Atherosclerotic plaques and aneurysms are injuries associated with areas of low and oscillatory wall shear stress at vascular bifurcations1. Experimental animal models have been used extensively in the development of new therapeutic approaches to this pathophysiological process.2 In such models, flow at the artery is complicated by several features1–2; however, these effects are minor compared to anatomical variations of bifurcation.3 Therefore, identifying the geometric features of arterial bifurcation is a priority.
Angiography of small animal models has been extensively used as a technique to resolve geometric features of the vascular tree.4 Angiography offers unsurpassed spatial resolution of vasculature, and, furthermore, offers a rapid practical method for the evaluation of large experimental groups.5 Unfortunately, in angiography, the viewing angles are predetermined and limited in number, such that the method has an associated problem of blood vessel superimposition and overlapping; therefore, simple angiography cannot be used to accurately depict the spatial relationship between vessels and between the vasculature and surrounding tissues.6 These limitations have prompted us to consider rotational angiography and rendering reconstruction to evaluate 3D volumes and to resolve the arterial geometry necessary for the generation of a finite elements mesh that is indispensable to computational fluid dynamics studies (CFD).
MATERIALS AND METHODSThe institutional principles for research involving animals were followed and the experimental study was reviewed and approved by the local committee for ethics (Approval no. 382/06). Five Male Wistar rats, each weighing 500 g, were anesthetized by intraperitoneal application of xylazine hydrochloride (10 mg/kg body weight; Pfizer Pharma) and ketamine (100 mg/kg body weight; Pfizer Pharma) and were placed on a thermoregulated surgical table. Rat body temperature was maintained at 37°C. Supplemental doses of anesthetic were given as needed to maintain a uniform level of anesthesia that allowed spontaneous breathing. The animals breathed room air and were monitored with pulse oximetry. After positioning the animal supine, a cervical midline incision was made and the right common carotid artery was exposed, cannulated using PE-10 tubing (Becton-Dickinson), and ligated with 4-0 silk. Systemic arterial pressure was measured using a Viggo-Spectramed transducer (Viggo Spectramed) attached to a polygraph (Grass Instruments). The tubing was gently introduced into the heart until characteristics of ventricular waves were obtained and was then pushed away until systemic arterial pressure waves indicated that the tip was located in the aortic arch. All surgical procedures were performed under sterile conditions. Angiography was performed under automatic injection with an Angiomat 6000 infusion pump (Tyco/Covidien) in our clinical angiography unit (Integris Allura; Philips Medical Systems), using volumes ranging from 8 to 16 ml at 1 to 2 ml stream/sec and 150 to 650 PSI of contrast agent (Optiray; Tyco Healthcare). Angiography was repeated up to 4 times to obtain high quality images free of respiration artifacts. After identification of the best injection parameters, digital rotational angiography was performed (14 ml to 2 ml stream/sec and 650 PSI with 1 sec of delay). Three-dimensional images were reconstructed from data collected using a 180° rotational arc. A total of 120 images were obtained in this arc. Three-dimensional reconstructions were performed and made available for analysis and manipulation on a dedicated 3D RA Workstation. Animals were sacrificed after angiography by intraperitoneal injection of sodium pentobarbital (200 mg/kg body weight; Sanofi-Aventis).
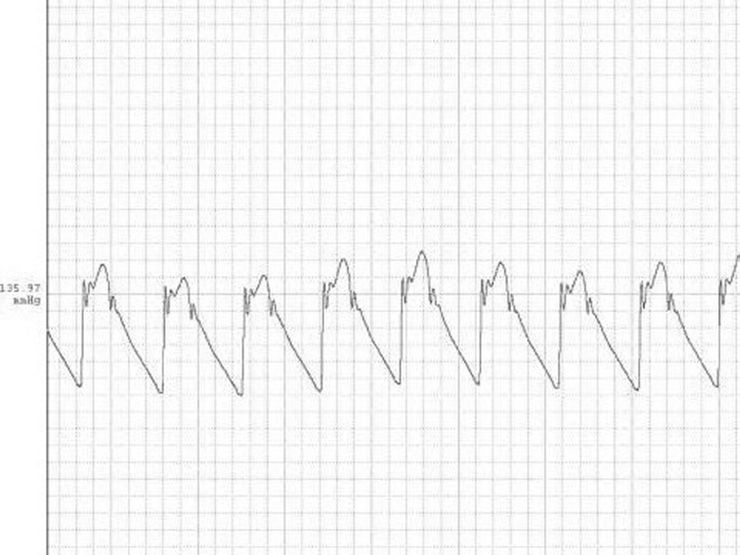
RESULTSThe tracing in Figure 1 depicts a systemic pressure waveform in the aortic arch. Figures 2 and 3 depict the angiography and the 3D reconstruction of the aortic bifurcation.
Due to the complexity of angiographic evaluation of arterial vessels in small animal models, the present study validates the use of 3D volume rendering reconstruction of the vascular tree. The results indicate that the resolution achieved by this technique in a single study is sufficient to solve the tridimensional geometry of all arterial segments in regions frequently involved in the development of aneurysms and atheromatous disease, and that it makes possible the generation of finite elements meshes for CFD studies by segmentation or image processing. The protocol is suitable for use in the investigation of systemic effects in different models of etiology and is compatible to the survival of specimens, consequently opening the possibility of sequential imaging and the facilitation of evaluating structural changes of the vascular tree over time.
CONCLUSIONSThe 3D RA technique can be used to resolve the geometries of arterial bifurcations in spontaneously breathing rats. These models should be particularly useful in providing outlet boundary conditions for CFD.
This work was supported by a grant from the FAPESP (06/03977-1).