerical scale (15) (Table 1). The parameters evaluated were inflammatory cell infiltration, fibroblast ingrowth, neovascularization and collagen deposition. Each parameter was individually assessed (Table 1).
Statistical analysisThe results are expressed as the mean±standard deviation of the mean for BP, HP, and histologic scores. The results of adhesion formation scores were expressed as the mean±standard error of the mean. The Shapiro-Wilk normality test indicated that the data had a normal distribution (p<0.05). The bursting pressures of the two groups were compared with an unpaired t-test. The HP contents of the groups were compared using ANOVA. The adhesion scores and histologic scores were compared using a one-way ANOVA, and multiple comparisons between the groups were performed using the least significant difference (LSD) post-hoc test. Differences were considered statistically significant if p<0.05. The data were analyzed with commercial statistical software (SPSS for Windows 17; SPSS, Chicago, IL, USA).
EthicsThe study protocol was approved by the Animal Ethics Committee of Inonu University Medical School.
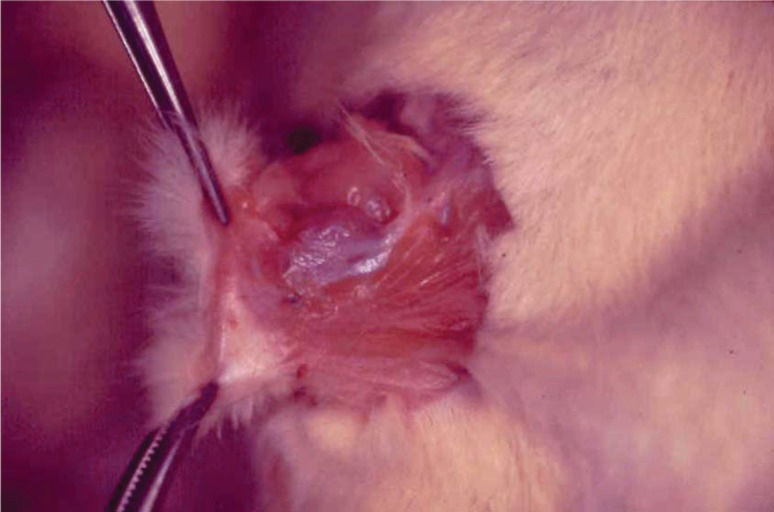
RESULTSAdhesion formationNo anastomotic dehiscence was detected in either group. The mean adhesion formation scores were 1.5±0.56 (standard error) for the Control Group and 0.2±0.13 for the Ghrelin Group. Loose adhesions were noted in the EO Ghrelin Group; however, four of the Control Group animals exhibited thicker and more numerous adhesions around the anastomoses (Figure 1). The adhesion formation scores were significantly higher in the Control Group than in the Ghrelin Group (p = 0.037).
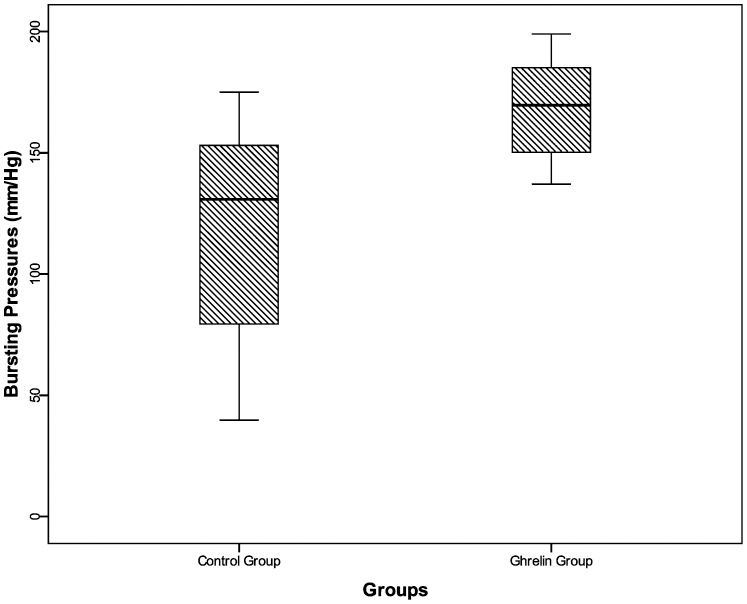
Bursting pressureThe mean BPs were significantly higher in the Ghrelin Group (168.3±20.8 mmHg) compared with the Control Group (117.8±46.5 mmHg) (Figure 2) (p<0.05).
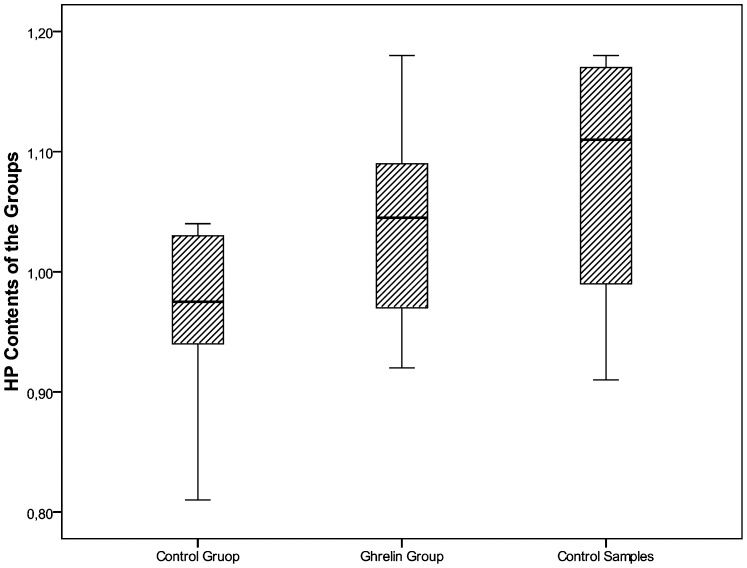
HP contentThe mean HP contents were 0.96±0.06 μg/mg wet tissue in the Control Group and 1.04±0.09 μg/mg in the Ghrelin Group. The HP content was significantly decreased in the Control Group compared with the mean control HP value of 1.8±0.26 μg/mg that was obtained in the control colonic tissues prior to anastomosis creation (p = 0.012). In the Ghrelin Group, the HP content was similar to the basal HP value (Figure 3).
The mean HP contents (μg/mg wet tissue) of the Ghrelin and Control Groups compared to the control HP levels. In the Control Group, the tissue HP contents were significantly decreased during anastomosis healing compared to the measurements performed prior to anastomosis creation (p<0.05). In the Ghrelin Group, the tissue HP contents during anastomosis healing were not different from those measured prior to anastomosis creation.
The mean inflammatory cell infiltration, fibroblast infiltration, collagen density and neovascularization scores are presented in Table 2.
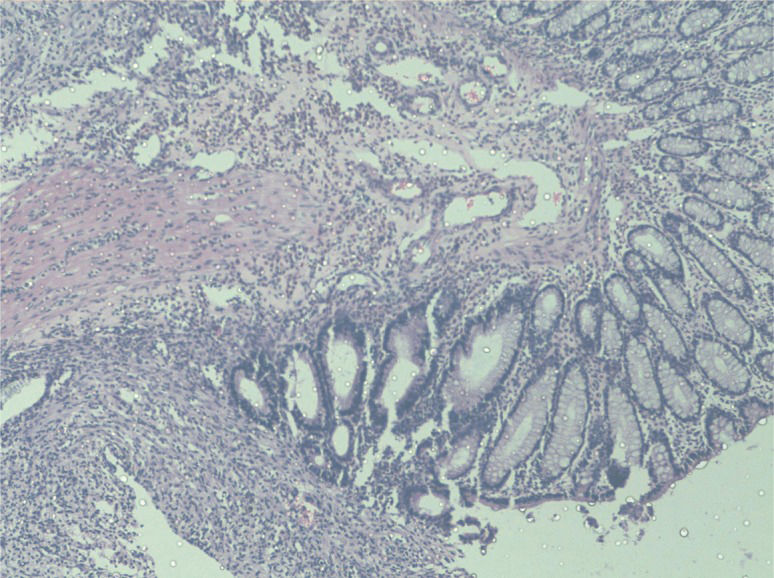
A comparison of the mean histological scores of the groups revealed a significant difference between the inflammatory cell infiltration scores (p<0.05). In the Ghrelin Group inflammatory cell infiltration scores were found to be lower than the Control Group. (Figure 4). There were no significant differences in fibroblast infiltration, collagen intensity or capillary formation between the groups.
DISCUSSIONGhrelin has been described as a natural ligand of GHS receptor type 1a. GHS receptors are expressed in several regions of the central nervous system and its periphery; this widespread distribution indicates that ghrelin has various effects in addition to stimulating the secretion of GH (16-19). The results of the current study, which included mechanical, biochemical, macroscopic and microscopic evaluations, demonstrated a beneficial effect of ghrelin on the healing of colonic anastomoses in an experimental model.
Ghrelin is mainly produced by X/A cells in the fundic glands of the stomach. Small amounts of ghrelin are also generated in the small intestine, pancreas, kidney and hypothalamus (7,16,17). Takachi et al. (20) found that following total gastrectomy, gastric cancer patients displayed significant weight loss and an immediate and remarkable decline in serum ghrelin, which did not recover even after a long postoperative period. In addition, gastrectomy patients are prone to anastomotic rupture and fistulas. Yu et al. (21) demonstrated in a rat model that in the early recovery phase after partial gastrectomy, exogenous ghrelin treatment increased food intake and body weight and promoted anastomotic healing. Ghrelin may have clinical implications for recovery from gastrectomy.
Various growth factors have been shown to enhance wound healing in several experimental models. Wheeless et al. (5) evaluated the effect of GH on the BP of radiation-injured terminal ileal anastomoses in a rat model. The BP in the GH-treated rats was significantly greater than that of the control group. Karahasanoğlu et al. (22) have suggested that GH strengthens left colonic anastomoses in rats. It has been shown that ghrelin stimulates GH release in humans and rats (23,24), which implies that the observed effect of ghrelin on anastomotic healing may be due to its GH secretagogue properties. The spectrum of biological activities attributed to growth factors includes mitogenic and chemoattractant properties, the ability to release cytokines, the promotion of angiogenesis and the stimulation of extracellular matrix production (25-27). Because ghrelin is a natural ligand of GHS receptor type 1a and stimulates GH release in humans and rats, we predicted that ghrelin would improve anastomotic healing. Consistent with this hypothesis, we found that anastomotic healing was improved following ghrelin administration. GH accelerates cell proliferation and enhances amino acid efflux into cells for protein synthesis or gluconeogenesis (28). In contrast, ghrelin, a natural ligand of GHSR, did not alter fibroblast infiltration or neovascularization at the anastomotic site of the colon in the present study.
Collagen synthesis, which may be evaluated by measuring the tissue HP content, is an essential feature of anastomotic healing. Therefore, we measured the tissue HP content. The HP levels were higher in the Ghrelin Group compared with the control group, suggesting increased collagen synthesis and therefore improved anastomotic wound healing following ghrelin administration. BP measurements indicate the mechanical strength of an anastomosis. It has been reported that the BP increases progressively immediately after the formation of a colonic anastomosis (29). The present study showed that the anastomotic BPs were increased in the Ghrelin Group compared with the Control Group. In a similar experimental study of post-gastrectomy rats, ghrelin effectively increased the BPs and HP contents of anastomoses on post-operative day seven (21).
In addition to measuring HP and BP, we evaluated the anastomotic area both macroscopically and microscopically. Macroscopic adhesion formation around the anastomosis was scored quantitatively, and the adhesion scores were found to be decreased following ghrelin administration. Anastomotic healing is affected by the strength of the primary inflammatory response; the rate of mucosal re-epithelialization; the amount, strength and maturation rate of the new collagen; and the presence of collagenolysis in the initial three days of the postanastomotic period (30). Inflammation is an integral element of wound healing. However, inflammation is aggravated, wound healing is delayed due to increased collagenolysis. It has been shown that ghrelin has anti-inflammatory properties (7-11), and this may be the cause of the reduced adhesion formation. In support of this hypothesized connection, the inflammatory cell infiltration scores were lower in the Ghrelin Group than in the Control Group.
The principal source of collagen during wound healing is fibroblasts. Fibroblast influx to the wound increases markedly on postoperative day two (29), after which it decreases to the normal level. In the present study, the extent of fibroblast infiltration did not differ between the two groups at the examined time point (Day 7). We believe that this result might be different at other time points, which merits further investigation.
The HP levels were higher in the Ghrelin Group compared with the control group, which suggests an increase in collagen synthesis. The anastomotic BP was also elevated in the Ghrelin Group. The observed effects of ghrelin on anastomotic healing may be partially due to the GH secretagogue properties of this substance. However, ghrelin, a natural ligand of GHSR, did not alter the fibroblast infiltration, collagen density or neovascularization at the anastomotic site of the colon on the seventh day after anastomosis formation. Interestingly, ghrelin administration reduced the adhesion formation around the anastomosis, which may be a result of the anti-inflammatory effect of ghrelin. In the Ghrelin Group, the inflammatory cell infiltration scores were found to be lower than in the Control Group. The results of this study indicate that ghrelin improves anastomotic healing. We propose that the underlying cause of this effect is, at least in part, a mechanism other than the GHS receptor agonist activity of ghrelin.
No potential conflict of interest was reported.
Ceran C planned the study, performed the animal experiments and wrote the manuscript. Aksoy RT performed the animal experiments and wrote the manuscript. Gülbahar O performed the biochemical evaluations. Öztürk F performed the histopathological assessments.