In 2020 the pandemic caused by SARS-COV-2 demanded an enormous number of healthcare resources in order to guarantee adequate treatment and support for those patients. This study aims to assess caloric and protein intake and evaluate its associations with relevant clinical outcomes in critically ill with coronavirus disease (COVID-19) patients.
MethodsA nationwide, multicentre prospective observational study including twelve Argentinian intensive care units (ICUs,) was conducted between March and October 2020. Inclusion criteria: Adult ICU patients>18 years admitted to the ICU with COVID-19 diagnosis and mechanical ventilation for at least 48h. Statistical analysis was carried out using IBM-SPSS© 24 programme.
ResultsOne hundred and eighty-five patients were included in the study. Those who died had lower protein intake (0.73g/kg/day (95% confidence interval (CI) 0.70–0.75 vs 0.97g/kg/day (CI 0.95–0.99), P<0.001), and lower caloric intake than those who survived (12.94kcal/kg/day (CI 12.48–13.39) vs 16.47kcal/kg/day (CI 16.09–16.8), P<0.001).
A model was built, and logistic regression showed that factors associated with the probability of achieving caloric and protein intake, were the early start of nutritional support, modified NUTRIC score higher than five points, and undernutrition (Subjective Global Assessment B or C). The patients that underwent mechanical ventilation in a prone position present less caloric and protein intake, similar to those with APACHE II>18.
ConclusionsCritically ill patients with COVID-19 associated respiratory failure requiring mechanical ventilation who died in ICU had less caloric and protein intake than those who survived. Early start on nutritional support and undernutrition increased the opportunity to achieve protein and caloric goals, whereas the severity of disease and mechanical ventilation in the prone position decreased the chance to reach caloric and protein targets.
En 2020, la pandemia provocada por el SARS-COV-2 demandó una enorme cantidad de recursos sanitarios para garantizar el tratamiento y apoyo adecuado a estos pacientes. Este estudio tiene como objetivo evaluar la ingesta de calorías/proteínas y evaluar sus asociaciones con resultados clínicos relevantes en pacientes críticamente enfermos con enfermedad por coronavirus (COVID-19).
MétodosSe realizó un estudio observacional prospectivo multicéntrico a nivel nacional que incluyó 12 unidades de cuidados intensivos (UCI) argentinas entre marzo y octubre de 2020. Criterios de inclusión: pacientes adultos de la UCI >18 años ingresados en la UCI con diagnóstico de COVID-19 y ventilación mecánica durante al menos 48h. El análisis estadístico se realizó mediante el programa IBM-SPSS© 24.
ResultadosEn el presente estudio se incluyeron 185 pacientes. Entre los que fallecieron se observó un aporte proteico más bajo (0,73g/kg/día [intervalo de confianza {IC} del 95% 0,70-0,75] vs. 0,97g/kg/día [IC 0,95-0,99], p<0,001), y menor aporte calórico que los que sobrevivieron (12,94kcal/kg/día [IC 12,48-13,39] vs. 16,47kcal/kg/día [IC 16,09-16,8], p<0,001).
Se construyó un modelo de regresión logística para analizar qué factores estaban asociados con la probabilidad de lograr los objetivos calóricos/proteicos. Se observó una mayor probabilidad de lograr dichos objetivos cuando el inicio del soporte nutricional era precoz, el puntaje NUTRIC modificado era superior a 5 puntos y el paciente tenía diagnóstico de desnutrición mediante la Evaluación Global Subjetiva(B o C). Por otra parte, en los pacientes que necesitaron ventilación mecánica en decúbito prono se observó menor aporte calórico y proteico, situación similar en aquellos con APACHE II>18.
ConclusionesLos pacientes críticos con insuficiencia respiratoria asociada a la enfermedad por COVID-19 que requerían ventilación mecánica y que fallecieron en la UCI tuvieron una ingesta calórica y proteica menor que los que sobrevivieron. El inicio temprano del soporte nutricional y la desnutrición aumentaron la posibilidad de alcanzar los objetivos calóricos y proteicos, mientras que la gravedad de la enfermedad y la ventilación mecánica en decúbito prono disminuyeron la posibilidad de alcanzar los objetivos calóricos y proteicos.
In 2020 the world was sieged by a pandemic caused by the novel coronavirus SARS-COV-2, which was responsible for the new coronavirus disease (COVID-19). This outbreak demanded a considerable number of intensive care unit (ICU) beds, hospital resources,1 and healthcare professionals, aimed to provide adequate treatment and organ support for ICU patients.2 SARS-COV 2 infected patients with respiratory failure due to severe pneumonia and acute respiratory distress syndrome (ARDS) who are admitted to the ICU exhibit severe systemic inflammation, hypermetabolism, and hypercatabolism, which expose these patients to high risk of malnutrition and sarcopenia.3 Moreover, these patients have increased energy expenditure, which is due to ventilatory workload, mechanical ventilation requirement, and the development of multiorgan failure.3 Recently, we have found that COVID-19 ICU patients are at high risk of malnutrition, and those who were previously malnourished showed a significantly higher overall mortality.4 Moreover, our recently published study found that malnourished ICU patients showed worse clinical outcomes compared with well-nourished COVID-19 critically ill patients.4
In 2020, the Metabolic and Nutritional Support Committee (COSONUME) and the Dietician Section (CALINU), both sections belonging to the Argentine Society of Intensive Care (SATI), developed a summary of clinical recommendations to provide the best nutritional therapy for COVID-19 ICU patients.5 This concise guideline, among others,6–9 is a basic framework of evidence mostly derived from observational and retrospective data, as well as data from non-COVID-19 patients.5
In the SATI guideline, we proposed that all ICU patients staying for more than 48h in the ICU should be considered at risk for malnutrition. In addition, a hypocaloric nutrition strategy over the early phase of critical illness, defined as less than 70% of the estimated requirements, was also suggested.5 Moreover, with the aim to determine the caloric requirement we proposed the use of predictive equations, as indirect calorimetry is not commonly available in most Argentine ICUs. Finally, after the seventh day of the ICU stay, we recommended progressing caloric delivery to up to 100% of energy requirements.5
Regarding protein intake, in agreement with the updated ESPEN guidelines,9 we suggested a daily dose of ≥1.3g/kg, which should be administered progressively. This intervention showed improvement mainly in the survival of elderly and frail critically ill patients.10
Therefore, this study aimed to evaluate the caloric and protein intake in the first 14 days in critically ill COVID-19 patients. The secondary outcome aimed to evaluate factors that could affect reaching a caloric intake higher than 25kcal/kg/day and protein intake higher than 1.3g/kg/day, although there is recent conflicting evidence.11–15
Material and methodsThis is a nationwide, multicentre, prospective, observational study conducted in 12 tertiary care Argentinian hospitals belonging to the public and private health system. It was performed by ICU physicians and dietitians who collected all variables. All the patients included in the present work were part of a database used in a previous paper.4 Each hospital Ethics Committee for Medical Research approved the protocol. Written Informed consent was obtained from all participants or their relatives before enrolment into the study.
Inclusion criteria- •
Patients older than 18 yr who were admitted to the ICU with a diagnosis of COVID-19 disease (defined by a positive nucleic acid nasopharyngeal test+symptoms), requiring invasive mechanical ventilation (MV) for more than 48h, and nutritional support requirement (enteral, parenteral, or both).
- •
Patients who were referred to another centre in which the investigators were unable to continue with the follow-up.
- •
Patients who received oral nutritional supplements or decided on limitation of care or refusal at the inclusion point.
- •
Pregnant women.
- •
Patients with do-not-resuscitate status and terminal illness.
- •
Patients with incomplete data (main outcome: daily caloric and protein intake) or who were still at the hospital at the end of the study.
Patients were included if they were admitted to the ICU from March 1st, 2020 to October 31st, 2020. At baseline clinical assessment was performed to assess malnutrition at ICU admission. In such way, actual body weight (ABW) (kg), height, body mass index (BMI) (kg/m2), BMI classification (<18.5 low weight; 18.5–24.9 normal weight; 25–29.9 overweight and >30 obese), the subjective global assessment (SGA) that was performed by dietitians and ICU physicians specialised in nutritional support, and the nutritional risk (NUTRIC) score without IL-6 (modified NUTRIC, mNUTRIC) were estimated. In addition, comorbidities such as cancer, history of hypertension, chronic obstructive pulmonary disease, diabetes mellitus, and chronic kidney disease were recorded, and the Charlson Comorbidity Index (CCI) was estimated. Moreover, the severity of illness based on the Acute Physiology and Chronic Health Evaluation II (APACHE II) score was calculated for each patient. Finally, clinical outcomes including ICU length of stay (LOS), ICU mortality, and hospital mortality were also collected.
During the study period, from admission until ICU discharge, daily records were kept regarding all ICU treatments and procedures. In addition, daily total caloric intake delivered by means of enteral and parenteral nutrition, dextrose and propofol infusions were recorded. Daily protein/amino acid intake from EN and PN was also recorded.
Nutritional goals were calculated using predictive equations. Each individual centre decided on the nutritional goal, but all centres calculated at least a minimal objective of 25kcal/kg and 1.3g/kg actual body weight of proteins.
Statistical analysisBaseline characteristics and outcomes were analysed depending on the nature of the variables. The normality of quantitative data was assessed by Mean, Median, Mode, and Kurtosis with a standard deviation (SD) or a confidence interval of 95% (CI 95%). Also, the Shapiro test for normality was performed. Quantitative data were described with the mean and 95% confidence interval. Normally distributed data were analysed with a 2-tailed t-test (P=0.05). Otherwise, the Wilcoxon rank sum test was used (P=0.05). For categorical variables, the Chi-Square test was used. The Kruskal Wallis test was used if the variable had three or more options.
Logistic regression was performed with protein intake ≥1.3g/kg/d and caloric intake ≥25kcal/kg/d after day four (the day on which the patient is expected to meet 100% of their caloric and protein intake goals) with the following variables: prone position, APACHE II score ≥18, SGA categories B or C, mNUTRIC ≥5, and early initiation of nutritional support (within the first 48h after ICU admission). A logistic regression model was built to analyse the possible effect of different factors that could affect the chance of reaching the nutritional goals of 25kcal/kg/day and 1.3g/kg/day of proteins. The factors analysed were the severity the of disease assessed with the APACHE II score, early start of nutritional support (before 48h of ICU stay), the nutritional risk with mNUTRIC score, undernutrition (SGA B or C), and MV in a prone position.
Also, in the logistic regression analysis, the ICU LOS was split into periods to address these factors on a time basis. There were three periods, the first range from day four to day seven, the second from day eight to day 11, and the last from day 12 to day 14P<0.05 was considered to be statistically significant. Statistical analysis was carried out using IBM-SPSS 24.0 software (SPSS, Chicago, IL, USA) for Windows.
ResultsA total of 290 critically ill patients with COVID-19 and nutritional support were assessed for eligibility. After applying inclusion and exclusion criteria, n=185 patients on MV met our inclusion criteria and were finally included in the study (Fig. 1). N=83 (44.9%) were obese (Table 1), and the most common comorbidity was a history of hypertension. Complete baseline demographic characteristics are expressed in Table 1. Fifty-one point nine percent of the population died in the ICU. There were no differences in BMI, and comorbidities between patients who died and survived in the ICU. Regarding nutritional status assessed through SGA, it was found that surviving patients presented more frequently with SGA “A”, and the patients that died presented more frequently with SGA “B” (Table 1). The route of NS was 96.7% enteral nutrition (EN), and 3.3% parenteral nutrition (Table 1). ICU LOS was 24.9±14.3 days, whereas the duration of MV was 21.3±11.6 in the whole population.
Baseline characteristics of ICU patients. Data are expressed as mean and standard deviation.
| Total population (n=185) | Dead (n=96) 51.9% | Alive (n=89) 48.1% | P value | |
|---|---|---|---|---|
| Gender (male) | 120 (64.5%) | 64 (66.7%) | 56 (62.9%) | 0.589 |
| Age (years) | 60.5 (14.4) | 64.3 (13.4) | 56.5 (14.5) | 0.0002 |
| Origin | ||||
| Ward | 80 (43.2%) | 37 (38.5%) | 42 (47.2%) | 0.233 |
| ED | 63 (34.1%) | 39 (40.6%) | 24 (27.0%) | 0.052 |
| Other Institution | 33 (17.8%) | 17 (17.7%) | 16 (18.0%) | 0.957 |
| ND | 9 (4.9%) | 3 (3.1%) | 7 (7.9%) | 0.154 |
| Weight (kg) | 89.5 (21.4) | 90.8 (25) | 88.1 (16.7) | 0.392 |
| Height (cm) | 169.4 (8.5) | 169.8 (8.7) | 169.1 (8.2) | 0.574 |
| BMI (kg/m2) | 31.2 (7.2) | 31.4 (8.3) | 30.8 (6.0) | 0.576 |
| BMI condition | ||||
| Low weight | 0 | 0 | 0 | NA |
| Normal | 21 (11.4%) | 12 (12.5%) | 9 (10.1%) | 0.608 |
| Over-weight | 81 (43.8%) | 41 (42.7%) | 40 (44.9%) | 0.763 |
| Obese | 83 (44.9%) | 43 (44.8%) | 40 (44.9%) | 0.989 |
| Comorbidities | ||||
| Hypertension | 92 (49.7%) | 44 (45.8%) | 36 (40.4%) | 0.460 |
| Oncologic | 15 (8.1%) | 9 (9.4%) | 6 (6.7%) | 0.502 |
| COPD/ASTHMA | 17 (9.2%) | 11 (11.5%) | 6 (6.7%) | 0.261 |
| T2D | 43 (23.2%) | 21 (21.9%) | 22 (24.7%) | 0.653 |
| CKD | 14 (7.6%) | 10 (10.4%) | 4 (4.5%) | 0.130 |
| 2 or more | 45 (24.3%) | 28 (29.2%) | 17 (19.1%) | 0.110 |
| NRS 2002 | 3.4 (1.2) | 3.5 (1.0) | 3.3 (1.4) | 0.262 |
| mNUTRIC score | 3.5 (1.8) | 3.8 (1.9) | 3.1 (1.8) | 0.011 |
| APACHE II score | 14.4 (6.1) | 14.9 (6.2) | 13.9 (6.0) | 0.267 |
| SGA | ||||
| A | 109 (58.9%) | 48 (50%) | 61 (68.5%) | 0.018 |
| B | 65 (35.1%) | 44 (45.8%) | 22 (23.6%) | 0.0016 |
| C | 2 (1.1%) | 0 | 2 (2.2%) | 0.145 |
| ND | 9 (4.9%) | 4 (4.2%) | 5 (5.6%) | 0.659 |
| Charlson comorbidity index | 2.25 (2.22) | 2.5 (2.2) | 2.0 (2.2) | 0.124 |
| Type of NS | ||||
| TPN | 2 (1.1%) | 1 (1.0%) | 1 (1.1%) | 0.937 |
| CPN+EN | 4 (2.2%) | 2 (2.1%) | 2 (2.2%) | 0.962 |
| EN | 179 (96.7%) | 93 (96.9%) | 86 (96.7%) | 0.938 |
| Kcal requirements (Kcal/day) | 1895.7 (434) | 1878.9 (465.3) | 1913.8 (399.4) | 0.568 |
| Protein requirements (g/day) | 117.8 (14.8) | 119.0 (15.8) | 116.5 (13.7) | 0.253 |
| ICU LOS | 24.9 (14.3) | 21.5 (11.6) | 28.8 (16.1) | 0.0005 |
| Length of mechanical ventilation | 21.31 (15.59) | 18.81 (11.65) | 24.01 (18.84) | <0.0001 |
| Hospital LOS | 30.7 (18.4) | 22.8 (13.2) | 39.2 (19.3) | <0.0001 |
SD: standard deviation; T2D: type II diabetes mellitus; ED: emergency department; CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; SGA: subjective global assessment; ND: no data; BMI: body mass index; BMI classification (<18.5 low weight; 18.5–24.9 normal weight; 25–29.9 overweight and >30 obese), MV: mechanical ventilation; NS: nutritional support; TPN: total parenteral nutrition; CPN: complementary parenteral nutrition; EN: enteral nutrition; LOS: length of stay; ICU: intensive care unit; cm: centimetres; kg: kilograms.
It was observed that patients who died in the ICU presented lower protein intake than those who survived (0.73g/kg/day (CI=0.70–0.75) vs 0.97g/kg/day (CI 0.95–0.99), P<0.001). Similarly, patients who died in the ICU had lower caloric intake 12.94kcal/kg/day (CI 12.48–13.39) vs 16.47kcal/kg/day (CI 16.09–16.8), P<0.001. During the first 14 days in ICU, those patients who survived in the ICU showed a statistically higher protein and caloric intake on days four, five, six, nine, and 11–14 (Table 2). Also, during the same period, patients who survived had higher caloric intake on the same days (Table 3). The adequacy of the caloric and protein intake was also analysed. The percentage of adequacy was calculated with the caloric target (25kcal/kg) and protein target (1.3g/kg), and the average of each day was calculated. It was observed that were differences in the adequacy of protein and caloric targets on days four, five, six, nine, and 11–14. Results are expressed in Tables 2 and 3.
Protein intake during the first 14 days of ICU.
| Day | Protein intake | Percentage of adequacy of protein intake | |||||
|---|---|---|---|---|---|---|---|
| All patients (n=185) | Dead (n=96) | Alive (n=89) | P value | Dead (n=96) | Alive (n=89) | P value | |
| 0 | 0.10 (0.025–0.168) | 0.09 (0.01–0.17) | 0.10 (0.0007–0.2) | 0.710 | 6.5 (0.75–13.15) | 7.64 (0.64–15.92) | 0.7 |
| 1 | 0.30 (0.23–0.37) | 0.30 (0.19–0.41) | 0.29 (0.2–0.39) | 1 | 23.0.6 (14.45–31.6) | 22.73 (15.34–30.11) | 0.98 |
| 2 | 0.51 (0.43–0.59) | 0.48 (0.35–0.6) | 0.54 (0.43–0.65) | 0.420 | 36.71 (27.03–46.38) | 41.08 (32.65–49.51) | 0.43 |
| 3 | 0.61 (0.53–0.7) | 0.56 (0.53–0.7) | 0.66 (0.54–0.78) | 0.160 | 43.33 (33.37–53.3) | 50.78 (41.67–59.9) | 0.16 |
| 4 | 0.73 (0.64–0.83) | 0.63 (0.51–0.76) | 0.84 (0.71–0.97) | 0.017 | 48.45 (38.89–58.01) | 64.4 (54.4–74.4) | 0.018 |
| 5 | 0.82 (0.74–0.91) | 0.73 (0.61–0.84) | 0.93 (0.8–1.05) | 0.025 | 55.76 (46.96–64.56) | 71.26 (61.75–80.78) | 0.025 |
| 6 | 0.87 (0.79–0.95) | 0.78 (0.67–0.9) | 0.95 (0.84–1.07) | 0.049 | 60.07 (51.46–68.67) | 73.13 (64.01–82.25) | 0.049 |
| 7 | 0.82 (0.73–0.9) | 0.74 (0.63–0.86) | 0.89 (0.78–1.01) | 0.097 | 57.13 (48.18–66.08) | 68.60 (59.91–77.3) | 0.098 |
| 8 | 0.82 (0.74–0.91) | 0.73 (0.6–0.85) | 0.92 (0.8–1.04) | 0.017 | 56.02 (46.53–65.51) | 70.25 (60.94–79.56) | 0.018 |
| 9 | 0.85 (0.77–0.93) | 0.75 (0.64–0.87) | 0.94 (0.83–1.06) | 0.016 | 57.73 (48.69–66.78) | 72.58 (63.65–81.51) | 0.016 |
| 10 | 0.91 (0.82–1) | 0.82 (0.69–0.96) | 1.00 (0.87–1.13) | 0.026 | 62.99 (52.55–73.43) | 76.39 (66.45–86.33) | 0.026 |
| 11 | 0.91 (0.82–1.01) | 0.79 (0.65–0.94) | 1.03 (0.92–1.15) | 0.005 | 60.81 (49.79–71.83) | 70.6 (79.51–88.43) | 0.006 |
| 12 | 0.89 (0.8–0.98) | 0.78 (0.65–0.9) | 1.01 (0.89–1.14) | 0.010 | 59.43 (49.59–69.26) | 77.64 (68.0.4–87.23) | 0.01 |
| 13 | 0.87 (0.76–0.98) | 0.74 (0.58–0.89) | 0.99 (0.83–1.16) | 0.012 | 56.6 (44.68–68.52) | 76.33 (63.99–88.67) | 0.013 |
| 14 | 0.89 (0.79–0.99) | 0.77 (0.61–0.92) | 1.00 (0.87–1.13) | 0.016 | 58.72 (46.59–70.84) | 77 (67.08–86.92) | 0.017 |
The relationship between daily protein intake and mortality during the first 14 days of hospitalisation is expressed. Values (mean and 95% CI) are expressed in g/kg ABW (actual body weight). The mean value of adequacy of the protein target is also expressed (1.3kcal/kg). Values are expressed as mean of percentage adequacy and (95% CI).
Caloric intake during the first 14 days of ICU.
| Day | Mean caloric intake | Percentage of adequacy of caloric intake | |||||
|---|---|---|---|---|---|---|---|
| All patients (n=185) | Dead (n=96) | Alive (n=85) | P value | Dead (n=96) | Alive (n=85) | P value | |
| 0 | 3.12 (1.87–4.37) | 2.92 (1.31–4.54) | 3.25 (1.42–5.08) | 0.83 | 11.82 (5.33–18.32) | 13 (5.65–20.34) | 0.7 |
| 1 | 6.6 (5.36–7.83) | 6.23 (4.35–8.11) | 6.88 (5.19–8.56) | 0.54 | 24.95 (17.43–32.48) | 27.51 (20.8–34.21) | 0.54 |
| 2 | 10.12 (8.70–11.53) | 9.65 (7.48–11.82) | 10.48 (8.58–12.98) | 0.50 | 38.64 (29.95–47.34) | 41.93 (34.33–49.53) | 0.5 |
| 3 | 11.65 (10.22–13.07) | 10.49 (8.32–12.66) | 12.68 (10.77–14.57) | 0.049 | 41.93 (33.25–50.62) | 50.76 (43.21–58.31) | 0.049 |
| 4 | 13.63 (11.93–14.76) | 11.69 (9.63–13.75) | 15.01 (13.13–16.89) | 0.012 | 46.75 (38.51–54.98) | 60.05 (52.53–67.56) | 0.013 |
| 5 | 14.66 (13.35–15.98) | 13.04 (11.16–14.92) | 16.34 (14.56–18.17) | 0.012 | 52.16 (44.65–59.67) | 65.47 (58.25–72.68) | 0.012 |
| 6 | 15.3 (13.98–16.62) | 13.96 (12.03–15.89) | 16.71 (14.92–18.49) | 0.027 | 55.87 (48.15–63.95) | 66.86 (59.72–73) | 0.027 |
| 7 | 14.6 (13.26–15.93) | 13.47 (11.51–15.44) | 15.85 (14.1–17.6) | 0.059 | 53.88 (46.03–61.73) | 63.39 (56.38–70.4) | 0.06 |
| 8 | 14.37 (13.05–15.96) | 13.09 (11.17–15) | 15.66 (13.84–17.47) | 0.052 | 52.4 (44.72–60.08) | 62.67 (55.4–69.93) | 0.053 |
| 9 | 14.73 (13.43–16.00) | 13.33 (11.36–15.29) | 16.08 (14.37–17.78) | 0.021 | 53.33 (45.45–61.2) | 64.33 (57.54–71.14) | 0.021 |
| 10 | 15.63 (14.19–17.07) | 14.54 (12.38–16.71) | 16.71 (14.8–18.63) | 0.074 | 58.2 (49.57–66.84) | 66.84 (59.16–74.51) | 0.075 |
| 11 | 15.26 (14.19–17.01) | 13.75 (11.54–15.97) | 17.52 (15.75–19.29) | 0.005 | 55.02 (46.17–63.88) | 70.13 (63.06–77.2) | 0.006 |
| 12 | 15.53 (14.08–16.99) | 13.59 (11.61–15.56) | 17.48 (15.39–19.57) | 0.008 | 54.33 (46.42–62.24) | 69.96 (61.6–78.32) | 0.008 |
| 13 | 15.29 (13.97–17.11) | 13.45 (10.94–15.97) | 17.03 (14.45–19.62) | 0.042 | 53.82 (43.76–63.89) | 68.17 (57.81–78.52) | 0.042 |
| 14 | 15.36 (13.74–16.97) | 13.31 (10.92–15.71) | 17.28 (15.15–19.42) | 0.016 | 53.23 (43.64–62.82) | 69.13 (60.16–77.65) | 0.016 |
The calories delivered in the first 14 days and mortality are expressed. Values of means and 95% confidence interval are expressed in kcal/kg ABW. The mean value of adequacy of the caloric target is also expressed (25kcal/kg). Values are expressed as mean of percentage adequacy and (95% CI).
Patients with an mNUTRIC score ≥5 had higher mean caloric intake than those without nutritional risk (1608kcal/kg/day (CI 15.54–16.61) vs 14.24kcal/kg/day (CI 13.88–14.59), P<0.001), as well as higher mean protein intake (0.92g/kg/day (CI 0.88–0.95) vs 0.83g/kg/day (CI 0.81–0.86), P<0.001) during the complete ICU stay. Patients who required the prone position had lower mean caloric intake than those who were ventilated in the supine position (14.36kcal/kg/day (CI 14.03–14.7) vs 16.34kcal/kg/day (CI 15.72–16.96), P<0.001), as well as lower mean protein intake (0.84g/kg/day, CI 0.81–0.86 vs 0.93g/kg/day, CI 0.9–0.97, P<0.001) during the complete ICU stay. Patients who started nutritional support within the first 48h after ICU admission had higher mean caloric intake than those with late nutrition therapy (16.68 vs 13.21kcal/kg/day, P<0.001) and higher mean protein intake (0.98 vs 0.75, P<0.001). Finally, the most seriously ill patients (APACHE II≥18 had higher mean caloric intake compared with those with lower APACHE II (15.56 vs 14.31–15.01, P<0.001). Nonetheless, there were no differences in the mean protein intake (P=0.46).
The severity of the disease influenced the caloric intake and protein intake differently. In the first four days, the patients with higher APACHE II had a higher chance of meeting the caloric target, but in the complete ICU stay the patients with higher APACHE II, had less chance of meeting the objective. With the protein target, similar results were observed. The patients with higher APACHE II had less chance of meeting the protein target. It was observed that higher severity of disease and prone position decreased the chance of meeting the daily caloric target during the complete ICU stay, and early start of nutritional support and undernutrition increased the chances of attaining the daily target for caloric intake (Table 4). Also, the higher severity of the disease and prone position decreased the chances of meeting the daily protein target during the ICU stay, whereas high nutritional risk and early start increased the chances of achieving the daily target (Table 5). Over the first period, the early start of nutritional support increased the chances of achieving daily caloric and protein targets, whereas MV prone position decreased the chances (Tables 4 and 5). Furthermore, over the second period, the early start of nutritional support and state of undernutrition continued to increase the chances of achieving the daily caloric and protein targets (Tables 4 and 5). Oddly, also in the second period, severity of the disease increased the chance of meeting the caloric intake target but not the protein intake target (Tables 4 and 5). Similar to the previous period, the prone position still decreased the chances of achieving both daily targets (Tables 4 and 5). Finally, in the later period, the severity of the disease and the need for a prone position MV decreased the chance of achieving the daily caloric target, while the early start of enteral nutrition and undernutrition continues to increase it (Tables 4 and 5).
Logistic regression of independent predictors of daily caloric intake>25kcal/kg/day.
| Complete ICU stay | Days 4–7 | Days 8–11 | Days 12–14 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | |
| APACHE II>18 | 0.77 | 0.61–0.96 | 0.02 | 1.83 | 1.08–3.11 | 0.023 | 0.79 | 0.46–1.38 | 0.42 | 0.55 | 0.27–1.13 | 0.1 |
| Early start of NS | 1.76 | 1.48–2.1 | <0.001 | 2.32 | 1.43–3.75 | <0.001 | 1.57 | 1.02–2.41 | 0.038 | 1.48 | 0.87–2.49 | 0.14 |
| mNUTRIC score>5 points | 1.18 | 0.97–1.44 | 0.1 | 1.19 | 0.7–2.03 | 0.51 | 1.39 | 0.84–2.31 | 0.19 | 1.02 | 0.55–1.9 | 0.93 |
| Undernutrition (SGA B or C) | 1.43 | 1.20–1.71 | <0.001 | 1.65 | 1.03–2.66 | 0.036 | 1.71 | 1.1–2.67 | 0.018 | 1.45 | 0.83–2.52 | 0.19 |
| Prone position | 0.55 | 0.46–0.65 | <0.001 | 0.48 | 0.31–0.78 | 0.0027 | 0.31 | 0.21–0.49 | <0.001 | 0.23 | 0.14–0.4 | <0.001 |
Abbreviations: CI: confidence interval; OR: odds ratio; NS: nutritional support; mNUTRIC: modified NUTRIC score; SGA: subjective global assessment.
Logistic regression of independent predictors of daily protein intake>1.3g/kg/day.
| Complete ICU stay | Days 4–7 | Days 8–11 | Days 12–14 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | |
| APACHE II>18 points | 0.82 | 0.69–0.97 | 0.022 | 1.52 | 0.99–2.33 | 0.056 | 0.95 | 0.6–1.5 | 0.83 | 0.9 | 0.5–1.62 | 0.73 |
| Early start of NS | 1.79 | 1.56–2.02 | <0.001 | 2.48 | 1.71–3.58 | <0.001 | 1.94 | 1.36–2.76 | <0.001 | 2.23 | 1.41–3.51 | <0.001 |
| mNUTRIC score>5 points | 1.44 | 1.23–1.69 | <0.001 | 1.01 | 0.65–1.54 | 0.98 | 1.53 | 1–2.35 | 0.047 | 1.34 | 0.78–2.3 | 0.28 |
| Undernutrition (SGA B or C) | 1.05 | 0.91–1.2 | 0.48 | 1.65 | 1.14–2.4 | 0.008 | 1.48 | 1.02–2.16 | 0.038 | 1.47 | 0.91–2.38 | 0.11 |
| Prone position | 0.78 | 0.67–0.9 | <0.001 | 0.6 | 0.41–0.88 | 0.0087 | 0.35 | 0.25–0.51 | <0.001 | 0.6 | 0.37–0.98 | 0.042 |
Abbreviations: OR: odd ratio; CI: confidence interval; NS: nutritional support mNUTRIC: modified NUTRIC score; SGA: subjective global assessment.
In this multicentre observational study conducted in critically ill ventilator-dependent patients with COVID-19, we have found that those patients who died in the ICU presented more frequently with SGA “B” and showed less caloric and protein intake than those who survived. Also, when we analysed the different factors that influence the chance of achieving the nutritional goals of 25kcal/kg/day and 1.3g/kg/day, we found that those who required prone position MV had less chance of reaching the daily goals. Conversely, malnourished patients and those who started nutrition before 48hs of admission had a greater chance of achieving the caloric and protein goals.
In our study, the protein caloric goals analysed were different in each centre, as well as the fact that they were different in the same patient throughout the hospitalisation. Therefore, for the analysis, 25kcal/kg/day and 1.3g/kg/day were chosen as goals since they are the minimum requirements recommended by the different nutritional guidelines published so far. According to current recommendations, protein requirements are expected to be in the range of 1.2–2.0g/kg ABW per day (6.11–13) during critical illness.
Another interesting finding is that progression of caloric and protein intake during the first 14 days in ICU showed no difference in the first four days between those who died and survived. It is well-known that prone position MV for adults with severe respiratory failure is usually needed during the first four days of ICU stay.16 Interestingly enough, MV in the prone position is a factor that negatively affected protein caloric intake. To the best of our knowledge, there is no similar data published so far regarding this population. In an observational study performed on critically ill COVID-19 patients, 56.9% of the patients reached the caloric target of 20kcal/kg on day three.17 It is interesting to observe that the percentage of the adequacy of the requirements was low. According to current guidelines, many patients probably should have received supplemental parenteral nutrition but did not receive it. This is observed in other similar studies where patients do not meet the requirements and do not receive parenteral nutrition.18–20
The factors that may affect caloric and protein intake during ICU stay were also analysed. Those protocols that start enteral nutrition early probably have a more aggressive approach and follow-up to meet the objectives throughout the hospitalisation. Early initiation of nutritional support is recommended by most of the recent guidelines.9,21,22 Also, recent studies have shown the benefits of early provision of proteins to critically ill patients.15,23
Another interesting finding is that undernutrition acts as a factor that increases the chances of achieving caloric and protein targets in the first two periods (from day four to day 11), which suggests that the progression of nutritional support is influenced by nutritional status. Also, nutritional risk assessed with mNUTRIC score was not a factor that influenced the chance of reaching the caloric and protein target. This finding was also observed in another observational study conducted on COVID-19 patients, in which it was found that patients at high nutritional risk were more likely to achieve the caloric target of 20kcal/kg/day within the first three days after ICU admission.17 Moreover, in a retrospective observational study Zhang et al.24 recently demonstrated that a large proportion of ICU COVID-19 patients with a high nutritional risk revealed by their mNUTRIC score at ICU admission exhibited significantly higher mortality.
Factors that negatively affected achieving daily caloric and protein targets were the severity of the disease and the need for MV in the prone position. The prone position compromises caloric and protein intake during the stay in the ICU. The negative influence of the prone position during MV could be explained by several factors not analysed in this study, such as the lack of feeding protocols in the prone position or the higher incidence of vomiting and high residual gastric volume. Considering that this technique is often used during the first few days of MV, its use would also condition subsequent periods of stay in the ICU. These could be due to the severity of respiratory failure with extreme hypoxaemia over the first days of critical illness, which may condition the whole ICU stay and its complications. It is important to recall that COVID-19 nutritional support guidelines recommend trying enteral nutrition in patients with mechanical ventilation in a prone position.25–27 So far, different clinical studies have shown the feasibility and safety of enteral feeding in the prone position. In 2010, an elegant “before and after” study in mechanically ventilated patients in a prone position showed that feeding protocols could increase enteral feeding volume without increasing residual gastric volume, vomiting, or ventilator-associated pneumonia (VAP).28
Similarly, other studies showed that mechanically ventilated patients in the prone position had the same enteral feeding volumes as those in supine but higher gastric residual volumes.29,30 Meanwhile, Reignier et al. showed that enterally fed patients in the prone position had higher gastric residual volume, vomiting, and a higher incidence of episodes of interrupted enteral feeding than those in a supine position.31
It was also observed that the severity of the disease influenced meeting the caloric and protein target in a negative way, except for the caloric target in the first four days where the severity of the disease influenced it in a positive way. These results could be influenced by the calories delivered with propofol and dextrose because those parenteral infusions are used at a higher rate in most of the cases during the first days of ICU stay. In the same way, the difference observed between the means of caloric intake in patients with APACHE II>18 vs <18 may be due to the fact that patients with higher APACHE II probably required higher doses of propofol for adequate adaptation to MV.
We are aware that this study has several limitations. First, complications associated with nutritional support provision such as diarrhoea, constipation, vomiting, and gastroparesis were not evaluated. Unfortunately, we know that these potential complications may be another reason for not achieving the daily nutritional goals. Second, the mean duration and the total number of cycles of prone position MV were not recorded. Third, since it was an observational multicentre study in which each centre had its own feeding protocol and nutritional goals, although based on ESPEN Critically Ill Patients Guidelines9 and the SATI COVID-19 protocol,5 the objectives of each centre were slightly different. Due to this situation, protein and caloric adequacy were analysed with a cut-off point of 25kcal/kg and 1.3g/kg, these being the objectives that all centres tried to meet. The study's observational design does not offer the strength of evidence that a randomised controlled trial would, to allow us to discern if the protein and caloric intake influenced overall mortality or if simply the severity of illness was the reason for not achieving the nutritional goals, and thus, these patients exhibited the highest mortality. Finally, another weak point is that this study is an analysis of a previous study where the nutritional characteristics of patients with COVID-19 on mechanical ventilation in the ICU were analysed, but the nutritional intake was not the main outcome, this being the cause of the significant exclusion of patients. Nonetheless, we strongly believe that the information provided by this study is important since nutritional characteristics and their contributions from a homogeneous patient population were analysed. Moreover, other strengths of the present study reside in the multicentre design and the sample size, which reduce the risk of bias due to different feeding protocols. Finally, the information gathered from ICU patients with ARDS secondary to COVID-19 allows for extracting important information that could be applied to other patients with ARDS needing respiratory support.
ConclusionA lower caloric and protein intake was observed in those critically ill patients with COVID-19 who died than in those who survived. We were not able to demonstrate a direct cause-effect relationship in the present study. Nonetheless, an early start on nutritional support and undernutrition increased the chance of achieving protein and caloric goals. The severity of disease and mechanical ventilation in the prone position decreases the chance of achieving caloric and protein targets. Therefore, more aggressive feeding protocols to ensure energy protein requirements may be needed in the most seriously ill COVID-19 patients.
Authors’ contributionAM, and SC designed the study protocol, participated in collecting data, performing statistical analysis, and preparing the manuscript. WM performed a critical review of the manuscript. All authors read and approved the final version of the manuscript.
Funding statementNeither grants nor funding were received for the development of the present research.
Conflict of interestFollowing our ethical obligation as researchers, we must report that SC, MD, Ph.D. received speaking honoraria from Nutricia and Fresenius Kabi, was a member of the Fresenius Kabi Advisory Board. AM is employee and Medical Director of NUTRIHOME-SA. CEK received speaking honoraria from Nutricia and Fresenius Kabi. WM received speaking honoraria and travel expenses from Baxter and Biosyn. No potential competing interest was reported by the other authors.
To our patients and their families, our sincere respect and affection.






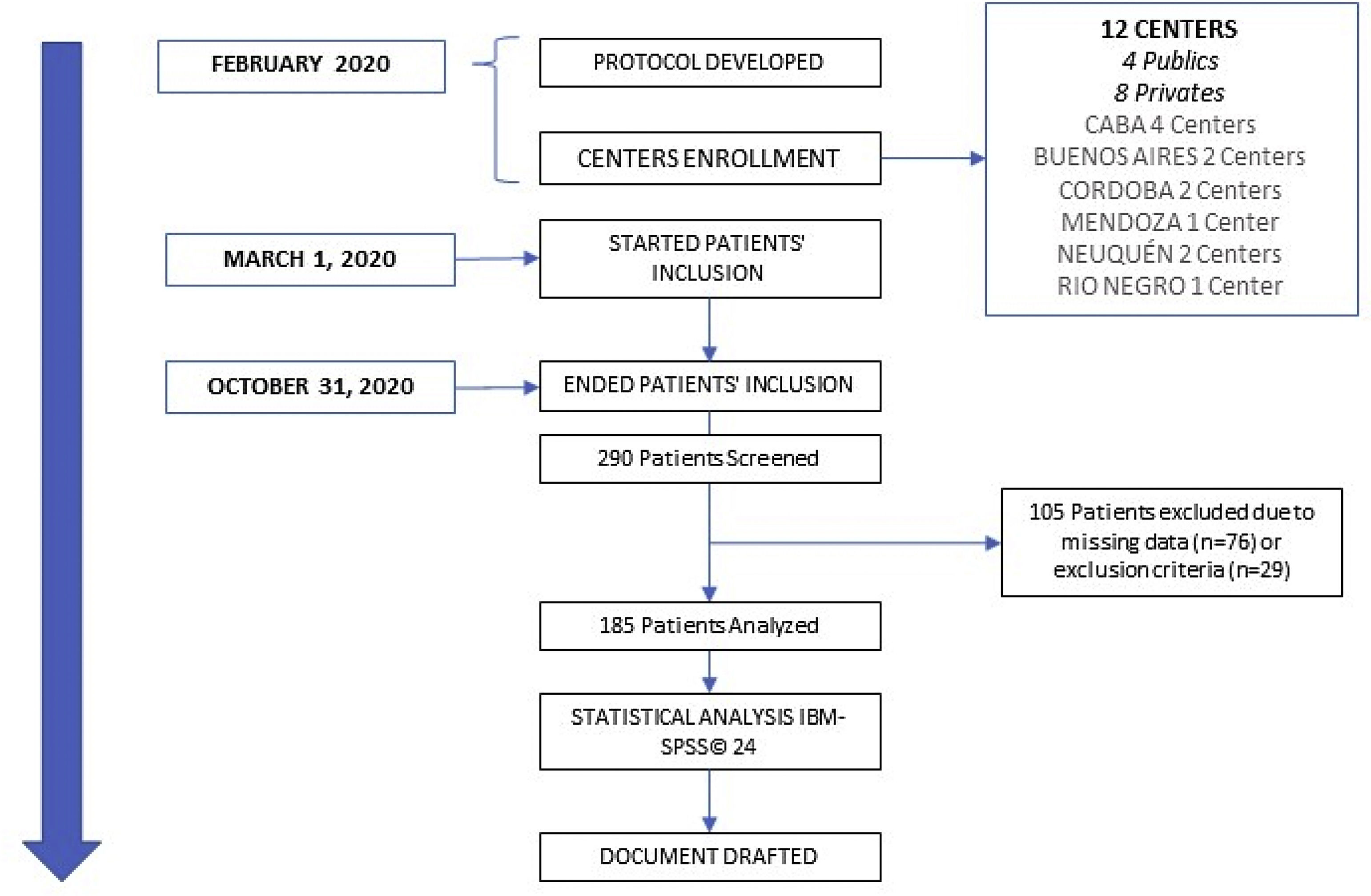
![Consort flow diagram. 290 patients were screened, and 185 were analysed. CABA: Ciudad Autónoma de Buenos Aires [Autonomous City of Buenos Aires]. Consort flow diagram. 290 patients were screened, and 185 were analysed. CABA: Ciudad Autónoma de Buenos Aires [Autonomous City of Buenos Aires].](https://static.elsevier.es/multimedia/25300164/0000007000000004/v2_202304080715/S2530016423000204/v2_202304080715/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
