The vulva is the primary site affected in lichen sclerosus, a chronic dermatosis in women that is histologically characterized by a zone of collagen remodeling in the superior dermis. The normal physiological properties of the vulva depend on the assembly of collagen types I (COLI), III (COLIII) and V (COLV), which form heterotypic fibers, and extracellular matrix protein interactions. COLV regulates the heterotypic fiber diameter, and the preservation of its properties is important for maintaining normal tissue architecture and function. In the current work, we analyzed the expression of COLV and its relationship with COLI, COLIII, elastic fibers and extracellular matrix protein 1 in vulvar biopsies from patients with lichen sclerosus.
METHODS:Skin biopsies from 21 patients with lichen sclerosus, classified according to Hewitt histological criteria, were studied and compared to clinically normal vulvar tissue (N=21). Morphology, immunohistochemistry, immunofluorescence, 3D reconstruction and morphometric analysis of COLI, COLIII, COLV deposition, elastic fibers and extracellular matrix 1 expression in a zone of collagen remodeling in the superior dermis were performed.
RESULTS:A significant decrease of elastic fibers and extracellular matrix 1 protein was present in the hyalinization zone of lichen sclerosus compared to healthy controls. The non-homogeneous distribution of collagen fibers visualized under immunofluorescence in the hyalinization zone of lichen sclerosus and control skin was confirmed by histomorphometry. Lichen sclerosus dermis shows a significant increase of COLI, COLIII and COLV expression compared to the healthy controls. Significant inverse associations were found between elastic fibers and COLV and between COLV and extracellular matrix 1 expression. A direct association was found between elastic fiber content and extracellular matrix 1 expression. Tridimensional reconstruction of the heterotypic fibers of the lichen sclerosus zone of collagen remodeling confirmed the presence of densely clustered COLV.
CONCLUSIONS:Increased deposition of abnormal COLV and its correlation with extracellular matrix 1 and elastic fibers suggest that COLV may be a trigger in the pathogenesis of lichen sclerosus.
Lichen sclerosus (LS) is a chronic inflammatory mucocutaneous dermatosis 1,2 that predominantly affects women in the perimenopausal and postmenopausal years. Genetic and autoimmune factors have been reported in the etiology of this dermatosis 3. The association with organ-specific autoimmune disease has suggested that LS may represent an autoimmune phenomenon 4. Histologically, the collagen hyalinization zone of the dermis has been used as a grading system for diagnosis 5. Several alterations of normal physiology could be responsible for the hyaline zone in LS dermatosis. For example, the spatial organization of collagen I (COLI) and III (COLIII) fibers 6 could be abnormally interlaced with heterotypic fibers and extracellular matrix (ECM) protein 1. Another possible cause is an absent elastic system 6. Finally, decreased CD44 expression and changes in cell-cell and cell-ECM interactions, followed by catabolism of these ECM components 7,8, could also be involved in this pathologic process.
Collagen V (COLV) is located in the dermis and basement membrane. This ECM component is a highly preserved protein found in many different animal species, which maintains the highly immunogenic NH2 terminal region 9,10. This protein is typically not exposed in the ECM, as it is hidden among COLI and COLIII, together comprising heterotypic fibers 10. COLV is also essential to the integrity of the connective tissue, exhibiting different functions depending on its distribution and molecular isoforms 10. The COLV molecules contribute to the binding of collagens to the basal membrane and are important for cell adhesion and extracellular matrix repair processes 11.
ECM protein 1 (ECM 1) was identified in 1994 as an 85 kDa glycoprotein secreted by a specific lineage of osteogenic stromal mouse cells, known as MN7 12. Subsequently, a human counterpart related to the regulation of endochondral bone formation and the proliferation of endothelial cells inducing angiogenesis was found 13. In addition, ECM 1 acts as ”biological glue“, thus contributing to the regulation of the basement membrane and organization of collagen fibers 14.
Considering that little is known about COLV in LS, in the present work, we analyzed the expression of this collagen and its relationships with COLI, COLIII, elastic fibers and ECM 1 component in vulvar biopsies from patients with LS.
MATERIALS AND METHODSPatient selection and sample collectionAll vulvar LS biopsies were obtained in the Division of Dermatology of the Clinics Hospital of the University of Sao Paulo, Brazil. Punch biopsies (4 mm-6 mm) included vulvar epidermis and dermis. LS biopsies (n=21) were obtained from untreated patients (67.29±12.34 years) with LS confirmed by histopathological analysis. Control vulvar biopsies (n=21) were obtained from normal vulva in autopsy cases (69.48±12.23 years). All patients in the study signed an informed consent to a research protocol that had been reviewed and approved by the Medical Ethical Committee of the Clinics Hospital from the School of Medicine of the University of São Paulo (No. 0233/09) for the specific study. Similarly, post-mortem sample use was approved by the Ethical Committee of the Coroner's Service of the University of São Paulo to obtain healthy controls from autopsy cases.
Histology, immunohistochemistry and immunofluorescenceFor histological, immunohistochemical and immunofluorescence evaluation, biopsies were fixed in 10% buffered formaldehyde for 24 h and embedded in paraffin. Five-micrometer-thick sections were obtained and stained with hematoxylin and eosin (H & E) and a Verhoeff stain for elastic fibers; they were then classified independently by two separate pathologists according to the Hewitt criteria 5.
For immunohistochemistry, the sections were hydrated and submitted to pepsin-mediated antigen retrieval for 20 min at 37°C. After that, endogenous peroxidase was blocked by immersing the sections in a 3% hydrogen peroxide solution (3 × 5 min) and washed twice with phosphate-buffered saline (PBS, pH 7.4). Nonspecific binding of immunoglobulins with tissue proteins was blocked by washing with casein for 5 min at room temperature, followed by incubation with rabbit polyclonal antibody directed against ECM 1 (Santa Cruz Biotechnology Inc., Dallas, Texas, USA, 1:50 dilution). The Novolink amplification kit (Leica Inc., Newcastle, UK) was used for signal amplification, and 3,3'-diaminobenzidine tetrachloride (0.25 mg dissolved in 1 mL of 0.02% hydrogen peroxide) was used as a precipitating substrate for signal detection. The specimens were then counterstained with Harris hematoxylin. The specificity of the primary antibody was confirmed with the appropriate reagent controls (omitting the primary antibody or substituting non-immune serum for the primary antibody in the staining protocol), which revealed no staining.
For immunofluorescence, the sections were mounted on 3-aminopropyltriethoxysilane (Sigma Chemical Co., St. Louis, MO, USA), dewaxed in xylol and hydrated in graded ethanol. Antigen retrieval was accomplished using enzymatic treatment with porcine pepsin (10.000 dry unit/ml) (Sigma Chemical Co., St. Louis, MO, USA) in acetic acid buffer at 0.5 N for 30 min at 37°C. For the immunodetection of COLI, COLIII and COLV, nonspecific sites were blocked with 3% hydrogen peroxide for 25 min, then blocked with avidin and biotin (Vector Laboratories, Inc., Burlingame, CA, USA) for 10 min each, and finally blocked with 5% skim milk in PBS for 30 min. The skin specimens were incubated overnight at 4°C with rabbit polyclonal antihuman COLV (1:50), rabbit polyclonal antihuman COLIII (1:200) or rabbit polyclonal antihuman COLI antibodies (Rockland Immunochemicals Inc., PA, USA, 1:150) 15. The skin sections were washed in PBS with 0.05% Tween20 and incubated for 90 min at room temperature with goat anti-rabbit IgG antibody conjugated with fluorescein (Sigma Chemical Co., St. Louis, MO, USA) 1:50 diluted in a PBS solution containing 0.006% Evans blue. Finally, the samples were mounted on buffered glycerol and analyzed using fluorescence microscopy (Olympus BX51, Olympus Co, Tokyo, Japan). For negative and autofluorescence controls, sections were incubated with normal rabbit serum and PBS instead of the specific antibody. We used tongue sections as positive controls.
HistomorphometryWe used the Image ProPlus software to quantify ECM 1 expression and COLI, COLIII, COLV, and elastic fibers in the hyalinization band. Briefly, the image analysis system consisted of an Olympus camera (Olympus Co, St. Laurent, Quebec, Canada) attached to an Olympus microscope (Olympus BX51, Olympus Co, Tokyo, Japan). The images taken were sent to an LG monitor (Oculus TCX, Coreco, Inc., St. Laurent, Quebec, Canada) and downloaded to a computer (Pentium 1330 MHz, Intel, Santa Clara, CA, USA). The images were then processed with software (Image Pro Plus 6.0, Media Cybernetics, Rockville, MD, USA) 16. The fraction area of collagen and elastic fibers was measured in an area delimited by the basement membrane, including papillary and reticular dermis partially covering the microscopic field observed at 400X magnification. The threshold for collagen and elastic fibers was established for all slides after the contrast was enhanced to the point at which the fibers were easily identified by the green (collagen fibers) and black (elastic fibers) bands. The collagen and elastic fibers' fraction area was expressed as the ratio of the numbers of measured fibers divided by the total area studied 16. All microscopic fields for each slide were quantified, and the results are expressed as the means of these fields.
The fraction area of ECM 1 was determined in five fields, including the superficial epidermis and small vessels from papillary and reticular dermis, which were randomly selected and analyzed under 400X magnification. We measured the intensity of immunostaining around blood vessels and in the epidermis. Only the vessels with strong expression and medium intensity were considered positive, and their sum was divided by the total number of vessels in the field. In the epidermis, these areas were also considered positive, and their area was divided by the total area of the epidermis in the field.
Three-dimensional reconstruction of collagen fibersCOLI, COLIII and COLV spatial organization was evaluated by confocal microscopy. Sections were processed in the same way as for immunofluorescence. The skin specimens were incubated overnight at 4°C with rabbit polyclonal antihuman COLV (1:50), rabbit polyclonal antihuman COLIII (1:200) or rabbit polyclonal antihuman COLI antibodies (Rockland Immunochemicals Inc., Limerick, PA, USA) (1:150) 15. The skin sections were washed in PBS with 0.05% Tween20 and incubated for 90 min at room temperature with goat anti-rabbit IgG antibody ALEXA 488 (Invitrogen, Life Technology, Grand Island, NY, USA) diluted 1:200 in PBS. The nuclei were stained with DAPI (4′,6-diamidino-2-phenylindole, Invitrogen, Life Technology, Grand Island, NY, USA; 1:200 in PBS) by incubating for 15 min at room temperature. Finally, the samples were mounted on buffered glycerol and analyzed using a confocal laser-scanning microscope (Zeiss LSM 510/UV GOAL, Jena, Germany) for the 3D reconstruction of collagen fibers. For negative and autofluorescence controls, the sections were incubated with normal rabbit serum and PBS instead of the specific antibody. We used tongue sections as positive controls.
Statistical analysisThe statistical analyses were performed using the Statistical Package for the Social Sciences version 20.0 (SPSS; Chicago, IL, USA). Data are expressed as means and standard deviations for continuous variables and percentages for categorical variables. The regular distribution of the data in the groups was assessed with the Kolmogorov-Smirnov test, and we used the Mann-Whitney U test to compare the continuous variables not paired and non-parametric and Student's t-test for paired and parametric continuous variables. When significant differences were identified between the groups for a given variable, the Pearson correlation coefficient for a parametric distribution of data and the Spearman correlation for non-parametric data were performed. The level of significance was p<0.05.
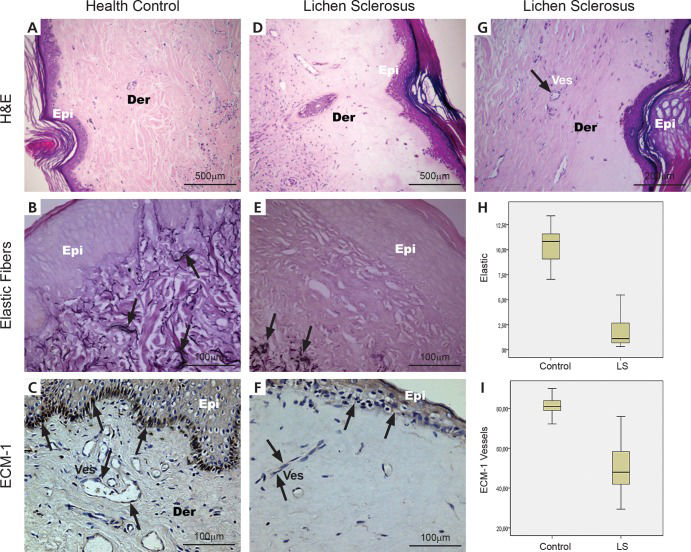
RESULTSThe typical histopathologic appearances of the normal control (n=21) and patient skin (n=21) with elastic fiber and ECM 1 expression are shown in Figure 1 (Panels A, B, C, E) stained by H & E, Verhoeff and immunohistochemistry.
Histological, histochemical, immunohistochemical and quantitative expression of elastic fibers and ECM 1 in the dermis of healthy controls and lichen sclerosus. (A) A health control biopsy shows a uniform pattern of papillary and reticular dermis; (B) fine black elastic fibers are seen in the papillary and reticular dermis (arrows) and (C) the regular brownish color of ECM 1 expression is in the superficial epidermis and small vessels of the reticular dermis (arrows). (D and G) Lichen sclerosus exhibits upper dermal homogenization with subepidermal separation; (E) disappearance of elastic fibers were seen, whereas (E) elastic fibers in the reticular to deep dermis mostly appeared normal or showed a mild increase just beneath the hyalinized zone (arrows); (F) mild or absent brownish color of ECM 1 expression in superficial epidermis and small vessels (arrows). (H) A significant decrease in elastic fibers is present in the hyalinization zone of LS compared to control skin. (I) In addition, the hyalinization zone from LS skin presents a significant decrease of ECM 1 protein expression compared to healthy controls (p<0.001). (H&E: A, X40; D, X40; G, X100 magnification); (Resorcin: B and E X200 magnification); (ECM immunostaining: C, X200; F, X200 magnification) (Der=dermis, Epi=epidermis, Ves=vessel).
In the normal control skin (Figure 1A), thin elastic fibers ran perpendicularly in the superficial dermis and horizontally in the papillary and reticular dermis (Figure 1B). In vulval LS, we found a subepidermic hyalinization zone (Figure 1D) with sparse elastic fibers (Figure 1E and 1G) and a discrete increase in elastic fibers just beneath the hyalinized superficial dermis.
In healthy controls, ECM 1 immunostaining occurs in the superficial epidermis and the small vessels of the papillary and reticular dermis (Figure 1C). In contrast, vulval LS tissue exhibits decreased immunoexpression of ECM 1 in the superficial epidermis and small vessels of the subepidermic hyalinization zone, with a discrete increase just beneath the hyalinized superficial dermis (Figure 1F).
Figure 1 (Panels H and I) shows the graphic expression of the measurements of elastic fibers and ECM 1 protein in the hyalinization zone of LS and in the control skin. As expected, a significant decrease in elastic fibers was found in the hyalinization zone of LS tissue compared to the control skin (Figure 1H; p<0.001). Furthermore, the hyalinization zone from LS skin presented significantly decreased ECM 1 protein expression compared to the healthy controls (Figure 1I). No difference was found for ECM 1 expression in the epidermis and beneath the hyalinized superficial dermis from LS and control specimens. No significant difference was revealed between the elastic fibers just beneath the hyalinized superficial dermis and those of the healthy controls.
Figure 2 (Panels A to F) presents COLI, COLIII and COLV fibers reconstructed by confocal microscopy in the hyalinization zone of LS and control specimens. Regarding COLI, in the control skin, the fibers were loosely arranged along the basement membrane (Figure 2A), assuming a honeycomb pattern in the papillar and part of the reticular layers and occasionally enhancing the walls of small vessels. In contrast, in the hyalinization zone of LS tissue, thick and fragmented COLI fibers were densely aggregated in the basement membrane (Figure 2D), the papillary layer, part of the reticular layer, and the small vessels (Figure 2D, arrows), modifying the typical histoarchitecture of the dermis. Thin fibers of COLIII were visualized predominantly around the small vessels and were sparsely distributed in the basement membrane and dermis from healthy controls (Figure 2B). In contrast, the hyalinization zone in LS skin exhibited thin fibers of COLIII irregularly distributed along the basement membrane, involving small vessels (Figure 2E, arrows) and diffusely permeating the papillary layer. Analysis of COLV in the control specimens indicated loose, thin fibers in a homogeneous and linear distribution along the basement membrane (Figure 2C) and around vessels (Figure 2C, thin arrow). In the dermis, COLV staining followed a wavy distribution coincident with the maintenance of normal tissue architecture (Figure 2C). In contrast, the hyalinization zone in LS specimens exhibited distorted and fragmented fibers of COLV (Figure 2F), as did the basement membrane and small vessels (Figure 2F, arrows).
Immunofluorescence, tridimensional reconstruction and quantitative expression of COLI, COLIII and COLV in the dermis of healthy controls and vulvar LS. (A) Dermis from a healthy control showing loosely arranged, green-stained COLI fibers assuming a basket-weave pattern in the dermis, along the basement membrane and sometimes around small vessels (thin arrows). (D) Vulvar LS dermis presents a similar pattern to the control, in which a basket-weave pattern of green-stained COLI fibers is also observed in the dermis (arrows), with loosely arranged fibers along the basement membrane (thick arrows), although small vessel enhancement is more prominent (thin arrows). (B) Dermis from a healthy control, showing COLIII thin fibers predominantly around the small vessels (thin arrows) and sparsely distributed in the basement membrane (thick arrows) and dermis (arrows). (E) In vulvar LS dermis, the thin green fibers are distributed around the small vessels (thin arrows); however, in contrast with the healthy control, this collagen permeates the dermis (arrows) and is irregularly distributed along the basement membrane (thick arrows). (C) COLV 3D-reconstruction in the dermis of a healthy control, with loosely arranged, thin COLV fibers showing a wavy distribution throughout the dermis (arrows), along the basement membrane (thick arrows) and around the small vessels (thin arrows). (F) Vulvar LS dermis presents dense COLV fibers forming irregular aggregates tightly packed in the dermis (arrows), exhibiting band distribution along the basement membrane (thick arrows) and present around the small vessels (thin arrows). (G, H, I) vulvar LS dermis shows a significant increase of COLI, COLIII and COLV expression compared to healthy controls (p=0.03, p<0.01 and p<0.01, respectively). (Immunofluorescence: A-F; X400, original magnification); (Der=dermis, Epi=epidermis, Ves=vessel).
Graphic representations of the measurements of COLI, COLIII and COLV in LS and control skin are presented in Figure 2 (Panels G, H, I). The non-homogeneous distribution of collagen fibers visualized under immunofluorescence in the hyalinization zone of LS and control skin was confirmed by histomorphometry. COLI, COLIII and COLV expression were significantly increased in the LS dermis compared to the healthy controls (p=0.03, p<0.01 and p<0.01, respectively; Figures 2 G, H, I).
Significant inverse associations were found between elastic fibers and COLV (R=-7.77; p<0.01) and between COLV and ECM 1 expression (R=-7.56; p<0.01). A direct association was found between elastic fibers and ECM 1 expression (R=6.50; p=0.01).
DISCUSSIONIn the current work, we observed a nearly complete absence of elastic fibers in the upper dermal homogenized zone in LS. Similar findings have been previously reported 17-19. The mechanism for the disappearance of elastic fibers has not been proven, but it may be triggered by the elastolytic changes induced by elastase-type proteases secreted from dermal fibroblasts or activated macrophages 17,20. This hypothesis is supported by Abbas and colleagues, who found prominent elastophagocytosis below the homogenized zone in extragenital LSA patients 21.
Using quantitative approaches, we found no significant difference between the elastic fibers just beneath the hyalinized dermis and those in the reticular deep dermis. This finding contrasts with previous reports, which employed subjective or qualitative approaches to show a prominent dermal increase in elastic fibers in some cases of vulval LS 22. These authors interpreted their case as the coexistence of nevus elasticus and vulval LSA. Another similar case was described by Allan and colleagues 23, who found LS involving a surgical scar, characterized by the uncommon presence of prominent nodules of elastin in the reticular dermis. These authors reported nodular elastosis as an atypical characteristic distinct to LS but related to scar formation. To further explore this relationship, Shiba and colleagues 19 reported an increase in elastic fibers in the mid- to lower dermis in contrast with a decrease or disappearance of elastic fibers in the superficial hyalinized dermis. The group found that the increase in elastic fibers varies and concluded that an increase in elastic fibers in the mid- to lower dermis may reflect a repairing process in response to the degraded upper dermal elastic fibers, which could be related to the pathogenesis of LS. The difference between our findings and these previous reports is likely related to our employ of objective quantitative approaches to evaluate the elastic fiber content in our cases.
In the present work, we also analyzed the expression of COLV and its relation with COLI, COLIII, elastic fibers and ECM 1 protein in vulvar biopsies from patients with LS. The main finding was that COLV was distorted and over-deposited in the hyalinization band of vulvar LS, suggesting that COLV is an important regulator of the cutaneous plaques and has potential as a target for future treatments. An increased amount of COLV was found and, according to tridimensional reconstruction, showed a structural disorganization of the fibrils with a different density clustering. In this context, COLV fibers may contribute to cutaneous plaque formation in LS, as the distribution and organization of the collagen system affects the mechanical properties of the tissues. Moreover, imperfect glycosylation of COLV fibrils may also explain the plaques, as glycosylated hydroxylysine residues avoid lateral triple-helical collagen assembly and thus the development of ordered fibrils 24. COLV fibrils are critical for controlling the development of a functional skin matrix 25. The triple helices of COLV are found hidden inside the heterotypic fibril, and the preserved amino terminal globular domain projects from the surface, regulating the diameter of these fibers 26. Interestingly, COLI and COLIII fibers were also densely clustered in LS tissue, indicating that COLV had likely lost its regulatory function. These findings were expected because COLI and COLIII are normally both up-regulated during skin remodeling. In normal skin, small amounts of COLV are expressed as thin fibrils in the basement membranes for binding with stromal collagen, which is important for cell adhesion and the matrix repair process 27. In the current work, the increased numbers of COLI, COLIII, and COLV fibers found in LS tissue were associated with the subepidermic hyalinization band, likely due to a high co-polymerization among the fibers, resulting in a heterotypic assembly incapable of controlling fibril structure and collagen fibrillogenesis. Existing reports have shown a direct correlation between the extent of the hyalinization band reaction and the progression of LS, which is similar to the increased COLV found in our study 26.
Our results also showed that an increase in COLV fibers was associated with a decrease in ECM 1 expression by cutaneous microvessels, suggesting that an increased binding of COLV to epitopes on endothelial cells reduces the synthesis of ECM 1 by these cells. COLV is a potential trigger antigen because it induces scleroderma in rabbits 28-32 and is related to lung transplant rejection in animals 33 and human patients 34. Furthermore, the induction of oral tolerance by COLV suppresses acute and chronic lung rejection in animals 35. Similarly, nasal tolerance induced by COLV in experimental SSc reverted skin 36 and pulmonary remodeling processes 37. These findings reinforce studies that suggested that immunological factors are involved in and responsible for all of the steps of disease. In fact, the relationship between organ-specific autoimmune disease and LS has indicated LS as an autoimmune phenomenon. Chan and colleagues 38 reported that the sera of affected individuals contain circulating IgG autoantibodies to the ECM 1 protein. The passive transfer of these autoantibodies may reproduce histological and immunophenotypical features of LS skin 39. Another question that could be resolved is the relationship between high COLV synthesis, the hyalinization band, and decreased ECM 1 blood vessel expression, which leads to decreased angiogenesis, increased hypoxia and decreased nutrient flow. Thus, the increased expression of COLV in LS may be a primary event, whereas decreased ECM 1 expression may be a secondary event. Regardless of the mechanism, immunostaining of LS for COLV and ECM 1 provided important information about LS.
To our knowledge, this study is the first to investigate COLV and ECM 1 signals in the skin from patients with LS, therefore limiting comparison of the results.
In summary, decreased staining of elastic fibers in the upper dermal homogenized zone in LS tissue was associated with highly and abnormally expressed COLV and decreased ECM 1 expression. The increased COLV and decreased ECM 1 expression may reflect a bad immunogenic repair process in response to the disappearance of elastic fibers in the upper homogenized dermis and may offer a better understanding of the pathogenesis of vulval LS. COLV fiber density and ECM 1 expression in vulval LS may have therapeutic implications for controlling this disorder. Furthermore, strategies aimed at preventing high COLV synthesis, or local responses to low ECM 1 expression, may be useful for LS treatment. Further confirmation of these conclusions will require a larger-scale study in a randomized and prospective trial.
AUTHOR CONTRIBUTIONSGodoy CA was involved in patient biopsy selection and control biopsy collection, data analysis and drafting the manuscript. Capelozzi VL and Teodoro WR conceived the study, participated in its design and organization and revised the article critically. Godoy CA, Capelozzi VL and Sotto MN evaluated and scored the histological assessments. Eher EM, Velosa AP and Garippo AL performed the experiments and were involved in the manuscript preparation. Parra ER analyzed the data and assisted in the manuscript preparation and statistical analyses.
This study was sponsored by the São Paulo Research Foundation (FAPESP 2011/52095-0; FAPESP 2012/03543-2) and National Center for Scientific and Technological Development (CNPq 471939/2010-2; CNPq 483005/2012-6; CNPq 300993/2010-2).
No potential conflict of interest was reported.