Early identification of children with metabolic syndrome (MS) is essential to decrease the risk of developing diabetes and cardiovascular disease in adulthood. Detection of MS is however challenging because of the different definitions for diagnosis; as a result, preventive actions are not taken in some children at risk. The study objective was therefore to compare prevalence of MS in children according to the IDF, NCEP-ATP-III, Cook, de Ferranti and Weiss definitions, considering insulin resistance (IR) markers such as HOMA-IR and/or metabolic index (MI).
MethodsA total of 508 Mexican children (aged 9–13 years) from seven schools were enrolled in a cross-sectional study. Somatometric, biochemical, and hormonal measurements were evaluated.
ResultsFrequency of MS was 2.4–45.9% depending on the definition used. Frequency of IR in children not diagnosed with MS was 12.4–25.2% using HOMA-IR and 4.0–16.3% using MI. When HOMA-IR or MI was included in each of the definitions, frequency of MS was 8.5–50.2% and 7.7–46.9% respectively. The kappa value including HOMA-IR and/or MI was greater than 0.8.
ConclusionsThis study demonstrated the poor effectiveness of the current criteria used to diagnose MS in Mexican children, as shown by the variability in the definitions and by the presence of IR in children who not diagnosed with MS. Inclusion of HOMA-IR and/or MI in definitions of MS (thus increasing agreement between them) decreases the chance of excluding children at risk and allows for MS prevalence between populations.
La identificación oportuna de niños con síndrome metabólico (SM) es la clave para disminuir el riesgo de desarrollar diabetes y enfermedad cardiovascular en la vida adulta, sin embargo, su detección representa un gran reto debido a las diversas definiciones para su diagnóstico dejando excluidos niños con factores de riesgo a los cuales no se les brindarán medidas preventivas. El objetivo es comparar la prevalencia de SM según las definiciones de la IDF, NCEP-ATP-III, Cook, de Ferranti y Weiss e incluir marcadores de resistencia a la insulina (RI) como el HOMA-IR y/o índice metabólico (IM).
MetodologíaEstudio transversal en 508 niños mexicanos de 9 a 13 años. Se registraron medidas somatométricas y evaluaron parámetros bioquímicos y hormonales.
ResultadosLa frecuencia de SM fue de 2,4-45,9% dependiendo de la definición utilizada. La RI en los niños sin diagnóstico de SM fue del 12,4-25,2% con HOMA-IR y 4,0-16,3% con IM. Al incluir el HOMA-IR o IM en cada una de las definiciones la frecuencia de SM fue 8,5-50,2% y 7,7-46,9% respectivamente. El valor de Kappa incluyendo HOMA-IR e IM fue mayor a 0,8.
ConclusionesEste trabajo revela la poca efectividad de las definiciones diagnósticas de SM empleadas actualmente, evidenciada por la variabilidad entre ellas y por la presencia de RI en niños que escapan al diagnóstico de SM. Incluir al HOMA-IR y/o IM en las definiciones, disminuye la probabilidad de excluir niños con SM y aumenta la concordancia entre ellas haciendo posible la comparación de la prevalencia de SM entre las poblaciones.
Metabolic syndrome (MS) is a public health problem giving rise to two main complications, type 2 diabetes mellitus (DM2) and cardiovascular disease (CVD), which have been the most frequent causes of death since the year 2000.1 Metabolic syndrome is associated with a two-fold increase in the risk of CVD and a 5-fold increase in the risk of DM2. It is defined by the presence of at least three of the following 5 risk factors: arterial hypertension, increased triglyceride levels, low HDL-cholesterol concentrations (i.e., atherogenic dyslipidemia), altered fasting glucose and abdominal obesity.2
In the case of the pediatric population, it is very important to identify those children at risk of developing MS, as these patients are more likely to develop DM2 and CVD over time. Relatively few studies have investigated the prevalence of MS in children and adolescents, though it is evident that MS is very common in the obese pediatric population.3,4
In Mexico and in certain Latin American countries such as Chile and Brazil, populations of obese children and adolescents have been studied to determine the prevalence of MS and of insulin resistance (IR).5–7 It has been postulated that IR and abdominal obesity are the main factors contributing to the manifestations of MS. In this regard, although abdominal obesity has shown a comparatively stronger correlation, many of the metabolic changes appear to be triggered by IR, since the latter exerts effects upon lipid metabolism, which manifest themselves as increased LDL-cholesterol, lowered HDL-cholesterol, and increased triglyceride and fatty acid levels.8,9
The diagnosis of MS in children and adolescents poses serious difficulties, bearing in mind that the cut-off points of the different components of the syndrome have not been precisely established in Mexico. As a result, diagnosis and prevalence vary according to the definition used.10 In this sense, discrepancies in prevalence can be found within the same population sample.3,4 The present study compares 5 definitions: those of the International Diabetes Federation (IDF), the National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATP-III), and those of Cook, Ferranti and Weiss, with a view to characterizing the diagnosis of MS in the Mexican pediatric population.4,10–12 In addition, in evaluating the role of IR in the development of DM2, the study proposes the inclusion of an independent criterion such as the homeostatic model assessment of insulin resistance (HOMA-IR) and/or the metabolic index (MI).13,14
MethodsThe present cross-sectional study consisted of 508 Mexican children between 9 and 13 years of age from 7 primary schools. The inclusion criteria were: children in the fourth, fifth and sixth year of primary school, the obtaining of written informed consent signed by either a parent or tutor, and the verbal consent of the children, themselves. The study was approved by the Ethics Committee of Hospital Juárez de México, and was carried out in accordance with the recommendations of the Declaration of Helsinki. The exclusion criteria were: the use of antihypertensive drugs, blood glucose-lowering medication or lipid-lowering drugs. The clinical evaluation consisted of the measurement of anthropometric parameters: height, weight and waist circumference according to standardized methods.15 The body mass index (BMI) percentile was calculated with the Pediatric Z-score Calculator (http://stokes.chop.edu/web/zscore/). In order to control inter-observer variability, a single person was in charge of obtaining the anthropometric measurements of all the participants. Waist circumference was recorded from the midpoint between the tenth rib and the iliac crests, followed its by transformation into percentiles according to age and gender reference tables.15 Arterial pressure was recorded in duplicate (two measurements separated by a 10-min resting period), followed by adjustment to percentiles (https://www.bcm.edu/bodycomplab/Flashapps/BPVAgeChartpage.html). In the case of the biochemical and hormonal parameters, the blood samples were obtained after an 8-h fasting period, centrifuged in the first 15min after sampling, and refrigerated (2–8°C) until processing. Total cholesterol, HDL-cholesterol, LDL-cholesterol, triglycerides and glucose were measured in the Molecular Endocrinology Laboratory of Hospital Juárez de México using commercial methods and following the instructions of the manufacturer. The insulin concentrations were determined by chemiluminescence using the Immulite 2000 kit from Siemens® in an Immulite 2000 system (Roche®), according to the manufacturer's instructions. All these methods were based on criteria established by the National Committee for Clinical Laboratory Standards, thereby guaranteeing precision both within and between tests. The sensitivity of the insulin test was 0.2μU/ml. The pediatric reference ranges for Mexican children are between 2 and 15μU/ml.8 The HOMA-IR index used to estimate IR was calculated by multiplying the insulin concentration (μU/ml) by the glucose concentration (mg/dl), and dividing the product by 405. Values of >3.4 were considered indicative of IR.14 Another IR indicator used was the MI, estimated on the basis of the glucose and triglyceride concentrations: MI=(triglycerides [mmol/l]×glucose [mmol/l])/HDL-cholesterol2 (mmol/l). Values of >7.0 were considered indicative of IR.13 Five definitions were used for diagnosing MS: those of the IDF, the NCEP-ATP-III, Cook, Weiss and Ferranti.4,10–12 A descriptive analysis was made, which involved calculating the frequencies of the 5 definitions of MS, with a determination of the differences between the obtained and expected frequencies using the chi-squared test. A value of p≤0.05 was considered statistically significant. The level of agreement among the 5 diagnostic definitions of MS was explored using the kappa index. The agreement levels were interpreted as follows: <0.2 (poor), 0.21–0.4 (weak), 0.41–0.6 (moderate), 0.61–0.8 (good), 0.81–1 (very good). A value of p<0.05 was considered statistically significant. The SPSS version 20.0 statistical package was used throughout.
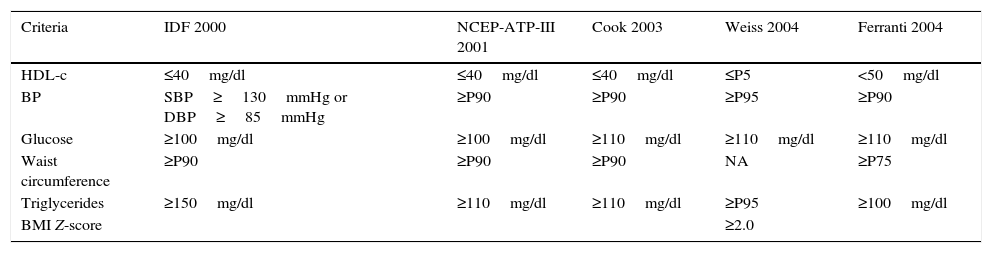
ResultsTable 1 shows the 5 definitions used for the diagnosis of MS in the pediatric population. Four of them include the same criteria (IDF, NCEP-ATP-III, Cook and Ferranti), though they use different cut-off points for each of the parameters studied. The definition of Weiss differs from the others in that it does not use the waist circumference percentile as a marker of obesity; instead, it uses the BMI Z-score for this purpose.
Criteria and cut-off points of the 5 definitions of metabolic syndrome in the pediatric population.
| Criteria | IDF 2000 | NCEP-ATP-III 2001 | Cook 2003 | Weiss 2004 | Ferranti 2004 |
|---|---|---|---|---|---|
| HDL-c | ≤40mg/dl | ≤40mg/dl | ≤40mg/dl | ≤P5 | <50mg/dl |
| BP | SBP≥130mmHg or DBP≥85mmHg | ≥P90 | ≥P90 | ≥P95 | ≥P90 |
| Glucose | ≥100mg/dl | ≥100mg/dl | ≥110mg/dl | ≥110mg/dl | ≥110mg/dl |
| Waist circumference | ≥P90 | ≥P90 | ≥P90 | NA | ≥P75 |
| Triglycerides | ≥150mg/dl | ≥110mg/dl | ≥110mg/dl | ≥P95 | ≥100mg/dl |
| BMI Z-score | ≥2.0 |
HDL-c: high-density lipoprotein cholesterol; BMI: body mass index; BP: blood pressure; SBP: systolic blood pressure; DBP: diastolic blood pressure; P5: percentile 5; P75: percentile 75; P90: percentile 90; P95: percentile 95; MS: metabolic syndrome.
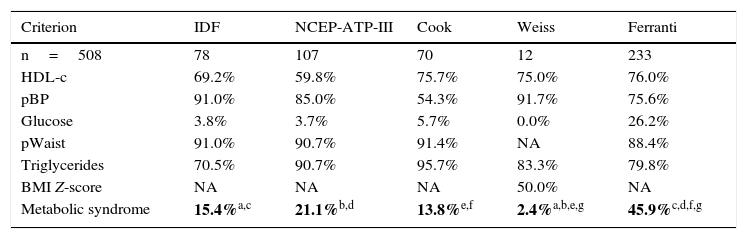
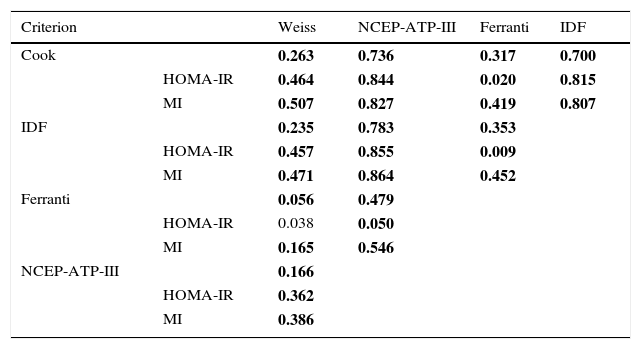
In this study the frequency of MS was found to range between 2.4% and 45.9%, depending on the parameters and cut-off points of each of the 5 definitions used (Table 2). The frequencies of MS according to the criteria of Weiss and Ferranti differed significantly (p=0.005). By contrast, there were no significant differences between the definitions of the IDF, the NCEP-ATP-III and Cook with regard to the frequencies of MS (p=0.55). It is important to emphasize that central obesity and increased triglyceride levels are the most frequent components using the definitions of the NCEP-ATP-III, Cook and Ferranti. The analysis of the level of agreement between the diagnostic definitions of MS revealed close agreement between the definition of Cook and that of the NCEP-ATP-III and the IDF, as well as between the definition of the IDF and that of the NCEP-ATP-III (Table 3). By contrast, the definitions of Weiss and Ferranti showed only weak agreement with the other definitions used. On examining HOMA-IR and/or the MI in the MS criteria, the disparity among definitions was seen to decrease, showing an agreement (kappa index) of 0.8 (p=0.0001), with the exception of the definitions of Weiss and Ferranti. Unexpectedly, greater agreement was observed among the definitions on including the MI.
Prevalence of metabolic syndrome according to the 5 definitions used.
| Criterion | IDF | NCEP-ATP-III | Cook | Weiss | Ferranti |
|---|---|---|---|---|---|
| n=508 | 78 | 107 | 70 | 12 | 233 |
| HDL-c | 69.2% | 59.8% | 75.7% | 75.0% | 76.0% |
| pBP | 91.0% | 85.0% | 54.3% | 91.7% | 75.6% |
| Glucose | 3.8% | 3.7% | 5.7% | 0.0% | 26.2% |
| pWaist | 91.0% | 90.7% | 91.4% | NA | 88.4% |
| Triglycerides | 70.5% | 90.7% | 95.7% | 83.3% | 79.8% |
| BMI Z-score | NA | NA | NA | 50.0% | NA |
| Metabolic syndrome | 15.4%a,c | 21.1%b,d | 13.8%e,f | 2.4%a,b,e,g | 45.9%c,d,f,g |
HDL-c: high-density lipoprotein cholesterol; BMI: body mass index; pWaist: waist circumference percentile; pBP: blood pressure percentile; MS: metabolic syndrome.
Statistical test: chi2p<0.05. Differences p<0.005 are shown in boldface.
Agreement between the 5 definitions used for the diagnosis of metabolic syndrome.
| Criterion | Weiss | NCEP-ATP-III | Ferranti | IDF | |
|---|---|---|---|---|---|
| Cook | 0.263 | 0.736 | 0.317 | 0.700 | |
| HOMA-IR | 0.464 | 0.844 | 0.020 | 0.815 | |
| MI | 0.507 | 0.827 | 0.419 | 0.807 | |
| IDF | 0.235 | 0.783 | 0.353 | ||
| HOMA-IR | 0.457 | 0.855 | 0.009 | ||
| MI | 0.471 | 0.864 | 0.452 | ||
| Ferranti | 0.056 | 0.479 | |||
| HOMA-IR | 0.038 | 0.050 | |||
| MI | 0.165 | 0.546 | |||
| NCEP-ATP-III | 0.166 | ||||
| HOMA-IR | 0.362 | ||||
| MI | 0.386 |
MI: metabolic index; MS: metabolic syndrome.
Interpretation of the agreement values (kappa index): <0.2 (poor), 0.21–0.4 (weak), 0.41–0.6 (moderate), 0.61–0.8 (good), 0.81–1 (very good). Statistical test: Kappa p<0.05; values corresponding to p<0.0001 are shown in boldface.
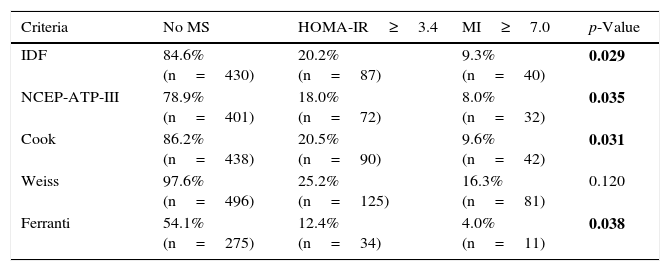
The frequency of IR among the 508 children was 25.8% as assessed using HOMA-IR vs 18.3% as determined by the MI, with moderate agreement between HOMA-IR and the MI (p=0.001). We also determined whether or not the children not diagnosed with MS according to each of the definitions presented IR (Table 4). In this regard, the percentage of children with IR ranged between 12.4–25.2% and 4.0–16.3% when using HOMA-IR and the MI, respectively, with a significantly greater frequency of IR as assessed with HOMA-IR among the children not diagnosed with MS according to the definitions of the IDF (p=0.029), NCEP-ATP-III (p=0.035), Cook (p=0.031) and Ferranti (p=0.038), vs IR as evaluated with the MI.
Frequency of children with insulin resistance that were not classified as presenting metabolic syndrome according to the 5 definitions studied.
| Criteria | No MS | HOMA-IR≥3.4 | MI≥7.0 | p-Value |
|---|---|---|---|---|
| IDF | 84.6% (n=430) | 20.2% (n=87) | 9.3% (n=40) | 0.029 |
| NCEP-ATP-III | 78.9% (n=401) | 18.0% (n=72) | 8.0% (n=32) | 0.035 |
| Cook | 86.2% (n=438) | 20.5% (n=90) | 9.6% (n=42) | 0.031 |
| Weiss | 97.6% (n=496) | 25.2% (n=125) | 16.3% (n=81) | 0.120 |
| Ferranti | 54.1% (n=275) | 12.4% (n=34) | 4.0% (n=11) | 0.038 |
HOMA-IR: homeostatic model assessment-insulin resistance; MI: metabolic index; IR: insulin resistance; MS: metabolic syndrome.
Statistical test: chi2p<0.05; values corresponding to p<0.05 are shown in boldface; comparison of the frequency of IR with MI≥7.0 vs HOMA-IR≥3.4.
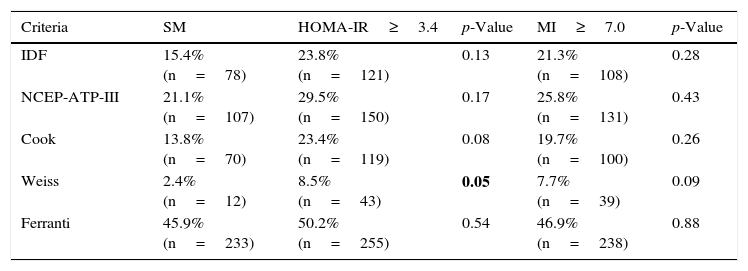
After including IR estimated with HOMA-IR as an additional criterion in each of the 5 definitions of MS, we observed an increase in frequency when we examined both IR parameters—though statistical significance was only reached when using the definition of Weiss with HOMA-IR (p=0.05). Interestingly, on comparing the definitions with the inclusion of HOMA-IR and/or the MI, a similar distribution was noted, with no significant differences between the observed frequencies (Table 5).
Frequency of metabolic syndrome in the pediatric population, according to the 5 definitions used, with insulin resistance according to HOMA-IR and the MI considered as an additional factor.
| Criteria | SM | HOMA-IR≥3.4 | p-Value | MI≥7.0 | p-Value |
|---|---|---|---|---|---|
| IDF | 15.4% (n=78) | 23.8% (n=121) | 0.13 | 21.3% (n=108) | 0.28 |
| NCEP-ATP-III | 21.1% (n=107) | 29.5% (n=150) | 0.17 | 25.8% (n=131) | 0.43 |
| Cook | 13.8% (n=70) | 23.4% (n=119) | 0.08 | 19.7% (n=100) | 0.26 |
| Weiss | 2.4% (n=12) | 8.5% (n=43) | 0.05 | 7.7% (n=39) | 0.09 |
| Ferranti | 45.9% (n=233) | 50.2% (n=255) | 0.54 | 46.9% (n=238) | 0.88 |
HOMA-IR: homeostatic model assessment-insulin resistance; MI: metabolic index; IR: insulin resistance; MS: metabolic syndrome.
Statistical test: chi2p<0.05; values corresponding to p<0.05 are shown in boldface; comparison of the frequency of MS and IR (MI≥7.0) vs MS and IR (HOMA-IR≥3.4).
The prevalence of MS in the pediatric population depends on the definition used to diagnose the syndrome. In this regard, the definitions used in our study were adapted from the range of definitions proposed for adult individuals by the World Health Organization (WHO), the NCEP-ATP-III, the European Group for the Study of Insulin Resistance and the IDF, and they are all in agreement as to the essential components of MS (glucose intolerance, central obesity, arterial hypertension and dyslipidemia).4,16,17 In the case of children and adolescents, over 40 definitions of MS have been adapted, and these differ both in the criteria used and in the cut-off points applied. The 5 definitions used in our study are derived from the two definitions most commonly used worldwide, namely those of the IDF and the NCEP-ATP-III. On the other hand, the definitions of Cook and Ferranti are the result of modifications made in the cut-off points referring to arterial pressure, glucose and waist circumference percentile of the NCEP-ATP-III.10,11,18 In turn, the definition of Weiss is based on modifications of the European Group for the Study of Insulin Resistance.16,19Table 1 shows that the definition of Weiss uses stricter cut-off points for the diagnosis of MS. Thus, taking HDL-cholesterol concentration as an example, it establishes the cut-off point at percentile 5, while the least restrictive definition (that of Ferranti) establishes the cut-off point at percentile 40.20
At present, the diagnosis and interpretation of data referring to MS in children and adolescents is complicated by the differences that result from the use of different defining criteria. The consequence of this is a considerable variability in the prevalence of MS among populations, which generates diagnostic problems and therefore difficulty in identifying the pediatric population at risk of developing DM2 and CVD.21
Four of the 5 definitions evaluated in our study coincide in terms of the individual components but differ widely in the cut-off points used to define biological risk, these cut-off points actually being the result of statistical projections of adult values rather than a biological effect.22 This explains the differences found in the prevalence of MS in the pediatric population (2.4–45.9%). Table 2 shows that the highest frequency of MS was obtained using the criteria of Ferranti (45.9%). The children with MS diagnosed according to this definition presented central obesity (88.4%), elevated triglyceride levels (79.8%) and low HDL-cholesterol concentrations (76.0%). In this regard, it should be mentioned that high triglyceride levels and low HDL-cholesterol concentrations are characteristic of the Mexican population,1 a fact that introduces data bias into the analysis. On the other hand, the lowest prevalence of MS was obtained when using the definition of Weiss, which establishes the highest cut-off point for triglyceride concentration (150mg/dl), corresponding to percentile 95 in adolescents between 15 and 19 years of age.20 Despite the above, 83.3% of the children had elevated triglyceride levels, this percentage being higher than that obtained with the definition of the IDF (70.5%).
It is important to emphasize that Weiss used a BMI Z-score ≥2.00 as an indicator of obesity, discarding central obesity measured according to the waist circumference percentile. Nevertheless, the IDF considers central obesity as the main criterion for the diagnosis of MS, since it contributes to the development of insulin resistance, hyperinsulinism, altered fibrinolysis and endothelial dysfunction, and is associated with cardiovascular risk.23,24 In our study, 20.3% of the children presented a BMI percentile≥95, and 34.4% had central obesity (waist circumference percentile≥90). However, only 16.3% of these children were classified as presenting MS according to the definition of the IDF. These data indicate that MS in this population is not conditioned by central obesity estimated from the waist circumference percentile.
On the other hand, the definition developed by Weiss attributes equivalent relevance to three of the 5 key components of MS related to cardiovascular events. Accordingly, this definition uses the highest cut-off point for arterial pressure (percentile 95), while the definition of Cook uses the NCEP-ATP-III cut-off point for arterial pressure (percentile 90), thereby ensuring the early identification of children that show changes in this parameter.
The definitions of Cook and the NCEP-ATP-III were the most similar in terms of the cut-off points corresponding to the parameters that define MS, with the exception of glucose concentration. Nevertheless, the observed frequency differed without reaching statistical significance, and exhibited good agreement (kappa index=0.736). On taking into consideration HOMA-IR and the MI, agreement was seen to be very good (kappa index=0.844 and 0.827, respectively). The agreement among the different definitions of MS improved on using the MI. This is a promising observation, since the MI may be a parameter that is both useful and easy to calculate. The results referring to the definitions of Weiss and Ferranti reveal a low level of agreement, thereby showing that some people could be excluded from a diagnosis of MS, which would increase their future risk of suffering from DM2 or CVD. This underlines the need for a definition that can allow us to more reliably classify children with MS and also compare the results between populations.
Bearing in mind that MS is based on the appearance of IR as the central metabolic event in the etiopathogenesis of the syndrome, as described by Reaven in 1988, the present study analyzed those children that were not classified as presenting MS. In this regard, a high percentage of them were seen to present IR, a fact that entails a potential risk of developing DM2 in adulthood. We therefore included IR in addition to the criteria used in each of the definitions of MS (Tables 4 and 5). The gold standard for measuring IR is the hyperinsulinemic – euglycemic clamp technique. However, since this is a complex and invasive procedure, the clamp was used to validate and verify IR through HOMA-IR, which is simpler and exhibits a significant correlation to clamp. The determination of fasting insulin can offer information on compensatory hyperinsulinemia, though many studies use glucose levels as an IR surrogate. This was not applicable here, however, since they do not always correlate to IR in children.
No standards for determining IR in children have been developed to date. There are a number of techniques for measuring insulin sensitivity, exhibiting an up to 53-fold variation in fasting insulin concentrations. As a result, the best method for evaluating IR and its correlation to clinical alterations and treatments has not been clearly established. However, HOMA-IR continues to exhibit a close correlation to the fasting insulin concentrations in children (r≥0.95).25 In view of the above, in this study we calculated IR based on two indices: HOMA-IR and the MI. The HOMA-IR index describes the homeostasis between glucose and insulin levels. However, one of the inconveniences of this index is the difficulty of measuring insulin concentrations, due to their great biological variability, together with the selection of the most adequate cut-off point, adapted to the age and gender of the children (the cut-off points range from 2.16 to 3.43). A strict cut-off point was selected for the diagnosis of IR (HOMA-IR≥3.4),14,26 revealing that 26% of the 508 children had IR. The other index proposed is the MI, which constitutes an indirect, effective and inexpensive method for evaluating IR, without the need for measuring insulin concentrations.13,14,26–29 With an MI cut-off point of >7.0,13 18.5% of the 508 children were seen to have IR. It is important to stress the results shown in Table 4, which show the presence of IR in children that were not classified as presenting MS according to some of the 5 definitions used. The frequency of IR was >23% with HOMA-IR and >7% with the MI. With the exception of the definition of Weiss, the frequencies of MS found with each of the definitions, including IR as calculated with HOMA-IR and/or the MI, showed statistically significant differences. The definition of Ferranti contemplated broader cut-off points and therefore included the largest number of children with some metabolic disorder. The children not classified as presenting MS according to the definition of Ferranti presented IR in only 12.4% and 4.0% of the cases as determined with HOMA-IR and the MI, respectively. The definition of Weiss classified the smallest number of children as presenting IR (HOMA-IR 25.2% and MI 16.3%). On the other hand, the definition of the IDF, which is based on the presence of central obesity due to its association with IR, did not classify 20.2% of the children with a high HOMA-IR index (Table 4).
The frequency of IR as estimated with the MI showed significant differences with respect to the frequency obtained with HOMA-IR in our study. This may be because the cut-off point for the MI was established for a population between 18 and 65 years of age,13 thus providing further evidence of the need to establish cut-off points for the pediatric population. Despite this, however, the MI is a parameter worth considering for identifying children with IR. Table 5 shows that the inclusion of an IR estimating factor (whether HOMA-IR or the MI) increases the chances of identifying the largest possible number of children with metabolic alterations. It is, therefore, important to note that including HOMA-IR in the definition of Weiss significantly increases the percentage of children with MS.
A very important point is that our results show that the inclusion of HOMA-IR or the MI in the different definitions of MS increases the level of agreement among them (kappa index>0.8). This makes it possible to eliminate the difficulties in establishing a diagnosis of MS in the pediatric population. In the present study, the definition of Ferranti was the definition that classified the largest number of children as presenting metabolic alterations, as few children with IR were left out of the classification, compared with the other definitions.
The above results illustrate the complexity of establishing a reliable diagnosis of MS in the pediatric population, since the currently used definitions do not take IR into account. As a result, they fail to adequately identify a large percentage of children at risk of suffering DM2 or CVD later in life. It is important to note that despite the existence of studies that compare different diagnostic definitions of MS in children, none of them evaluated the use of alternatives to HOMA-IR in assessing IR among children that did not present MS. A limitation of our study is the fact that the Tanner test could not be applied to assess puberty. However, Hirschler et al.30 found no significant differences between the groups with and without MS and the distribution of the Tanner stages.
The results of this study show that the definition of Ferranti classified the largest number of children as presenting metabolic alterations. This suggests that it is the most useful definition for identifying children with MS and with an increased risk of developing DM2 and CVD in adulthood. We also found that the inclusion of HOMA-IR and/or the MI in MS definitions eliminated discrepancies between the criteria. This favors the comparison of studies in different populations without the requirement of a universal definition, thus allowing for the early identification of cardiometabolic risk factors and prevention of the progression of MS toward diabetes.
Conflicts of interestThe authors state that they have no conflicts of interest.
This study was carried out with the support of the Dirección General de Asuntos del Personal Académico UNAM UNAM (Projects IN221014 and IN231511).
Please cite this article as: Peña-Espinoza BI, Granados-Silvestre MÁ, Sánchez-Pozos K, Ortiz-López MG, Menjivar M. Síndrome metabólico en niños mexicanos: poca efectividad de las definiciones diagnósticas. Endocrinol Diabetes Nutr. 2017;64:369–376.