Brachial plexus injuries, in all their severity and complexity, have been extensively studied. Although brachial plexus injuries are associated with serious and often definitive sequelae, many concepts have changed since the 1950s, when this pathological condition began to be treated more aggressively. Looking back over the last 20 years, it can be seen that the entire approach, from diagnosis to treatment, has changed significantly. Some concepts have become better established, while others have been introduced; thus, it can be said that currently, something can always be offered in terms of functional recovery, regardless of the degree of injury. Advances in microsurgical techniques have enabled improved results after neurolysis and have made it possible to perform neurotization, which has undoubtedly become the greatest differential in treating brachial plexus injuries. Improvements in imaging devices and electrical studies have allowed quick decisions that are reflected in better surgical outcomes.
In this review, we intend to show the many developments in brachial plexus surgery that have significantly changed the results and have provided hope to the victims of this serious injury.
Brachial plexus injuries have been a challenge throughout the history of medical knowledge in the sense of both the need to understand the neural anatomical structures affected and the aim of proposing treatment that may restore function to the injured upper limb.
The brachial plexus is formed by the roots from C5 to T1, and it may or may not receive contributions from C4 and T2 (pre- or post-fixed). Its anatomy is complex and is characterized by nerves coming from the plexus that interrelate to form fascicles and finally the nerves that head to all parts of the upper limb.
Despite the few studies that have been conducted in Brazil, it is apparent that there has been a large increase in the number of brachial plexus injuries occurring, which is a consequence of significant increases in the use of motorcycles as a means of transport (1,2). American and European studies have demonstrated that 10 to 20% of peripheral nerve injuries are brachial plexus injuries and that 80 to 90% of these injuries are caused by motorcycle and car accidents (3). In our outpatient clinic at the Hospital das Clínicas de São Paulo, 95% of the cases of brachial plexus injury result from motorcycle accidents.
It is very important to classify the injury for treatment purposes. It seems most intuitive to us to divide the injuries into upper trunk (Erb-Duchene; C5/C6), extended upper trunk (Erb-Duchene; C5/C6/C7), lower trunk (Dejerine-Klumpke; C8/T1) and swinging limb (all roots). In terms of prognostic factors relating to the level of the injury, Rorabeck and Harris (4) indicated that isolated injuries of the upper trunk have a better prognosis than do isolated lesions of the divisions, upper roots or lower trunk. Complete injuries and pain persisting for more than six months indicate a poor prognosis in terms of neurological recovery, independent of the level of the injury.
The first serious approaches towards brachial plexus treatment were begun in the 1940s and 1950s by pioneers such as Seddon (5) and Bateman (6), among others. At that time, the parameter used for surgical indications was an attempt to clinically identify whether the injury was an avulsion or a neuroma with continuity. The first was characterized as an irreversible injury, while the second would have a better prognosis. Among the signs of a poor prognosis was the Claude-Bernard-Horner sign, which was claimed by several authors (4,7) to be very reliable for diagnosing avulsion injuries of the lower roots. Presence of the Tinel sign in the supraclavicular region indicated that graftable roots were possibly present.
The concept that reigned until the 1970s in relation to treatments for avulsion injuries was that these were cases for amputation procedures or arthrodesis of the shoulder, elbow and wrist, depending on the level of the injury. In cases of amputation, attempts were made to create limb prostheses. In non-avulsion cases, an expectant approach was taken for two years that awaited any possible return to functioning, a state from which attempts to perform orthopedic surgery could be made. In addition, in 1965, Seddon (8) described a surgical procedure for cases of avulsion simply as a means of accelerating the diagnosis and being able to bring forward amputation surgery.
Although in the 1990s it was considered prudent to wait five or six months before indicating surgery, the improvements in imaging examinations and electrical studies that have now become established mean that today, there is a tendency to indicate surgery earlier rather than more than three months after the injury because it is now known that nerve regeneration has a better prognosis the earlier it occurs. Bertelli and Ghizoni (7) backed indications of exploratory surgery three to six months after the injury and, similarly to Narakas and Hentz (9), emphasized that cases operated upon more than nine months after the injury have worse prognoses.
In surgical procedures for cases of neuroma with continuity, coaptation of the injured stumps was often performed while keeping the patient in a forced position or even through shortening the clavicular bone. This situation only changed with the advent of the use of the nerve graft that was proposed by Seddon (10) in 1963, followed by several studies at the beginning of the 1970s (3). These confirmed that this procedure was effective for reconstructing the loss of a nerve segment. Recently, several studies on the possibility of using neurotrophic factors for increasing the efficacy of the nerve regeneration process have been published, although there remains no standardization regarding use of these factors (11).
In addition to repairs on injured nerve structures in the plexus, the technique of neurotization (which consists of suturing a remaining intact nerve to another that is injured) has constituted a major advance for brachial plexus reconstructions. It has evolved from the first descriptions of use of the intercostal nerve (9,12) to the use of many other nerves (13-15), thereby contributing greatly towards improving the results from brachial plexus surgery.
ARTICLE SELECTION CRITERIAThe selection criteria for the articles we reviewed were a) original articles describing the different techniques cited throughout the text, and b) articles based on developments of original techniques, mainly those proposing comparisons between operative outcomes.
Although the authors privileged more recent studies (edited in the last 10 years), there was no strict time interval for a paper to be considered in this work.
DIAGNOSTIC METHODSBefore the surgeryStaging the brachial plexus injury is of enormous importance for making an indication of exploratory surgery and for helping to plan it. It is known that nerve reconstruction should be performed as early as possible because after this procedure has been accomplished, functional recovery remains dependent on axonal growth to reach the effector organ (muscle or sensory unit). According to some authors, this growth should not take more than 18 to 24 months. After this time, the fibrotic muscle tissue should be replaced (16).
For diagnosing the injury, there needs to be a clinical assessment made in conjunction with an imaging examination and an electrical study on nerve conduction. Among the imaging methods, the standard is myelotomography, which has been shown to be very good for detecting root avulsion and has a sensitivity of 85% and a specificity of 95% (17). Nonetheless, our experience shows that magnetic resonance imaging is now an effective method for demonstrating not only the avulsion but also the level of the injury along the plexus. Therefore, this procedure is now our preferred imaging examination.
Electroneuromyography is used with the aim of differentiating plexopathy from root injury and for establishing a prognosis for nerve injuries, including post-reconstruction surgeries. The motor amplitude in electroneuromyography is not a good means for evaluating the injury level, but sensory studies in comparison with the contralateral site provide important information about the injury location and whether avulsion is present.
During the surgeryIt is now possible to conduct studies on the condition of the brachial plexus nerves in greater detail during the surgery (18). With the aid of electrical studies conducted during the operation, it should be possible to achieve greater sureness with regard to making decisions such as whether to preserve an injured neural segment. Electrical stimulation of the brachial plexus can be performed, although there may be “contamination” of the stimulus to other nerve branches, thus making it difficult to make a selective assessment on each nerve. Electroneuromyography examinations can also be performed, despite the logistic difficulty of doing so during the surgery.
If a neuroma is identified, there is some doubt regarding whether it should be resected or whether simple neurolysis should be performed. Studying the nerve action potential (NAP) (19) using one electrode proximally to the neuroma and one distally to it enables quantitative assessment of the number of viable nerve fibers and thus assists in decision-making. Studying the sensory evoked potential (SEP) using one electrode distally to the nerve root studied and picking up the stimulus in the contralateral cortex makes it possible to diagnose intraforaminal avulsion.
Surgical indicationBrachial plexus injuries are generally associated with severe sensory and motor deficits of the upper limb. Therefore, all efforts towards treatment should take into consideration the correct staging of the lesion so that the best type of treatment can be chosen. In our experience, considering that the trauma that led to the brachial plexus injury generally involved high energy, spontaneous regeneration of the injury is unusual. Thus, we very often indicate a surgical procedure as a means of therapy and staging. Even in cases of avulsion, there are options such as neurotization that can be used with the aim of restoring some degree of functionality. Therefore, brachial plexus surgery is important both for performing reconstruction and for resolving doubts.
Surgical proceduresDecisions regarding brachial plexus surgery should be made as a function of the predefined priorities. Because of the small number of nerve units available, decisions regarding whether to prioritize the shoulder or the elbow often have to be made. In our view, shoulder stability is fundamental for good functioning of the elbow; therefore, the concept of seeking to reconstruct from proximal to distal applies. It should be kept in mind that the results obtained in relation to C8 and T1 are very unfavorable, which provides justification for some authors' opinion that these roots should not be reconstructed (7).
NeurolysisNerve reconstructions of the brachial plexus have historically gone through the stages of neurolysis, direct nerve repair, nerve grafts (20) and, lastly, neurotization. Neurolysis consists of promoting dissection of the nerve that presents a neuroma with continuity. This technique is performed with the aim of decompressing any viable fascicles in relation to the fibrotic tissue. Today, it is possible to have a quantitative assessment of the number of viable nerve fibers. This ability is due to the advent of intraoperative electrical studies (18), especially the nerve action potential (NAP), which uses one electrode proximally to the neuroma and one distally to it. This information may help in making the decision regarding whether to perform neurolysis or to resect the neuroma and perform a nerve graft.
Nerve graftNerve grafting started in 1963 by Seddon (10) who introduced the concept of using nerve grafts to reconstruct nerve losses. Good results were subsequently confirmed by other authors (21-24). Until then, attempts to perform direct suturing of injured nerves were made at any cost despite bone shortening or maintenance of the limb in forced positions, which compromised nerve regeneration. The use of grafts initially required suturing of the ends of the sural nerve individually, which made the procedure very tedious and less precise. However, with the advent of fibrin glue (25), it became possible to form a group of graft ends, thus constituting a single structure. Fewer stitches were needed in the proximal and distal sutures, which made the grafting procedure much simpler and much more secure.
NeurotizationThe procedure of neurotization consists of transferring an undamaged motor nerve to another nerve that is injured. The nerves used are remainders from the trauma to the brachial plexus and may come from the brachial plexus (intra-plexular) or from elsewhere (extra-plexular).
Through neurotization, the conditions for a quality suture close to the effector area (muscle unit) are provided, which diminishes the distance for nerve regeneration. We agree with other authors (26-28) who have affirmed that direct suturing should always be attempted in cases of neurotization because using a graft leads to a worsening of the results from nerve regeneration. Another point in relation to neurotization is that although recent neurotization techniques have generally shown good results, some authors (7,29) have emphasized the importance of primary reconstruction of the brachial plexus whenever possible when performing neurotization, given that the chances of reinnervation of the antagonist musculature are thus increased, whereas this outcome is less likely with neurotization alone.
Intra-plexular neurotizationThere are several options for neurotization of the remaining nerve units, and many of them have been described recently.
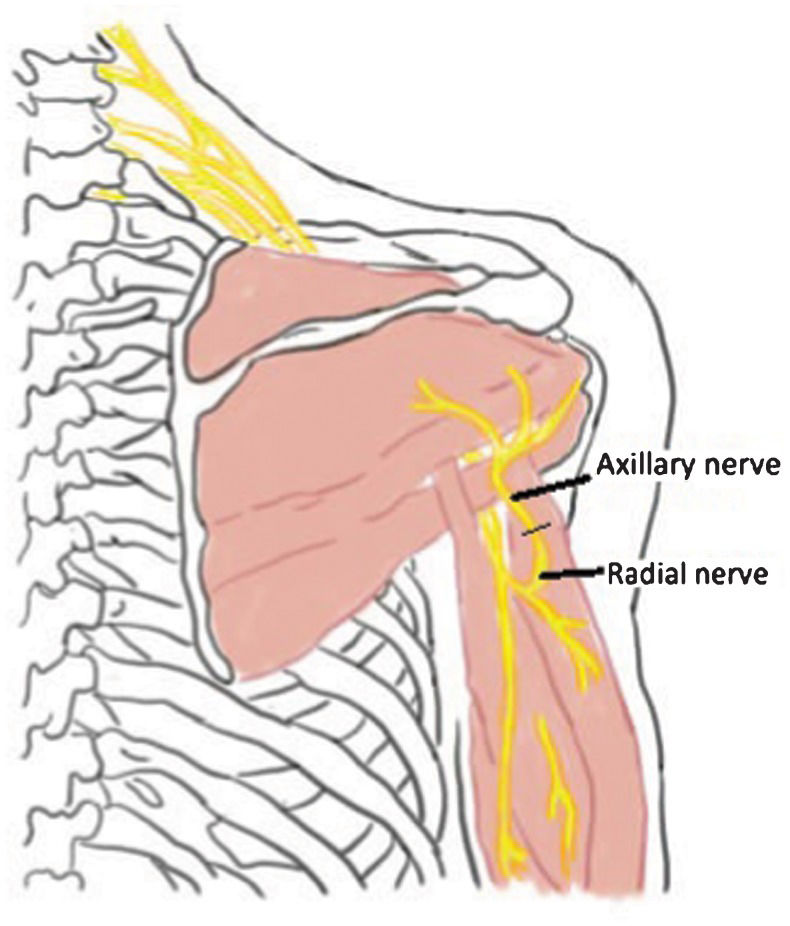
Radial nerve to axillary nerve – This was described in 2003. The authors (30-32) demonstrated through an anatomical and clinical study that it was possible to obtain a branch of the radial nerve for suturing to the posterior branch of the injured axillary nerve (Figure 1. Subsequent clinical studies demonstrated good results with this technique (approximately 124 degrees of abduction), especially if in association with neurotization of the spinal accessory nerve with the suprascapular nerve (33).
Ulnar nerve to musculocutaneous nerve – The first description of this technique was provided by Oberlin et al. (34) in 1994. It consists of neurotization of a fascicle of the ulnar nerve to a motor branch of the musculocutaneous nerve that heads towards the biceps. Excellent clinical results have been confirmed by different authors (13,26,27,33,35-39). Our experience with this type of neurotization has also been very good: in most cases, we have achieved a biceps of at least grade 3, which could be given more potential through possible transfer of the flexion-pronation musculature (Steindler). The meta-analysis study conducted by Merrel et al. (33) indicated that the best results were achieved when the procedure was performed up to six months after the trauma, while there was a sharp worsening of the results if performed more than 12 months after the trauma.
Despite the good results from neurotization of the biceps muscles, double neurotization was proposed (40) and was named Oberlin2 surgery. In this technique, in addition to the usual procedure, a fascicle from the median nerve would be used for neurotization in the motor branch of the brachial muscle. In a study comparing these two techniques, Sungpet et al. (38) did not find any difference regarding the final functional result.
Medial pectoral nerve to musculocutaneous nerve – This procedure was described by Brandt and Mackinnon (41) in 2003 and consists of suturing the medial pectoral nerve directly to the musculocutaneous nerve with the aim of restoring the elbow flexion. It should be remembered that the pectoral nerve receives contributions from the lateral and medial fascicle and may thus be compromised in many cases (Figure 2.
Extra-plexular neurotizationPhrenic nerve to musculocutaneous nerve – This transfer was first described by Gu et al. (42) in 1990. It can be performed because the phrenic nerve is generally preserved in cases of brachial plexus injury, given that its biggest contribution comes from C3 and C4. To avoid using grafts, the dissection on the phrenic nerve needs to be performed as distally as possible. According to Monreal (43), the phrenic nerve is eminently motor and presents 800 neurons. Some divergences persist regarding the morbidity ensuing from its removal: this removal could lead to breathlessness when making effort. This procedure is not recommended for patients with previous pulmonary diseases or for children under the age of two years.
Phrenic nerve to suprascapular nerve – This type of transfer is an alternative to the spinal accessory nerve and can be used for neurotization of another injured nerve. In some clinics, this procedure is used routinely with good results (42), and it can be highlighted that function returns after eight months. Morbidity relating to the removal of the phrenic nerve needs to be taken into consideration. We do not have much experience with this procedure, but because the phrenic nerve is an eminently motor nerve and the distance to the effector area is short, it is reasonable to expect that good results are possible.
Spinal accessory nerve to suprascapular - The spinal accessory nerve is a pure motor nerve without approximately 1500 axons (44) (Figure 3. This neurotization was first described in the 1980s (9,12,45) and is perhaps the neurotization that is used most often, given the frequency of injuries to the suprascapularis nerve and the ease of execution of this procedure. These nerves are close together, and the same route is taken as the one used for exploration of the brachial plexus. The result from this procedure has been shown to be below expectations considering the proximity of the supra- and infraspinatus muscles. Venkatramani et al. (27) reported that there was a gain in shoulder abduction of 66 degrees in 60% of the cases that they studied and that the external rotation was generally unsatisfactory. Even with these results showing partial recovery, Narakas and Hentz (9) reaffirmed that the shoulder range of motion was twice what was obtained through arthrodesis on the shoulder. We believe that the prime function of this type of neurotization is shoulder stabilization, which may ensure a better result with regard to gains in elbow flexion and may provide greater synchronism of gait to avoid swinging limbs.
Contralateral C7 to median nerve – This transfer was first described by Gu et al. (46) in 1992, who proposed harvesting the root of the contralateral C7 and transferring this root to the median nerve of the injured side by means of a nerve graft (Figure 4. The first problem posed related to the morbidity caused by sectioning the C7 root, but subsequent studies revealed that this morbidity was minimal. Another issue posed was that the graft was from a long nerve and that there was a long distance to the effector area. This concern was confirmed through the results presented that showed that protective sensitivity was recovered in 83% of the cases, but with an unfavorable motor result (15). In view of these points, we have not used this procedure at our clinic.
Intercostal nerve to musculocutaneous nerve – This procedure was described in the 1980s (9) and uses the intercostal nerves, which each present approximately 1200 axons transferred to the musculocutaneous nerve with the aim of restoring shoulder flexion (Figure 5. This surgery implies accessing not only the upper limb but also the thoracic region just below the nipples. In general, at least two intercostal nerves are dissected as far as proximally to the sternal region to gain length, thus favoring not using a graft (28). The study by Merrel et al. (33) confirmed positive functional results with the return of elbow flexion against gravity in more than 65% of cases. This proportion is only lower than the result from neurotization of the ulnar nerve to the musculocutaneous nerve. The intercostal nerve is of mixed type; therefore, identifying the motor branch is fundamental to the success of the procedure. We have considerable experience with this procedure, which allows us to affirm that if it is impossible to use the ulnar nerve as a source of neurotization, the second choice is to take the intercostal nerve to the musculocutaneous nerve.
Complementary orthopedic treatmentUntil the 1960s, bone surgery had an important role in treating patients with brachial plexus injuries, especially among those with complete injuries. Certain procedures, such as shoulder arthrodesis (47), external derotation osteotomy and wrist arthrodesis, were routine procedures (48), along with limb amputation, for which the level was defined according to the degree of functional recovery of the limb.
This scenario has changed drastically with improvements in the results from nerve reconstructions, especially through neurotization, which has enabled joint stabilization and a return to functioning for some muscles. This advance, together with conventional free muscle transfers, has made arthrodesis and amputation exceptional approaches in treating brachial plexus injuries.
Arthrodesis: Although some clinics advocate using shoulder arthrodesis as a means of stabilizing this joint, we are now giving preference to the transfer of the trapezium to the humerus, as first described by Saha (49).
We reserve wrist arthrodesis for situations in which, because of the small number of tendons available for transfer, we can use the carpal flexors and extensors for the finger tendons in performing the arthrodesis.
Tendon transfersTrapezium muscle to humerus (shoulder abduction) – In this type of transfer, the acromial insertion of the trapezium muscle is transposed to the proximal humerus together with a segment of the acromion with the aim of achieving a gain in shoulder abduction. Despite unsatisfactory results in terms of shoulder abduction, we have observed that this transfer has an important role as a shoulder stabilizer. It has positive results, particularly in cases of severe shoulder instability with inferior subluxation of the glenohumeral joint.
Trapezium muscle to humerus (external rotation of the arm) – In this transfer, the lower segment of the trapezium is dissected while maintaining its vascularization and innervation. With complementation using a graft from the fascia lata or by expanding the muscle segment as far as the external border of the acromion using the aponeurotic tissue, the trapezium is inserted into the intertuberous sulcus with the aim of gaining external rotation. Few reports are yet available in the worldwide literature (50) regarding functional results, but based on our experience with this technique, the initial results are very promising.
Pectoralis major to biceps (Clark) – This procedure was described by Clark (51) and is an option for gaining elbow flexion when the pectoralis major muscle is preserved. This situation is unusual because its innervation comes from the medial and lateral fascicles, which are very commonly affected in brachial plexus injuries. Another negative factor is that the distal suture of the muscle in the biceps is of the muscle-to-muscle type, which compromises its quality, particularly with regard to adjusting the necessary tension.
Latissimus dorsi to elbow flexor – Success in this transfer also depends on the integrity of the muscle and its innervation through the thoracodorsal nerve, which is often injured. When undamaged, this muscle is a very efficient transfer that can result in restitution of flexion strength and even cosmetic improvement regarding the muscle outline in the anterior region of the arm. Our preference has been for a bipolar transfer in which the muscle is fully lifted and then reinserted both proximally and distally, which facilitates adjustment of tension on the transferred muscle.
Triceps to biceps – In this type of transfer, the triceps is used to act as an elbow flexor, for which the basic condition is that the C7 root and therefore the triceps should be undamaged. Through this complete distal deinsertion of the tricipital tendon and its transfer to the bicipital tendon, a good elbow flexion result can be obtained after a period of rehabilitation for functional readaptation of the triceps. The major inconvenience of this technique is in relation to the loss of active extension of the elbow, thereby leading to dependence on passive extension through the action of gravity. For patients who depend on elbow extension (with use of crutches or a wheelchair), this technique is contraindicated. We believe that it should be used when other transfers are not possible.
Flexor-pronator musculature to humerus – Steindler (elbow flexion) – This technique was described by Steindler in 1918 and consists of transfer of the flexor-pronator musculature to the metaphyseal region of the humerus (52). To achieve success in this procedure, it is essential for the C8 and T1 roots to be undamaged. In performing this technique, this musculature is made to act as an elbow flexor. Our experience with this procedure confirms other authors' experience (53) that indicated that this transfer is efficient for boosting elbow flexion, i.e., when the existing elbow flexor musculature is at least grade 2 (54). This transfer has been shown to be insufficient for achieving functional elbow flexion on its own.
Free muscle transfer – The first report of free muscle transfer was made by Tamai et al. (55) in 1970, who reported that this type of procedure was successful in dogs. Specifically for brachial plexus injuries, this type of transfer has been performed using the gracilis muscle to restore elbow flexion (Figure 6. There is no doubt that this procedure represents a major advance in cases of chronic lesions that did not have any prognosis. Such cases have come to be viewed as having very favorable functional results (13,56-58). For this procedure to be successful, not only are vascular sutures needed; additionally, a good donor motor nerve has to be chosen to perform neurotization. The intercostal nerve, the spinal accessory nerve and even fascicles from the ulnar nerve have been used for this procedure, each with advantages and disadvantages. One point that we judge to be important is to always seek direct suturing and avoid using nerve grafts at all costs.
One of the points that gives rise to discussion is in relation to the limits between expecting the flexor musculature to become reinnervated or deciding to perform free muscle transfer. In our clinic, based on previous studies that indicated that the nerve and muscle receptors were significantly impaired in lesions that were more than 12 months old (59-60), we have chosen to use free muscle transfer. We use the gracilis muscle with very satisfactory results.
In conclusion, the treatment of brachial plexus injuries has evolved over recent decades. Many new procedures have been incorporated with known ones, allowing a better perspective for functional recovery after surgical approaches to treat this severe injury.
AUTHOR CONTRIBUTIONSRezende MR was the originator and primary contributor to text and data collection. Silva GB was the secondary contributor to text and data collection. Paula EJ contributed to the text formatting and revision. Mattar Junior R and Camargo OP revised the text.
No potential conflict of interest was reported.