The anesthetic gas xenon is reported to preserve hemodynamic stability during general anesthesia. However, the effects of the gas during shock are unclear. The objective of this study was to evaluate the effect of Xe on hemodynamic stability and tissue perfusion in a canine model of hemorrhagic shock.
METHOD:Twenty-six dogs, mechanically ventilated with a fraction of inspired oxygen of 21% and anesthetized with etomidate and vecuronium, were randomized into Xenon (Xe; n = 13) or Control (C; n = 13) groups. Following hemodynamic monitoring, a pressure-driven shock was induced to reach an arterial pressure of 40 mmHg. Hemodynamic data and blood samples were collected prior to bleeding, immediately after bleeding and 5, 20 and 40 minutes following shock. The Xe group was treated with 79% Xe diluted in ambient air, inhaled for 20 minutes after shock.
RESULT:The mean bleeding volume was 44 mL.kg−1 in the C group and 40 mL.kg−1 in the Xe group. Hemorrhage promoted a decrease in both the cardiac index (p<0.001) and mean arterial pressure (p<0.001). These changes were associated with an increase in lactate levels and worsening of oxygen transport variables in both groups (p<0.05). Inhalation of xenon did not cause further worsening of hemodynamics or tissue perfusion markers.
CONCLUSIONS:Xenon did not alter hemodynamic stability or tissue perfusion in an experimentally controlled hemorrhagic shock model. However, further studies are necessary to validate this drug in other contexts.
Xenon (Xe) is an inert gas that is present in the atmosphere at very low concentrations and has potent hypnotic and analgesic properties that have been known for several decades (1-4). This anesthetic gas is neither toxic nor metabolized within the body, as it is quickly eliminated through the lungs (4-6) and can be used in patients with impaired renal or hepatic function with minimal or no side effects (5). In heart failure conditions, a number of publications have shown that inhaled xenon is an adequate alternative to other inhaled anesthetics, as it does not interfere with cardiac contractility, cardiac autonomic control or vascular tonic regulation (7-11).
However, few studies have evaluated the impact of xenon on hemodynamics, oxygen transport and tissue perfusion during circulatory shock (12,13). It is well known that most inhalational anesthetics used in clinical practice may hinder hemodynamic responses to shock, either by directly interfering with cardiac function or the vascular contractile response or by indirectly decreasing sympathetic activity. Due to the absence of hemodynamic effects caused by xenon, this gas is a suitable option in severe shock conditions under which the cardiovascular depressant effects of anesthesia would further worsen tissue hypoperfusion.
The aim of this study was to assess the impact of xenon on hemodynamics, oxygen transport and tissue perfusion markers in dogs undergoing severe hemorrhagic shock.
MATERIALS AND METHODSThis research project was approved by the Ethics Committee of the Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (process CAPPesq 655/97) and was funded through a research grant from FAPESP (Fundação de Amparo à Pesquisa do Estado São Paulo; Process 00/10122-6). The study included 26 mixed-breed dogs weighing between 10 and 20 kg. The study met the international criteria for animal care and abided by the rules of COBEA (The Brazilian College of Animal Experimentation).
Animal preparation and measurementsA clinical veterinarian evaluated the health status of the animals following their arrival at the laboratory. Food was withheld from the animals for 12 hours prior to the experiment and free access to water was available at all times. Following the arrival of the animals to the research laboratory, a venous line was inserted into one limb using a 20-G Teflon catheter. General anesthesia was induced using etomidate (2 mg.kg−1), followed by tracheal intubation with an 8.0-mm ID cuffed cannula. A continuous infusion of etomidate (50 μg.kg−1.min−1) and vecuronium (1 μg.kg−1.min−1) was initiated to facilitate pressure-controlled ventilation and to enable the installation of vascular lines and was maintained throughout the study. Mechanical ventilation was performed using a Cicero EM (Drager, Lübeck, Germany). The inspiratory pressure was set at 15 cmH2O and the respiratory rate was set at 20 breaths per minute. The fraction of inspired oxygen (FiO2) was set at 21% (room air) in a semi-closed circuit with a CO2 absorber. Respiratory system compliance; tidal volume; and peak, plateau and end-expiratory pressures were directly monitored with the anesthesia equipment after pneumotachometer calibration.
After the induction of anesthesia, small-bore polyethylene catheters (P260) were inserted by dissection into both femoral arteries. One of the arterial catheters was used exclusively to measure systemic arterial pressure, whereas the other catheter was used to remove blood and collect arterial blood samples. The right internal jugular vein was dissected and a pulmonary artery catheter (Edwards Lifesciences, Irvine, CA, USA) was inserted. The position was confirmed by obtaining pulmonary artery pressure (PAP) and pulmonary artery occlusion pressure (PAOP) curves. Heart rate, systolic arterial pressure (SAP), diastolic arterial pressure (DAP), mean arterial pressure (MAP), mean PAP and PAOP were measured using a multiparameter monitor (Viridia 885, Hewlett-Packard, Palo Alto, CA, USA). All data from the monitor were stored on a personal computer. Intravascular pressures were computed using disposable transducers (Edwards Lifesciences, Irvine, CA, USA), which were filled with a 0.9% sodium chloride solution and zeroed at the mid-axillary line. Mixed central venous blood samples were collected from the distal port of the pulmonary artery catheter. Cardiac output (CO) was measured using the standard thermodilution technique. The measurement was repeated three times and the average value was divided by body surface area (BSA) to obtain the cardiac index (CI). Body surface area was calculated using the following formula: BSA = 0.122×weight2/3. Systemic and pulmonary hemodynamic calculations, arterial and mixed venous blood oxygen contents (CaO2 and CvO2), the venous admixture and oxygen transport variables were calculated using standard equation. A pulse oximeter probe was applied to the tongue of the animal and a gas analyzer (Criticare Systems Inc., Waukesha, WI, USA) was placed between the endotracheal cannula and respiratory circuit Y piece to continuously monitor FiO2 and ETCO2 levels.
Arterial and mixed venous oxygen (PaO2, PvO2), carbon dioxide (PaCO2, PvCO2) tensions, arterial lactate concentration and pH were measured using a blood-gas analyzer (Radiometer ABL Gas Analyzer, Copenhagen, Denmark). All results were corrected for body temperature. The bicarbonate concentration, hemoglobin (Hb) concentration and base excess were also calculated.
Study protocol and data collectionAnimals were randomly allocated to the Control or Xenon group. A random treatment sequence was obtained using the Stata 11 statistical software (StataCorp, College Station, TX, USA). The allocation of each animal was enclosed in a brown numbered envelope and revealed at the start of each experiment. Following animal preparation, blood samples and respiratory and hemodynamic data were collected before the induction of shock, immediately after induction and 5, 20 and 40 minutes after induction.
The hemorrhagic shock model used in this study is described elsewhere (14). Briefly, the animals were subjected to continuous bleeding (25 mL.min−1) to achieve a MAP of 40 mmHg. When the MAP reached 40 mmHg, continuous bleeding was interrupted and small aliquots of blood were removed to maintain the target pressure for 2 minutes. Individual variations in the total amount of blood volume lost were not expected to significantly alter the MAP. Immediately after establishing circulatory shock in the Experimental group, xenon inhalation was initiated. First, the breathing circuit was saturated with a specially prepared gas mixture (White Martins, São Paulo, Brazil) containing 79% xenon and 21% oxygen, with a flow rate of 10 L.min−1. Following initial saturation of the breathing circuit, the animals were ventilated for 20 minutes at a constant 1-L.min−1 flow rate. An additional variable flow rate of O2 was used as needed to maintain the inspired fraction of O2 at 21% according to the gas analyzer. The Control group received room air ventilation at a flow rate of 1 L.min−1 and additional O2 ventilation to maintain the FiO2 at 21%. After data were collected at the 20-minute time point, the configuration of the respiratory circuit was changed from closed to open to allow for the rapid clearance of xenon. The fresh gas flow was then adjusted to 10 L.min−1 for both groups. The ventilatory settings were kept constant throughout the experiment. After the end of the experiment, the animals were euthanized by injecting KCl (25 mEq.L−1) intravenously and sent to the animal care facility for adequate disposal.
Statistical analysisThe distribution of the data was tested for normality using the Kolmogorov-Smirnov test. Weight, body surface area and blood volume lost were compared between groups using Student’s t-test for unpaired samples. The temporal behavior of hemodynamic, respiratory, metabolic and oxygen transport variables was evaluated using a two-way analysis of variance for repeated measures, followed by a Newman-Keuls post-hoc test when indicated. P-values less than 0.05 were considered statistically significant. The results are expressed as the mean ± standard deviation (SD). All statistical analyses were performed using the statistical packages Aabel 3.0.5 (Gigawiz Ltd. Co., Tulsa, OK, USA) and G Power 3 (Heinrich-Heine-Universität, Dusseldorf, Germany).
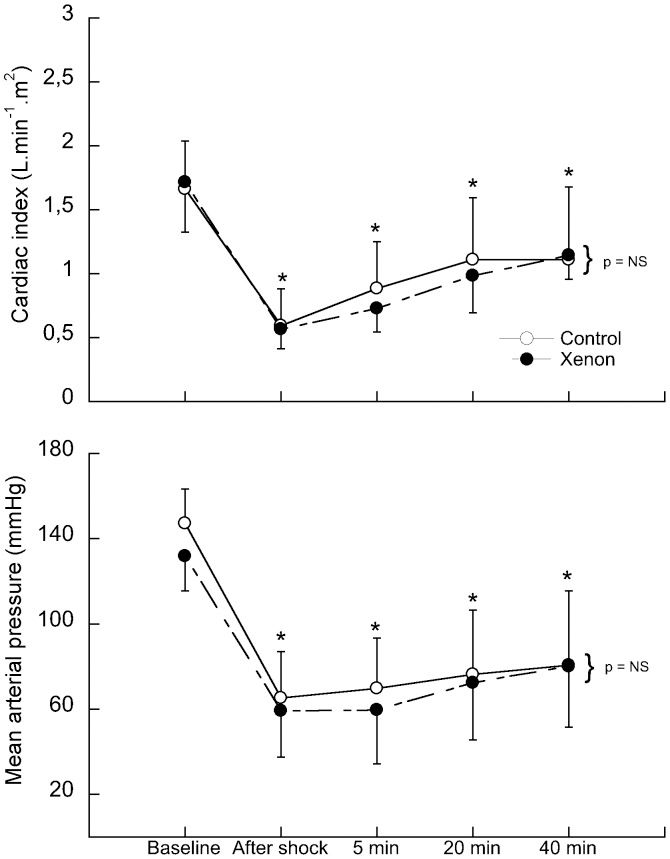
RESULTSThe Control and Experimental groups were similar with respect to weight, BSA and blood volume losses, as indicated in Table 1. The volume of blood removed was 44 mL.kg−1 in the Control group and 40 mL.kg−1 in the Xenon group and the blood removal caused a significant reduction in the cardiac index in both groups (64 and 67%, respectively), measured immediately after shock (upper panel of Figure 1). Following the shock induction phase, a non-significant increase in the cardiac index was observed that was similar in both groups. At 20 minutes post-blood loss, the cardiac indexes were 33 and 42% lower than baseline measurements in the control and Xenon groups, respectively (p<0.05). The removal of xenon inhalation at 20 minutes post-shock did not alter the cardiac index. The mean arterial pressure decreased in both groups by 55% following the blood loss phase compared with baseline values. These values remained stable throughout the remainder of the experiment (lower panel of Figure 1). As seen in Table 2, there was a significant and persistent increase in heart rate and pulmonary vascular resistance index after shock that was similar in both groups. The systemic vascular resistance index transiently increased after shock, returning to baseline levels during the observation period, but there was no significant difference between the groups. The mean pulmonary arterial pressure, cardiac filling pressures and left and right ventricular systolic work indexes exhibited a marked reduction until the last measurement was taken. The interruption of xenon inhalation did not produce any significant alterations or improvement in hemodynamics compared with measurements taken at the 20-minute time point.
Characteristics of the groups.
| Control Group | Xenon Group | p-value | |
|---|---|---|---|
| Weight (kg) | 15±2 | 15±2 | NS |
| BSA (m2) | 0.71±0.07 | 0.72±0.07 | NS |
| Volume removed (ml) | 670±155 | 602±156 | NS |
BSA – body surface area. NS indicates no significant difference between groups. Data are expressed as the mean±SD. n = 13 for both the Control and Xenon groups.
Differences in the cardiac index (upper panel) and mean arterial pressure (lower panel) between the Control (open circles; n = 13) and Xenon (closed circles; n = 13) groups during the study. ∗ indicates a significant difference from baseline, p<0.05. NS indicates no significant difference. The x-axes of the graphs are not linear, so the curves are not true to scale. Data are expressed as the mean±SD.
Hemodynamic behavior of the animals in the Control and Xenon groups during the study.
| Group | Baseline | After shock | 5 min | 20 min | 40 min | p-value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (bpm) | Control | 75 | ± | 19 | 123 | ± | 38∗ | 124 | ± | 37∗ | 133 | ± | 31∗ | 140 | ± | 28∗ | NS |
| Xenon | 73 | ± | 18 | 137 | ± | 32∗ | 139 | ± | 34∗ | 149 | ± | 35∗ | 142 | ± | 35∗ | ||
| CVP (mmHg) | Control | 7 | ± | 2 | 3 | ± | 1∗ | 3 | ± | 1∗ | 3 | ± | 1∗ | 3 | ± | 1∗ | NS |
| Xenon | 7 | ± | 1 | 3 | ± | 1∗ | 3 | ± | 1∗ | 4 | ± | 1∗ | 3 | ± | 2∗ | ||
| mPAP (mmHg) | Control | 18 | ± | 3 | 10 | ± | 3∗! | 11 | ± | 2∗! | 11 | ± | 3∗! | 14 | ± | 3∗ | NS |
| Xenon | 17 | ± | 4 | 9 | ± | 2∗! | 11 | ± | 3∗! | 12 | ± | 3∗! | 13 | ± | 4∗ | ||
| POAP (mmHg) | Control | 11 | ± | 3 | 5 | ± | 2∗ | 5 | ± | 2∗ | 4 | ± | 2∗ | 6 | ± | 2∗ | NS |
| Xenon | 10 | ± | 2 | 4 | ± | 2∗ | 5 | ± | 2∗ | 6 | ± | 3∗ | 5 | ± | 2∗ | ||
| SVRi (dyne.s.cm−5.m−2) | Control | 7067 | ± | 1675 | 9924 | ± | 4464∗ | 6651 | ± | 2723 | 5574 | ± | 2156 | 6080 | ± | 1971 | NS |
| Xenon | 6108 | ± | 1565 | 8202 | ± | 3298∗ | 6206 | ± | 2311 | 5659 | ± | 1571 | 5370 | ± | 1935 | ||
| PVRi (dyne.s.cm−5.m−2) | Control | 366 | ± | 97 | 676 | ± | 332∗ | 553 | ± | 161∗ | 553 | ± | 232∗ | 729 | ± | 478∗ | NS |
| Xenon | 304 | ± | 158 | 672 | ± | 246∗ | 629 | ± | 244∗ | 502 | ± | 228∗ | 594 | ± | 289∗ | ||
| LVSWi (g·m.m−2) | Control | 45.8 | ± | 11.8 | 5.3 | ± | 5.3∗ | 8.5 | ± | 7.9∗ | 9.8 | ± | 7.6∗ | 10.3 | ± | 7.5∗ | NS |
| Xenon | 43.4 | ± | 11.8 | 3.7 | ± | 2.7∗ | 4.8 | ± | 3.4∗ | 7.5 | ± | 5.2∗ | 9.3 | ± | 4.3∗ | ||
| RVSWi (g·m.m−2) | Control | 5.6 | ± | 1.4 | 0.7 | ± | 0.7∗ | 1.3 | ± | 1∗ | 1.4 | ± | 0.9∗ | 1.5 | ± | 1∗ | NS |
| Xenon | 5.5 | ± | 2.1 | 0.5 | ± | 0.3∗ | 0.8 | ± | 0.4∗ | 1.1 | ± | 0.6∗ | 1.5 | ± | 0.7∗ | ||
HR – heart rate; CVP – central venous pressure; mPAP – mean pulmonary artery pressure; PAOP – pulmonary artery occlusion pressure; SVRi – systemic vascular resistance index; PVRi – pulmonary vascular resistance index; LVSWi – left ventricular systolic work index; RVSWi – right ventricular systolic work index. ∗ indicates a difference from baseline, p ± 0.05. ! indicates a difference at the 40-min time point, p<0.05. NS indicates no significant difference. Data are expressed as the mean ± SD. n = 13 for both the Control and Xenon groups.
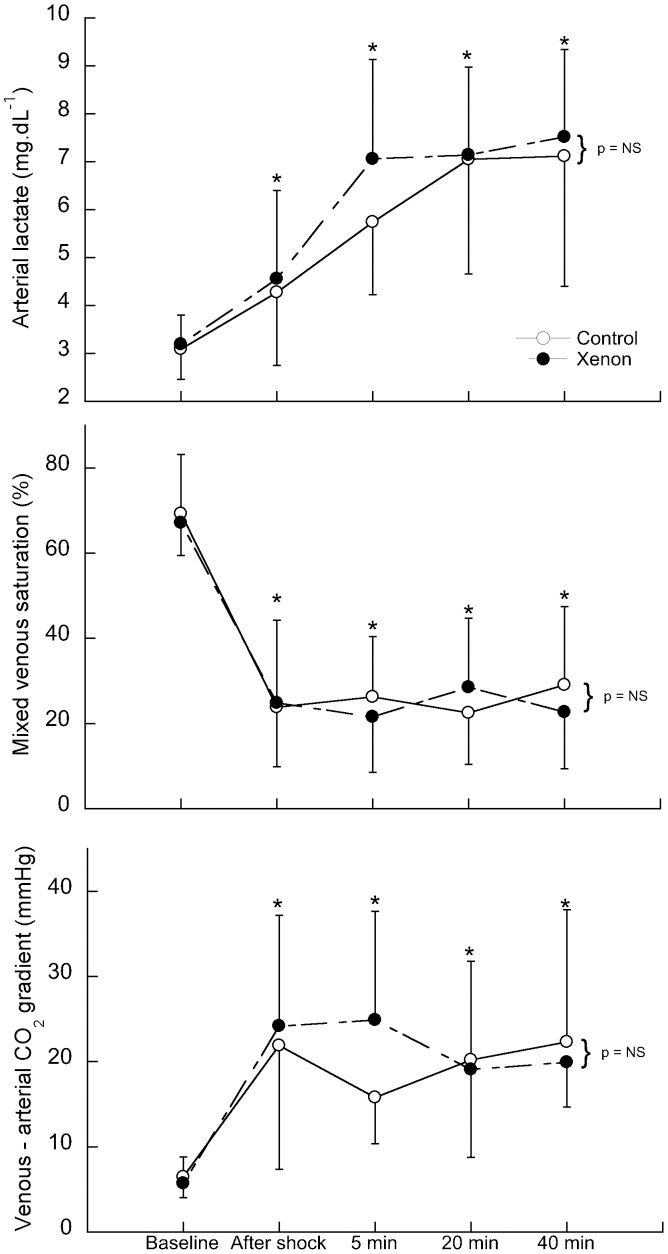
The induction of shock promoted a significant and progressive increase in lactate levels compared with the baseline levels that was similar in both groups (upper panel of Figure 2. The mixed central venous saturation decreased from 69 and 67% to 23 and 24% in the Control and Xenon groups, respectively. Saturation remained low in both groups through the end of the study (middle panel of Figure 2). The veno-arterial CO2 difference increased four-fold in both groups compared with baseline values and remained elevated throughout the study (lower panel of Figure 2). As noted in Table 3, there was a similar and significant four-fold reduction in oxygen delivery, accompanied by an important and sustained augmentation of the oxygen extraction rate, from 29 and 30% to 76 and 74% in the Control and Xenon groups, respectively. The initiation of shock induced a significant decrease in arterial pH, arterial base excess and bicarbonate concentrations that were similar in both groups (Table 3). The removal of xenon from the respiratory mixture did not result in any changes in tissue perfusion markers. No changes in other metabolic parameters were observed. Hemoglobin levels did not change throughout the study, as shown in Table 3.
The arterial lactate (upper panel), mixed venous saturation (middle panel) and venous-arterial CO2 (lower panel) differences between the Control (open circles; n = 13) and Xenon (closed circles; n = 13) groups during the study. ∗ indicates a significant difference from baseline, p<0.05. NS indicates no significant difference. The x-axes of the graphs are not linear, so the curves are not true to scale. Data are expressed as the mean±SD.
Values of the metabolic, hemoglobin and oxygen transport variables during the study in the Control and Xenon groups.
| Group | Baseline | After shock | 5 min | 20 min | 40 min | p-value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arterial pH | Control | 7.36 | ± | 0.05 | 7.39 | ± | 0.09 | 7.26 | ± | 0.09∗ | 7.15 | ± | 0.11∗ | 7.15 | ± | 0.11∗ | NS |
| Xenon | 7.35 | ± | 0.08 | 7.35 | ± | 0.08 | 7.2 | ± | 0.1∗ | 7.19 | ± | 0.08∗ | 7.15 | ± | 0.06∗ | ||
| Arterial HCO3 | Control | 18.1 | ± | 1.4 | 13.7 | ± | 1.9∗ | 12.5 | ± | 1.8∗ | 11.7 | ± | 2.6∗! | 11.8 | ± | 3.8∗! | NS |
| (mmol.L−1) | Xenon | 19.2 | ± | 2.2 | 14.6 | ± | 3∗ | 12.2 | ± | 3.2∗ | 12.6 | ± | 2.5∗! | 11.8 | ± | 2.2∗! | |
| Arterial base excess | Control | -5.5 | ± | 2.1 | -9 | ± | 2.7 | -13 | ± | 3 | -16.3 | ± | 4.7 | -13.7 | ± | 5.8 | NS |
| (mmol.L−1) | Xenon | -4.6 | ± | 2.6 | -9.1 | ± | 4.2 | -14.6 | ± | 5 | -14.6 | ± | 4 | -16 | ± | 3.3 | |
| Hemoglobin | Control | 12.3 | ± | 1.8 | 11.4 | ± | 1.8 | 11.1 | ± | 1.9 | 10.4 | ± | 1.9 | 10.3 | ± | 2.1 | NS |
| (g.dL−1) | Xenon | 11.6 | ± | 2.2 | 11.6 | ± | 2.5 | 10.6 | ± | 1.8 | 10.7 | ± | 1.7 | 10.7 | ± | 1.7 | |
| DO2i | Control | 266 | ± | 54 | 88 | ± | 38 | 125 | ± | 46 | 147 | ± | 66 | 151 | ± | 78 | NS |
| (mL.min−1.m−2) | Xenon | 265 | ± | 85 | 86 | ± | 23 | 102 | ± | 33 | 140 | ± | 47 | 158 | ± | 45 | |
| VO2i | Control | 75 | ± | 22 | 67 | ± | 33 | 89 | ± | 34 | 107 | ± | 34 | 93 | ± | 40 | NS |
| (mL.min−1.m−2) | Xenon | 72 | ± | 34 | 63 | ± | 22 | 78 | ± | 26 | 94 | ± | 30 | 117 | ± | 36 | |
| O2ER | Control | 29 | ± | 10 | 76 | ± | 14 | 75 | ± | 14 | 76 | ± | 12 | 69 | ± | 19 | NS |
| (%) | Xenon | 3 | ± | 16 | 74 | ± | 2 | 77 | ± | 14 | 70 | ± | 17 | 75 | ± | 14 | |
DO2i – systemic oxygen delivery; VO2i – systemic oxygen consumption; O2ER – oxygen extraction rate; ∗ indicates a difference from baseline, p<0.05. ! indicates a difference from the value immediately after shock, p<0.05. NS indicates no significant difference. The data are expressed as the mean ± SD. n = 13 for both the Control and Xenon groups.
Table 4 shows the values of the gas exchange and respiratory mechanics variables observed during the study. As seen in the table, none of the studied variables were affected by the induction of shock or the initiation or cessation of xenon inhalation.
Values of the gas exchange and respiratory mechanics variables during the study in the Control and Xenon groups.
| Group | Baseline | After shock | 5 min | 20 min | 40 min | p-value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PaO2 (mmHg) | Control | 97 | ± | 16 | 110 | ± | 16 | 98 | ± | 15 | 94 | ± | 11 | 99 | ± | 14 | NS |
| Xenon | 91 | ± | 7 | 108 | ± | 18 | 103 | ± | 15 | 103 | ± | 14 | 87 | ± | 9 | ||
| PaCO2 (mmHg) | Control | 33 | ± | 3 | 23 | ± | 5 | 29 | ± | 7 | 33 | ± | 7 | 34 | ± | 7 | NS |
| Xenon | 34 | ± | 4 | 28 | ± | 4 | 31 | ± | 5 | 34 | ± | 5 | 34 | ± | 5 | ||
| ETCO2 (mmHg) | Control | 30 | ± | 3 | 18 | ± | 4 | 19 | ± | 8 | 22 | ± | 9 | 24 | ± | 10 | NS |
| Xenon | 29 | ± | 10 | 17 | ± | 6 | 23 | ± | 4 | 24 | ± | 7 | 29 | ± | 5 | ||
| GAaO2 (mmHg) | Control | 10.8 | ± | 9.3 | 9.4 | ± | 9.9 | 13.7 | ± | 11.3 | 12 | ± | 8.7 | 8.7 | ± | 7.7 | NS |
| Xenon | 12.8 | ± | 7.8 | 10.2 | ± | 15.9 | 6.9 | ± | 5.3 | 6.8 | ± | 5.6 | 15.4 | ± | 7.2 | ||
| Shunt (%) | Control | 11.4 | ± | 5.4 | 3.3 | ± | 2.2 | 6.5 | ± | 3.9 | 7.8 | ± | 3.5 | 7.5 | ± | 4.3 | NS |
| Xenon | 15.8 | ± | 11.8 | 4.5 | ± | 5.2 | 5.6 | ± | 3.5 | 6.5 | ± | 4.8 | 9.7 | ± | 4.3 | ||
| Peak pressure (cmH2O) | Control | 13.5 | ± | 2.4 | 14.3 | ± | 1.3 | 14.8 | ± | 2.7 | 15.5 | ± | 2.6 | 14.7 | ± | 2.4 | NS |
| Xenon | 12.6 | ± | 1.2 | 15.1 | ± | 2.8 | 15.6 | ± | 1.6 | 15.3 | ± | 2.3 | 14.6 | ± | 2.1 | ||
| Plateau pressure (cmH2O) | Control | 14.3 | ± | 5.4 | 14.6 | ± | 5.1 | 15 | ± | 5.3 | 14.3 | ± | 2.6 | 15.3 | ± | 4.9 | NS |
| Xenon | 11.6 | ± | 0.9 | 14 | ± | 2.5 | 14.3 | ± | 1.7 | 15.4 | ± | 5.2 | 13.8 | ± | 2.3 | ||
| PEEP (cmH2O) | Control | 1.7 | ± | 0.7 | 1.6 | ± | 0.6 | 1.8 | ± | 0.6 | 1.6 | ± | 0.7 | 1.9 | ± | 0.6 | NS |
| Xenon | 1.7 | ± | 0.4 | 1.5 | ± | 0.6 | 1.9 | ± | 0.4 | 2 | ± | 0.6 | 1.5 | ± | 0.6 | ||
| VTexp (mL) | Control | 278 | ± | 44 | 274 | ± | 39 | 275 | ± | 42 | 285 | ± | 64 | 285 | ± | 39 | NS |
| Xenon | 282 | ± | 51 | 290 | ± | 53 | 305 | ± | 67 | 304 | ± | 76 | 279 | ± | 53 | ||
| Cstat (cmH2O.L−1) | Control | 46 | ± | 21 | 48 | ± | 18 | 49 | ± | 20 | 46 | ± | 15 | 48 | ± | 18 | NS |
| Xenon | 36 | ± | 8 | 45 | ± | 16 | 43 | ± | 13 | 46 | ± | 21 | 46 | ± | 15 | ||
ETCO2 – end-tidal CO2; GAaO2 – alveolar-arterial oxygen gradient; VTexp – expired tidal volume; Cstat – static compliance of the respiratory system. NS indicates no significant difference. Data are expressed as the mean ± SD. n = 13 for both the Control and Xenon groups.
In this study, we observed that xenon inhalational anesthesia did not decrease hemodynamic variables or tissue perfusion markers in dogs experiencing severe hemorrhagic shock. Gas exchange and respiratory mechanics were not affected by the initiation of inhaled xenon. In addition, the removal of xenon from the respiratory mixture did not produce any improvement in systemic hemodynamics or tissue perfusion.
The properties of xenon anesthesia have been well established (3). The gas has been shown to cause minimal to no cardiovascular instability in experimental and clinical studies in healthy subjects (15,16). Luttropp et al. studied the effects of xenon anesthesia (65% xenon in oxygen) on myocardial function. In their study, the cerebral blood flow velocities in 17 ASA 1 patients undergoing open abdominal surgery were investigated using transesophageal echocardiography and transcranial Doppler sonography (17). The authors did not observe any adverse effects of xenon anesthesia on myocardial function. However, they observed increased blood flow in cerebral arteries 15 and 30 minutes after the inhalation of xenon. Recently, a multicenter study was conducted to investigate left ventricular function during anesthesia with xenon compared with isoflurane. Using transesophageal echocardiography, 252 patients were evaluated. It was observed that xenon did not reduce contractility; however, isoflurane did decrease the contractile index in patients even in the absence of cardiac dysfunction (18). Even in patients with heart failure undergoing implantation of a cardioverter-defibrillator, xenon use during anesthesia has been proven to better maintain mean arterial pressure and the left ventricular ejection fraction compared with propofol/remifentanil anesthesia (10). In recent years, several other studies have been published that have associated xenon inhalation with cardiac preconditioning and myocardial protection in patients with ischemia and myocardial infarction (8,19-22).
Despite the many studies that have been conducted on the hemodynamic safety profile of xenon anesthesia in healthy subjects and those with cardiac dysfunction, very few studies have tested the impact of xenon on hemodynamic stability and tissue perfusion in the context of severe hemorrhagic shock. Baumert et al. investigated the hemodynamic variables in pigs anesthetized with xenon/remifentanil or isoflurane/remifentanil and subjected to hemorrhagic shock (20 mL.kg−1 bleeding volume), followed by reinfusion (12). These authors observed that the hemodynamic response to acute hemorrhage appeared faster and was more severe with xenon/remifentanil than with isoflurane/remifentanil anesthesia. Despite the fact that the model we used in this study was different from that used by Baumert et al., our results point in the opposite direction of their observations. In our study, the hemorrhagic shock was more profound and the bleeding volume was 44 mL.kg−1 in the Control group and 40 mL.kg−1 in the Xenon group, corresponding to 46 and 42% of the total blood volume, respectively (23). Second, the maximum possible concentration of xenon (inhalation of 79% Xe in room air) was selected to elicit the maximum hemodynamic interference during severe shock conditions. Finally, because our objective was to evaluate the impact of xenon anesthesia during shock, we did not test the hemodynamic behavior of other inhalational anesthetics.
In the Experimental group, xenon anesthesia was introduced when the dogs were in shock and it did not induce any further deterioration in hemodynamics or tissue oxygenation compared with the Control group. One possible explanation for this absence of hemodynamic interference is that the actual autonomic nervous system activity was preserved during xenon inhalation. This is suggested by the absence of a difference in heart rate and either pulmonary or systemic vascular resistance between the groups. According to Baumert et al., autonomic beat-to-beat HR modulation was better preserved with xenon-based anesthesia than with propofol-based anesthesia (9). The authors believe this was a result of xenon’s limited effects on sympathetic-vagal activity (12). On the other hand, Hanss et al. showed that xenon increased parasympathetic activity and decreased sympathetic activity, in contrast to the effects of total intravenous anesthesia (24). Marx et al. observed that plasma adrenaline concentrations were decreased in pigs anesthetized with xenon anesthesia compared with the Control group, a finding that is potentially explained by the analgesic properties of xenon. Although adrenaline concentrations were decreased, circulating catecholamines remained at normal levels (25). Based on these studies and our results, we hypothesize that in subjects submitted to circulatory shock, the minimal sympathetic inhibition induced by xenon in high inspiratory concentrations is not capable of offsetting the intense autonomic response to the shock. Therefore, hemodynamic variables and tissue perfusion were not negatively affected by the administration of xenon.
A second point that may also contribute to the well-tolerated hemodynamic profile of xenon during shock is that xenon inhalation does not hamper myocardial contractility or its response to inotropic stimuli (18). Using isolated guinea pig ventricular muscle bundles, Schroth et al. observed that, in contrast to isoflurane, xenon did not alter the force of myocardial contractions or their response to inotropic stimuli, such as calcium, isoproterenol and increased pacing frequency (15). As described above, several other authors also described the absence of significant effects of xenon on myocardial contractility in either experimental or clinical contexts (7,17,26). Experimentally, xenon has a minimal impact on autonomic nervous system activity and myocardial function, making it a suitable choice for general anesthesia in subjects in shock.
An important point about this study is that splenectomy was not performed to simulate a real trauma situation, in which patients with shock are immediately transported to the operating room to undergo damage control surgery (27). As a consequence, there was a partial recovery in mean artery pressure values within the 1 to 2 minutes necessary to collect blood samples for laboratory analysis and perform hemodynamic measurements after interrupting blood withdrawal. As the magnitude of shock was comparable in both groups, as can be observed from the similar MAP values after shock and blood volume removed (Control group, 40 mL.kg−1; Experimental group, 44 mL.kg−1), this partial recovery of MAP values does not alter the results of the study.
In conclusion, the use of xenon at the maximum inhaled concentration in a canine model of severe hemorrhagic shock did not promote a decrease in hemodynamic stability, oxygen transport or tissue perfusion. However, clinical studies are necessary to confirm the applicability of xenon-based anesthesia in hemorrhagic shock patients.
No potential conflict of interest was reported.
Franceschi RC contributed to the study design, data collection and manuscript drafting. Malbouisson LM contributed to the data analysis, manuscript drafting and reviewing. Yoshinaga EM performed data collection. Auler Jr JO contributed to the study design. Carmona MJ contributed to the study design, data collection and manuscript drafting and reviewing.