Continuous glucose monitoring (CGM) systems are a tool to facilitate the attainment of control targets compared to capillary blood glucose (CBG) monitoring.1 Its use is currently widespread in patients with type 1 diabetes (T1DM), and it is expected to be increasingly adopted in patients with type 2 diabetes (T2DM) following the publication of the Resolution of the General Directorate for the Common Portfolio of Services of the National Health and Pharmacy System regulating public coverage and the accessibility criteria for CGM systems in patients with T2DM treated with multiple insulin doses.2 This resolution establishes that patients with T2DM should have access to these systems in the healthcare setting where the patient is regularly and continuously monitored, which in many cases is primary care (PC).
However, the level of knowledge and the perceptions of primary care providers (PCPs) on the use of CGM are unknown. To fill this gap, in late 2021 and early 2022, a cross-sectional study was conducted through the SEMERGEN, SEMFyC, SEMG and SED scientific societies in the form of an online survey targeting PCPs clinically active in Spain at the time of the study. An anonymous online survey of 26 questions was developed to gather information on the type of consultation, number and profile of patients with diabetes seen, T2DM management strategies, frequency of complications, and knowledge, use and difficulties in the use of CGM systems in T2DM patients. A total of 438 PCPs started the survey. Of these, 137 (31.3%) completed the survey, and they are the main focus of this report. The level of participation, which is difficult to quantify precisely because of the access method to doctors (social media from their own societies), was low, and the results certainly reflect the current landscape of professionals interested in T2DM, which makes them all the more striking.
The results show that a PCP sees around 130–160 patients with DM in their quota. Of them, about 10% are treated with two or more doses of insulin and they perform between seven and 15 CBG measurements per week, whereas 2%–8% have had severe hypoglycaemia in the last year.
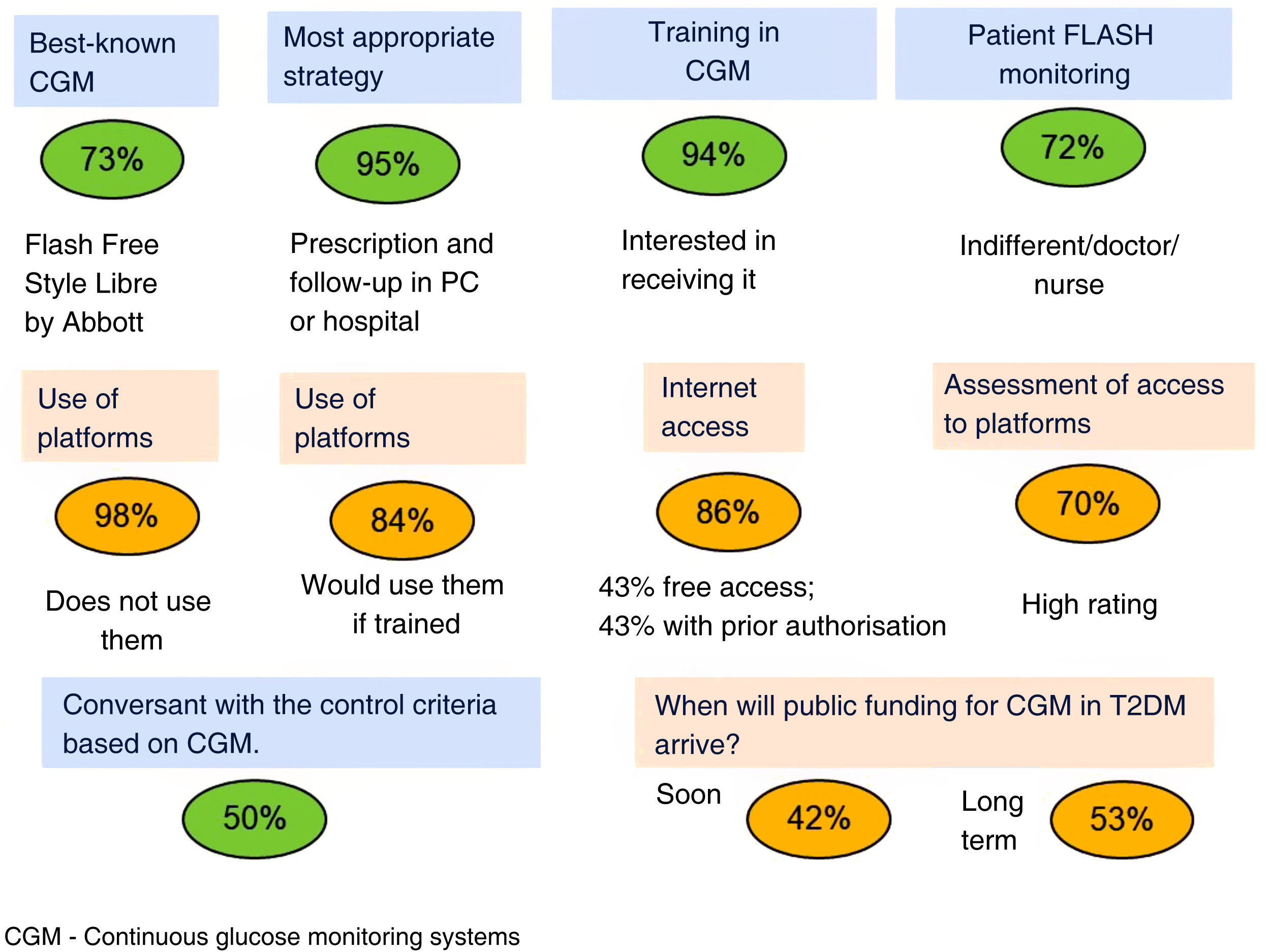
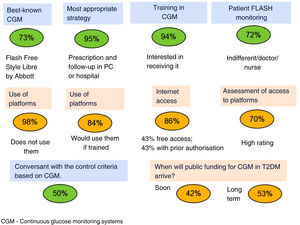
Fig. 1 summarises the answers regarding use of CGM systems. FreeStyle Libre is the best-known system, and most of the respondents believe that prescribing and monitoring should be conducted in primary care or in the hospital by both doctor and nurse. Only half of the PCPs are conversant with the criteria for glycaemic control based on CGM, and while most of them do not use the platforms, they do regard the latter as important and express a willingness to use them. There would therefore seem to be a patent need for continuing education on CGM in PC.
The benefits of CGM are well established in T1DM, as well as in subjects with T2DM on insulin treatment: it improves glycaemic control, increases diabetes-related quality of life and satisfaction and reduces the costs of acute complications.1,3,4 These benefits for different glycaemic control parameters were recently confirmed in a study in routine clinical practice in patients with T2DM with different treatment modalities.5 One of the main benefits of CGM is the reduction in hypoglycaemia.4,6 As reflected in the survey, severe hypoglycaemia is still a major challenge in T2DM patients treated with insulin. In Spain, although hospital admissions for hypoglycaemia decreased between 2005 and 2015, in 2015 there were 8331 hospital admissions for hypoglycaemia and 244 in-hospital deaths, with higher admission and mortality rates in men.7 In another study conducted between 2000 and 2014 in 109 countries, the age-standardised hypoglycaemia-related death rate was 4.49 (95% CI: 4.44–4.55) for every 1000 total diabetes deaths.8
In this context, increasing knowledge and empowering PC teams in these technological tools, as well as establishing virtual cross-consultation systems with the hospital's endocrinology department and shared access to CGM data for professionals from both healthcare settings are key strategies to effectively manage the use of this new tool.
FundingThe study was funded by an unconditional grant from Abbott Laboratories to the Spanish Diabetes Society (SED).