Initial evaluation of adrenal incidentalomas (AIs) should be aimed at ruling out malignancy and functionality. For this, a detailed clinical history should be taken, and an adequate radiographic assessment and a complete blood chemistry and hormone study should be performed. The most controversial condition, because of the lack of consensus in its definition, is autonomous cortisol secretion (ACS). Our recommendation is that, except when cortisol levels <1.8 µg/dL in the dexamethasone suppression test (DST) rule out diagnosis and levels ≥5 µg/dL establish the presence of ACS, diagnosis should be based on a combined definition of DST ≥ 3 µg/dL and at least one of the following: elevated urinary free cortisol (UFC), ACTH level <10 pg/mL, or elevated nocturnal cortisol (in serum and/or saliva). During follow-up, DST should be repeated, usually every year, on an individual basis depending on the results of prior tests and the presence of comorbidities potentially related to hypercortisolism.
The initial radiographic test of choice for characterization of AIs is a computed tomography (CT) scan without contrast, but there is no unanimous agreement on subsequent monitoring. Our general recommendation is a repeat imaging test 6−12 months after diagnosis (based on the radiographic characteristics of the lesion). If the lesion remains stable and there are no indeterminate characteristics, no additional radiographic studies would be needed.
We think that patients with ACS with comorbidities potentially related to hypercortisolism, particularly if they are young and there is a poor control, may benefit from unilateral adrenalectomy (UA). The indication for UA is clear in patients with overt hormonal syndromes or suspected malignancy.
In conclusion, AIs require a comprehensive evaluation that takes into account the possible clinical signs and comorbidities related to hormonal syndromes or malignancy; a complete hormone profile (taking into account the conditions that may lead to falsely positive and negative results); and an adequate radiographic study. Monitoring and/or treatment will be decided based on the results of the initial evaluation.
La evaluación inicial de los incidentalomas adrenales (IA) se centra en dos objetivos: descartar malignidad y descartar funcionalidad. Para ello se debe realizar una historia clínica detallada, obtener una valoración radiológica adecuada y un estudio bioquímico-hormonal completo. La entidad que más dudas genera, por la falta de consenso en su definición, es la secreción autónoma de cortisol (SAC). Nuestra recomendación es que, salvo para valores de cortisol <1.8 µg/dl en el test de supresión con dexametasona (TSD) que descartan SAC, y ≥5 µg/dl que establecen el diagnóstico; se debe emplear una definición combinada de TSD ≥ 3 µg/dl y al menos uno de los siguientes: cortisol libre urinario (CLU) elevado, ACTH < 10 pg/mL o cortisol nocturno (sérico y/o salival) elevado para establecer el diagnóstico de SAC. En el seguimiento se debe repetir el TSD, generalmente de forma anual, individualizando en función de los resultados de las pruebas previas y de la presencia de comorbilidades potencialmente relacionadas con el hipercortisolismo.
La prueba radiológica inicial de elección para la caracterización de los IA es la tomografía axial computarizada (TAC) sin contraste, pero no existe acuerdo unánime sobre el seguimiento posterior. Nuestra recomendación general es repetir la prueba de imagen a los 6-12 meses del diagnóstico (en función de las características radiológicas de la lesión). Si la lesión se mantiene estable y no existen características indeterminadas, no serían necesarios más estudios radiológicos.
Consideramos que los pacientes con SAC con comorbilidades potencialmente relacionadas con el hipercortisolismo, especialmente si existe un control deficiente y se trata de pacientes jóvenes, se pueden beneficiar de una suprarrenalectomía unilateral (SRU). La indicación de SRU es clara en pacientes con síndromes hormonales manifiestos o sospecha de malignidad.
Como conclusión, los IA deben ser valorados de forma integral, teniendo en cuenta las posibles manifestaciones clínicas y comorbilidades relacionadas con síndromes hormonales o malignidad; un estudio hormonal completo (teniendo en cuenta las situaciones que pueden conllevar resultados falsamente positivos y negativos) y radiológico adecuado. En base a los resultados de la evaluación inicial se planificará el seguimiento y/o tratamiento.
Adrenal incidentalomas (AIs) are defined as asymptomatic adrenal lesions measuring 1 cm or more in size and detected from imaging studies carried out for reasons unrelated to suspected adrenal disease or evaluation of the spread of non-adrenal tumor disease.1,2 Adrenal incidentalomas are a very common reason for consultation in endocrinology clinics because of their high prevalence (estimated to be 2% in the general population and up to 7% in the population over 70 years of age).1
Once AI is detected, adequate hormone screening and radiographic assessment are important, and a complete clinical history should be compiled. The aim is to identify functioning and/or malignant lesions, associated with increased morbidity-mortality, and thus candidates for treatment, generally in the form of adrenalectomy.3
The prevalence of malignancy ranges from 1.2 to 12% according to the different series,2 though some authors consider that the prevalence is probably overestimated due to selection bias.4,5 Although most AIs are benign and do not secrete excess hormones, up to 20–30 % of all such lesions are characterized by hormonal hypersecretion, with autonomous cortisol secretion (ACS) being the most common alteration.6 This term refers to AI carriers with biochemical evidence of excess cortisol, but without the “specific” clinical signs of Cushing’s syndrome (CS).7 These patients are at increased cardiometabolic risk,2,6,8–11 and their correct identification is therefore very important. However, the hormone tests currently available for diagnostic purposes have multiple limitations, with a large percentage of false positive and false negative results.6 Consensus is lacking, and although most experts consider suppression testing with 1 mg of dexamethasone (the dexamethasone suppression test [DST]) to be the most adequate tool in screening for ACS, there is no agreement as to which cut-off point should be used.2,3,12–14
The aim of the present study was to reach a consensus on a number of practical recommendations, based on the greatest available scientific-clinical evidence, regarding the initial evaluation, follow-up and treatment of patients with AI, both non-functioning and with ACS, possible ACS, and other special situations such as indeterminate AIs and bilateral adrenal hyperplasia with ACS.
Beyond screening, the document will not address pheochromocytoma, primary hyperaldosteronism (PHA), florid CS or malignant adrenal lesions, since these fall outside the scope of the study, and specific guidelines for these conditions are already available.
Initial evaluationThe initial study of AI has two basic objectives:
- •
To discard malignancy based on clinical data and imaging techniques.
- •
To discard the functionality of the lesion based on the clinical history and biochemical-hormonal studies.
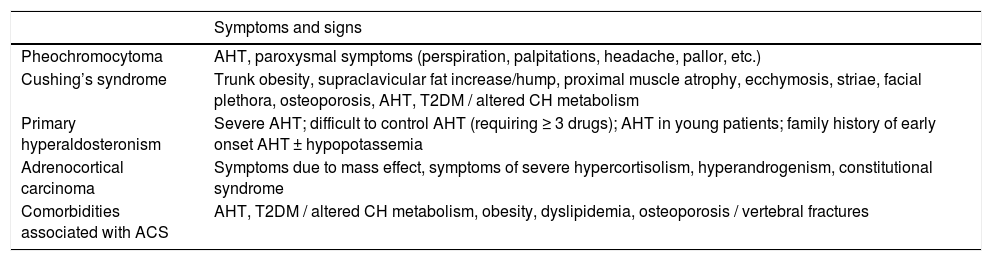
The clinical history should be studied in order to rule out symptoms and signs suggesting malignancy and endocrine functionality of the lesion, to assess the presence of comorbidities associated with excess cortisol (Table 1), to record weight, height, abdominal circumference and blood pressure, and to take into account any potential conditions that may give rise to interference with the results of functional tests, in order to adapt the diagnostic protocol to each individual case.
Clinical signs associated with biochemically functioning adrenal lesions and comorbidities associated with autonomous cortisol secretion.
| Symptoms and signs | |
|---|---|
| Pheochromocytoma | AHT, paroxysmal symptoms (perspiration, palpitations, headache, pallor, etc.) |
| Cushing’s syndrome | Trunk obesity, supraclavicular fat increase/hump, proximal muscle atrophy, ecchymosis, striae, facial plethora, osteoporosis, AHT, T2DM / altered CH metabolism |
| Primary hyperaldosteronism | Severe AHT; difficult to control AHT (requiring ≥ 3 drugs); AHT in young patients; family history of early onset AHT ± hypopotassemia |
| Adrenocortical carcinoma | Symptoms due to mass effect, symptoms of severe hypercortisolism, hyperandrogenism, constitutional syndrome |
| Comorbidities associated with ACS | AHT, T2DM / altered CH metabolism, obesity, dyslipidemia, osteoporosis / vertebral fractures |
T2DM: type 2 diabetes; CH: carbohydrates; AHT: arterial hypertension; ACS: autonomous cortisol secretion.
It is also necessary to assess the need to complete the diagnostic study in patients with a poor prognosis because of other disease conditions or advanced age, where the etiological diagnosis of the adrenal lesion will not improve patient life expectancy or quality of life, or will not result in changes in the treatment approach. On the other hand, the patient should be informed about the probably benign nature of the lesion and the studies that need to be made. A possibly useful tool in this regard is the document for patients containing information on AI available on the website of the Spanish Society of Endocrinology and Nutrition (Sociedad Española de Endocrinología y Nutrición [SEEN])(ht**tps://ww*w.seen.es/docs/apartados/2376/Seen_OK.pdf).
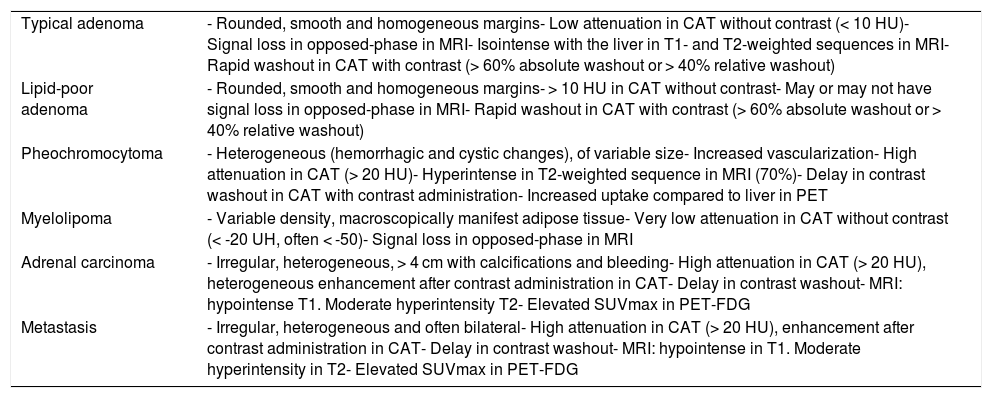
Radiological studyIn order to characterize the adrenal lesion and rule out malignancy, the initial evaluation should include the use of an imaging technique of sufficient precision. Computed axial tomography (CAT) without contrast administration is the recommended technique for characterizing AIs.2 The main criterion for defining benignity is the density of the lesion expressed in Hounsfield units (HU). A density of ≤ 10 HU in a solid area of the nodule without necrosis rules out malignancy with a high probability, and is seen in adenomas and tumors with a large fat content, such as myelolipoma. Thus, in the absence of oncological disease, a homogeneous lesion with smooth margins and ≤ 10 HU may be classified as an adenoma (and therefore as a benign lesion).15 Likewise, other characteristics of the lesion, such as its size, appearance or stability can be suggestive of its benign nature16–20 (Table 2).
Typical radiological characteristics of the most important adrenal lesions.
| Typical adenoma | - Rounded, smooth and homogeneous margins- Low attenuation in CAT without contrast (< 10 HU)- Signal loss in opposed-phase in MRI- Isointense with the liver in T1- and T2-weighted sequences in MRI- Rapid washout in CAT with contrast (> 60% absolute washout or > 40% relative washout) |
| Lipid-poor adenoma | - Rounded, smooth and homogeneous margins- > 10 HU in CAT without contrast- May or may not have signal loss in opposed-phase in MRI- Rapid washout in CAT with contrast (> 60% absolute washout or > 40% relative washout) |
| Pheochromocytoma | - Heterogeneous (hemorrhagic and cystic changes), of variable size- Increased vascularization- High attenuation in CAT (> 20 HU)- Hyperintense in T2-weighted sequence in MRI (70%)- Delay in contrast washout in CAT with contrast administration- Increased uptake compared to liver in PET |
| Myelolipoma | - Variable density, macroscopically manifest adipose tissue- Very low attenuation in CAT without contrast (< -20 UH, often < -50)- Signal loss in opposed-phase in MRI |
| Adrenal carcinoma | - Irregular, heterogeneous, > 4 cm with calcifications and bleeding- High attenuation in CAT (> 20 HU), heterogeneous enhancement after contrast administration in CAT- Delay in contrast washout- MRI: hypointense T1. Moderate hyperintensity T2- Elevated SUVmax in PET-FDG |
| Metastasis | - Irregular, heterogeneous and often bilateral- High attenuation in CAT (> 20 HU), enhancement after contrast administration in CAT- Delay in contrast washout- MRI: hypointense in T1. Moderate hyperintensity in T2- Elevated SUVmax in PET-FDG |
PET-FDG: fluorodeoxyglucose positron emission tomography; MRI: magnetic resonance imaging; CAT: computed axial tomography.
However, 30% of all adenomas have a density of > 10 HU. These are the so-called lipid-poor adenomas, which cannot be characterized by density alone in the CAT scan without contrast. Other characteristics16–20 (Table 2) and/or different imaging techniques are required in such cases for adequate characterization of the lesion. The most standardized test is CAT with contrast administration (with contrast washout measurement), though magnetic resonance imaging (MRI) may be useful as a second imaging test in adenomas with densities between 10–30 HU.21
Historically, a cut-off point of >4 cm has been established as corresponding to suspected malignancy and as a criterion for indicating surgery. However, many benign lesions that may not require therapeutic actions can measure > 4 cm in size (myelolipoma, cysts, etc.). The current recommendation is to pay more attention to the density characteristics and to place less emphasis on lesion size in establishing suspected malignancy.22
In CAT with contrast, adenomas typically show rapid enhancement and washout after intravenous contrast injection. In this respect, adenomas may be characterized by a calculation of the absolute and relative washout values of the lesion 15 min after contrast administration. An absolute washout of ≥ 60% and a relative washout of ≥ 40% are typical of adenoma. These results have a high positive predictive value for the diagnosis of adenoma, with a sensitivity of 98% and a specificity of 92%.23 It should be noted that washout calculations are of no use in characterizing masses with non-homogeneous low attenuation foci (necrosis or cystic areas).
Magnetic resonance imaging using the chemical shift technique, based on the acquisition of T1-weighted gradient-echo in-phase and opposed-phase sequences, allows for the detection of the presence of intracytoplasmic lipids, and has been the most widely studied approach for the characterization of AIs. Lipid-rich adenomas lose signal in opposed-phase sequences compared to in-phase images, while malignant lesions and pheochromocytomas are unchanged. Dynamic studies involving contrast administration and protocols characterized by T1- and T2-weighted sequences with fat saturation and following contrast injection may be useful. Magnetic resonance imaging is considered to have a sensitivity and specificity similar to that of CAT without contrast in diagnosing adenoma, with a somewhat greater sensitivity in diagnosing some lipid-poor adenomas with > 10 HU. However, this sensitivity decreases in the case of lesions with > 30 HU, where CAT with contrast is more useful.18,24 Magnetic resonance imaging is recommended as the first imaging test when ionizing radiation is to be avoided (as in children or pregnant women).
Nuclear medicine techniques are of limited usefulness in the characterization of AIs. Positron emission tomography with 18-fluoro-deoxy-glucose associated with CAT (PET-CAT with 18FDG) has a high negative predictive value and may be useful for ruling out malignancy when other imaging techniques have not been effective. However, it should not be used as a routine technique due to the possibility of false positive results in conditions such as infections, pheochromocytoma or infiltrating lesions. The current recommendations limit its indication almost exclusively to patients with lesions that cannot be characterized by other imaging techniques and a history of extra-adrenal neoplastic disease, to discard metastasis.15 Positron emission tomography may also be considered for indeterminate AIs measuring under 6 cm in size. The results obtained may be of help in selecting the therapeutic approach and surgical management.25–27
Meta-iodobenzylguanidine scintigraphy is reserved, together with other tracers in PET, for the study and treatment of metastatic pheochromocytomas or extra-adrenal paragangliomas, and is less recommended for the characterization of AIs because of the possibility of false positive results.27
Iodine-norcholesterol scintigraphy associated with SPECT-CAT (single photon emission computed tomography associated with CAT) is a time-consuming technique used by some groups for the diagnosis of PHA and ACS.28
Positron emission tomography with metomidate is under study.29
BiopsyGuided biopsy has been of limited value in the differential diagnosis between adenoma and adrenocortical carcinoma, and can give rise to tumor spread. The non-diagnostic biopsy rate is around 8.7%, with a complications rate of 2.5%.30 Sensitivity appears to be somewhat greater in the diagnosis of metastasis in patients with extra-adrenal oncological disease, reaching 87%.29 The current clinical guidelines therefore limit the indication for biopsy to patients with known non-adrenal oncological disease, when it has not been possible to characterize the lesion by imaging techniques, and provided the result is going to modify management of the oncological disease.2
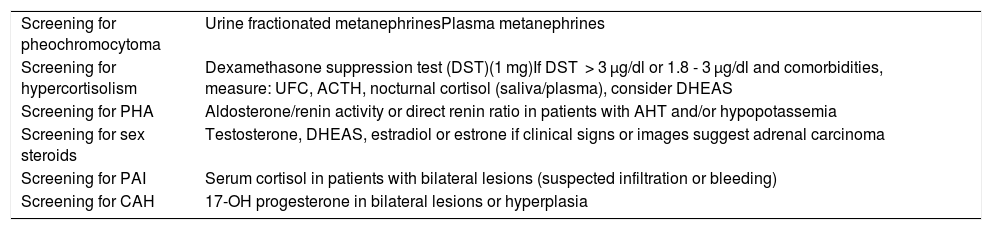
Biochemical-hormonal studyThe objective of the endocrine study is to identify those patients eligible for surgery. It should include a basic biochemical evaluation and a screening test for pheochromocytoma and hypercortisolism in all patients. Furthermore, a screening test for primary hyperaldosteronism should be included in the case of patients with arterial hypertension and/or hypopotassemia.2,3,12–14,31 Some authors consider that hormone testing is not necessary in patients with adrenal cysts and myelolipoma.2,3,12–14 However, considering that DST is inexpensive and easy to perform, and that there have been reports of tumors radiologically reminiscent of cortisol-producing myelolipoma,32 in the event of clinical suspicion or comorbidities potentially related to hypercortisolism, we recommend screening for hypercortisolism in this group of patients (Table 3).
- a)
Screening for pheochromocytoma. Such screening is generally recommended in all patients, even in individuals with normal blood pressure, and even if the characteristics of the adrenal lesion are not suggestive of pheochromocytoma.2,3,12–14 However, some authors suggest that in AI with unequivocal radiographic characteristics of adenoma, the risk of pheochromocytoma is very low, and that screening could therefore be dispensed with for such patients.17,33,34
Initial hormone study.
| Screening for pheochromocytoma | Urine fractionated metanephrinesPlasma metanephrines |
| Screening for hypercortisolism | Dexamethasone suppression test (DST)(1 mg)If DST > 3 µg/dl or 1.8 - 3 µg/dl and comorbidities, measure: UFC, ACTH, nocturnal cortisol (saliva/plasma), consider DHEAS |
| Screening for PHA | Aldosterone/renin activity or direct renin ratio in patients with AHT and/or hypopotassemia |
| Screening for sex steroids | Testosterone, DHEAS, estradiol or estrone if clinical signs or images suggest adrenal carcinoma |
| Screening for PAI | Serum cortisol in patients with bilateral lesions (suspected infiltration or bleeding) |
| Screening for CAH | 17-OH progesterone in bilateral lesions or hyperplasia |
ACTH: adrenocorticotropic hormone; UFC : urinary free cortisol; DHEAS: dehydroepiandrosterone sulfate; PHA : primary hyperaldosteronism; CAH : congenital adrenal hyperplasia; AHT: arterial hypertension; PAI : primary adrenal insufficiency.
Screening requires the determination of fractionated metanephrines in 24 -h urine (a greater specificity with immunoassay, but use can also be made of liquid chromatography coupled to electrochemical detection and mass spectrometry: LC-MS/ECD) or plasma free metanephrines in the supine position (greater sensitivity).35–37 Due consideration is required of conditions prior to measurement (diet, drugs, etc.) that may cause false positive results. The specific guides should be consulted for more details on this subject.36
- b)
Screening for hypercortisolism. The diagnosis of florid CS is not usually more difficult in this context, due to its frequent association with specific clinical manifestations of hypercortisolism (proximal myopathy, ecchymosis, etc.) and clearly pathological urinary free cortisol (UFC) values.38 However, the diagnosis of ACS constitutes the greatest diagnostic challenge in patients with AI, given the lack of consensus on its definition and the cut-off points used in the different screening tests. The most widely accepted test for initial screening is suppression with dexamethasone 1 mg,2,3,12–14 but agreement is lacking as to which cut-off point to use (Table 4). Our recommendation is to perform DST for initial screening and to complete the study with nocturnal cortisol (in serum or saliva), UFC and ACTH in all patients with DST > 3 µg/dl or in those with > 1.8 µg/dl who present cardiometabolic complications potentially related to hypercortisolism (arterial hypertension, type 2 diabetes, osteoporosis, obesity, dyslipidemia). An ACTH concentration of < 10 pg/mL,39 elevated nocturnal salivary or serum cortisol and/or high UFC,6 support the diagnosis of ACS. In addition, the finding of low dehydroepiandrosterone sulfate (DHEAS) levels in these patients reinforces the diagnosis of ACS,13 though there is no defined cut-off point. In any case, age-standardized reference ranges should be used.
Table 4.Cut-off points in the dexamethasone suppression testa (in µg/dl) for the diagnosis of ACS according to different guides.
NIH (14) FES (12) AACE/AAES (13) AME (3) ESE/ENSAT (2) SEENb Possible ACS > 5 1.9 - 5 > 5 1.9 - 5 1.9 - 5 1.9 - 5 ACS > 5+ other criteriac > 5 > 5+ other criteriac > 5 > 5 > 5 or > 3+ other criteriac ACTH, elevated nocturnal cortisol or low DHEAS.
If DST is < 1.8 µg/dl or ranges between 1.8−3 µg/dl, and there are no cardiometabolic comorbidities potentially related to hypercortisolism, we consider that no further tests are needed in the initial study. However, possible conditions and/or drugs capable of interfering with the test results must be taken into account.6,38
- c)
Screening for primary hyperaldosteronism. In hypertensive patients and/or individuals with hypopotassemia not explained by other causes, it is advisable to determine the aldosterone (ALD)/plasma renin activity (PRA) or aldosterone/direct renin (PR) ratio in the supine position after two hours in the standing position for PHA screening.2,3,12–14,31 Before measurement, hypopotassemia should be corrected, a salt-free diet should be followed, and eplerenone, spironolactone and amiloride should be discontinued 4−6 weeks before testing.31 An ALD/PRA ratio of > 30 (ng/dl/ng/mL/hour) or an ALD/PR ratio of > 3.7 (ng/dl/ng/l) is usually considered suggestive of PHA (the normal ranges established in the reference laboratory should be taken into account).31,40 In these cases, it is advisable to complete the study with a confirmatory test. The specific guides should be consulted for further details.31
- d)
Screening for excess sex hormones. In women with rapidly developing hirsutism or virilization, measurements should be made of testosterone, DHEAS, and androgen precursors. In men with recently developing gynecomastia, a study including estradiol and estrone should be requested. In asymptomatic patients it is not necessary to perform these routine measurements, unless the imaging study suggests adrenal carcinoma, in which case the determination of adrenal androgens may be of help in establishing the diagnosis.2,12,13
- e)
Screening for adrenal insufficiency. In patients with bilateral AI, it is advisable to request a study involving basal serum cortisol in order to rule out primary adrenal insufficiency (AIn),2,12,13 particularly if infiltrative or hemorrhagic lesions are suspected. A value of < 5 µg/dl is diagnostic of AIn, a value of > 15 µg/dl rules out AIn, and intermediate values should cause us to consider expanding the study with functional tests (ACTH test with 250 or 1 µg of ACTH).41
- f)
Screening for congenital adrenal hyperplasia (CAH). In patients with bilateral AI / bilateral hyperplasia, 17-hydroxyprogesterone should be measured to rule out CAH.2,12,13
Based on the results of the hormone and radiological study, the patients are classified into the following groups:
Non-functioning adenoma: AI showing < 10 UH in the CAT scan without contrast, presenting signal loss in opposed-phase in MRI and/or contrast washout in CAT > 60% absolute and > 40% relative washout; with normal hormonal findings.2,3,12
Adenoma with ACS: AI with radiographic features of adenoma, and showing one or more of the following profiles*:
- •
DST > 5 µg/dl2.
- •
DST > 3 µg/dl and at least one of the following: Elevated UFC, low plasma ACTH, elevated nocturnal cortisol (in serum or saliva).
Adenoma with possible ACS: AI with radiographic features of adenoma and a hormone profile intermediate between non-functioning AI (NFAI) and AI with ACS*.
* In the absence of specific signs of hypercortisolism (ecchymosis, proximal myopathy, skin atrophy, broad wine-red striae) and clearly elevated UFC levels (2–3 times the upper limit of normal [ULN]), in which case a diagnosis of florid CS should be considered.
Pheochromocytoma, CS, PHA and adrenal carcinoma: based on the criteria established by the latest clinical practice guides.2,31,36,38
Bilateral adenomas: adrenal lesions with features of adenoma and measuring over 1 cm in size in both adrenal glands.
Indeterminate lesions: AI showing > 10 UH in the CAT scan without contrast, presenting no signal loss in opposed-phase in MRI and/or contrast washout in CAT < 60% absolute and < 40% relative washout.42
AI with myelolipoma features: a well-defined adrenal lesion with attenuation values under -20 HU in the CAT scan without contrast.16,18–20
Other less common lesions: cysts, hemorrhage, collision tumors, ganglioneuroma, hemangioma, ganglioneuroblastoma, neuroblastoma, etc.
Follow-upMost AIs are benign and non-functioning, and surgical treatment is therefore not required. The problem lies in uncertainty when deciding whether follow-up is necessary and how to perform it if needed.
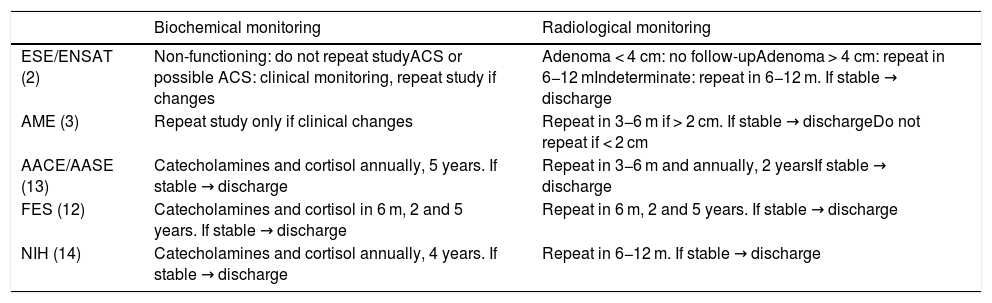
There is considerable discrepancy between the recommendations of the different guides as regards the indications for follow-up and its required duration2,3,12–14 (Table 5). This lack of consensus is mainly explained by the lack of solid scientific evidence, since most existing publications correspond to retrospective studies with limited case series, or to prospective studies with a short follow-up. Furthermore, the prevalence and incidence of adrenal carcinoma is very low, which makes it difficult to estimate the malignancy risk of AI over the course of follow-up.
Proposed follow-up according to the different guides.
| Biochemical monitoring | Radiological monitoring | |
|---|---|---|
| ESE/ENSAT (2) | Non-functioning: do not repeat studyACS or possible ACS: clinical monitoring, repeat study if changes | Adenoma < 4 cm: no follow-upAdenoma > 4 cm: repeat in 6−12 mIndeterminate: repeat in 6−12 m. If stable → discharge |
| AME (3) | Repeat study only if clinical changes | Repeat in 3−6 m if > 2 cm. If stable → dischargeDo not repeat if < 2 cm |
| AACE/AASE (13) | Catecholamines and cortisol annually, 5 years. If stable → discharge | Repeat in 3−6 m and annually, 2 yearsIf stable → discharge |
| FES (12) | Catecholamines and cortisol in 6 m, 2 and 5 years. If stable → discharge | Repeat in 6 m, 2 and 5 years. If stable → discharge |
| NIH (14) | Catecholamines and cortisol annually, 4 years. If stable → discharge | Repeat in 6−12 m. If stable → discharge |
ESE/ENSAT: European Society of Endocrinology/European Network for the Study of Adrenal Tumors; AME: Italian Association of Clinical Endocrinologists; AACE/AASE: American Association of Clinical Endocrinologists and Surgical Endocrinologists; FES: French Endocrinology Society; m: months; NIH: National Institutes of Health; ACS : autonomous cortisol secretion.
In a cohort of 4121 patients with a follow-up of 50.2 months, a recent meta-analysis found that only 2.5% of the patients with NFAI or ACS experienced significant changes in lesion size (≥ 10 mm) or function (4.3% of the NFAI developed ACS), and there were no cases of malignant transformation.43 One of the most relevant findings is the demonstration of an increased cardiometabolic risk and the exacerbation of such risk during follow-up in AI with ACS versus NFAI.43
Hormone monitoringFlorid CS, PHA or pheochromocytoma practically never develops from a mass correctly classified as a non-functioning lesion at initial study. According to the abovementioned meta-analysis, the incidence is < 0.1% in patients with NFAI and ACS.43 Similar rates of approximately 0.3% have been reported by other studies (Table 6).2
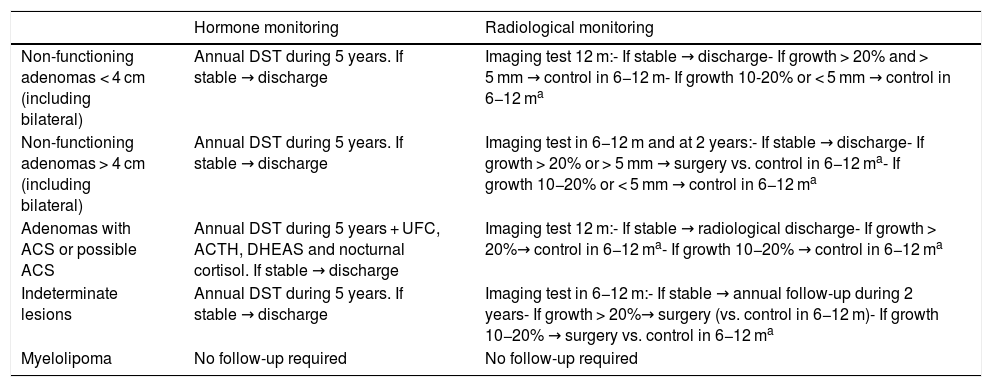
Proposed follow-up of adrenal lesions according to the present consensus document.
| Hormone monitoring | Radiological monitoring | |
|---|---|---|
| Non-functioning adenomas < 4 cm (including bilateral) | Annual DST during 5 years. If stable → discharge | Imaging test 12 m:- If stable → discharge- If growth > 20% and > 5 mm → control in 6−12 m- If growth 10-20% or < 5 mm → control in 6−12 ma |
| Non-functioning adenomas > 4 cm (including bilateral) | Annual DST during 5 years. If stable → discharge | Imaging test in 6−12 m and at 2 years:- If stable → discharge- If growth > 20% or > 5 mm → surgery vs. control in 6−12 ma- If growth 10−20% or < 5 mm → control in 6−12 ma |
| Adenomas with ACS or possible ACS | Annual DST during 5 years + UFC, ACTH, DHEAS and nocturnal cortisol. If stable → discharge | Imaging test 12 m:- If stable → radiological discharge- If growth > 20%→ control in 6−12 ma- If growth 10−20% → control in 6−12 ma |
| Indeterminate lesions | Annual DST during 5 years. If stable → discharge | Imaging test in 6−12 m:- If stable → annual follow-up during 2 years- If growth > 20%→ surgery (vs. control in 6−12 m)- If growth 10−20% → surgery vs. control in 6−12 ma |
| Myelolipoma | No follow-up required | No follow-up required |
UFC : urinary free cortisol; m: months; ACS: autonomous cortisol secretion; DST : dexamethasone suppression test.
However, the risk of developing ACS in NFAI and with possible ACS is significantly higher. The percentage risk varies among the different studies from 6.6 to 31%, depending on the criteria used to define ACS, and the follow-up period involved.6 An increased risk has been documented in lesions measuring over 2.5−3 cm in size.44,45 Based on these data, and until prospective studies with longer follow-up periods become available, our recommendation is to repeat DST annually for at least 5 years in patients with AI in general, and to consider including other studies (UFC, nocturnal cortisol, ACTH, DHEAS) in patients with possible ACS and with ACS. In these latter two groups, assessment moreover should also include screening and the control of comorbidities potentially related to hypercortisolism (type 2 diabetes, arterial hypertension, obesity, osteoporosis and dyslipidemia). In fact, this aspect should receive priority even over hormone testing, since it will condition the treatment decision in most patients with ACS and possible ACS. Repeat hormone testing to screen for PHA or pheochromocytoma is not recommended unless there are new clinical-biochemical data giving reason to suspect such disorders.
Radiological monitoringOne of the most important points in planning a follow-up is the information obtained from the initial radiological assessment, since it is the initial radiological characteristics of the lesion which determine the subsequent follow-up. As mentioned above, there is considerable discrepancy between the radiological monitoring recommendations offered by the different guides. In the case of lesions without initial radiological or hormonal criteria advising treatment, we consider that an intermediate approach should be adopted, based mainly on the results of the meta-analysis conducted by Elhassan44 and the European guides,2 as detailed below (Table 6).
In AI measuring < 4 cm in size and with unequivocally benign radiological features, no further imaging studies are strictly necessary.2 However, considering the possibility of false negative results in the radiological studies, repeat radiological control may be considered at 6−12 months, and if stability is confirmed, then no further radiological studies will be needed.
In AI measuring > 4 cm in size with benign radiological features, we recommend repeat imaging tests at 6−12 months and after two years. If radiological stability is confirmed, further radiological follow-up after these two years may be suspended.
Significant growth is defined as an increase in size of ≥ 20% in the maximum diameter of AI, with a minimum growth of 5 mm. The risk of growth appears to be greater in AI with ACS versus NFAI.44 If the increase is found to be 10−20% with a growth of < 5 mm, we recommend the repetition of imaging testing after 6−12 months. If subsequent stability is confirmed, the discontinuation of radiological monitoring may be considered, and if new growth is detected, we recommend the evaluation of adrenalectomy. In any case, it should be taken into account that the risk of malignancy in this group of patients is very low. According to the different studies that have analyzed this aspect, the risk of malignancy is less than 1% in patients subjected to surgery due to an increase in lesion size during follow-up.43,46–48
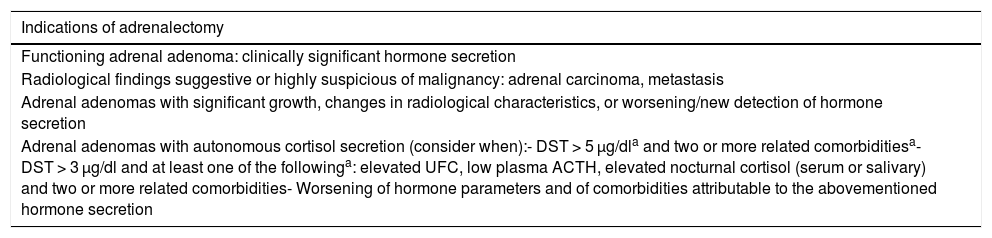
TreatmentAdrenalectomy indicationsSurgery is the first choice for the treatment of functioning AI (with overt hormone syndromes) and in the case of a diagnosis/suspicion of malignancy.2 Likewise, surgery, evaluated by a multidisciplinary team, should be considered in special situations such as a unilateral adrenal mass measuring over 4−6 cm in size with indeterminate findings or atypical features in the imaging studies; or a lesion showing changes in its radiological characteristics or with growth (a 20% increase in the major diameter or an absolute increase of over 5 mm in the major diameter) during follow-up2,3,12,14 (Table 7).
Indications of adrenalectomy in adrenal incidentalomas.
| Indications of adrenalectomy |
|---|
| Functioning adrenal adenoma: clinically significant hormone secretion |
| Radiological findings suggestive or highly suspicious of malignancy: adrenal carcinoma, metastasis |
| Adrenal adenomas with significant growth, changes in radiological characteristics, or worsening/new detection of hormone secretion |
| Adrenal adenomas with autonomous cortisol secretion (consider when):- DST > 5 μg/dla and two or more related comorbiditiesa- DST > 3 μg/dl and at least one of the followinga: elevated UFC, low plasma ACTH, elevated nocturnal cortisol (serum or salivary) and two or more related comorbidities- Worsening of hormone parameters and of comorbidities attributable to the abovementioned hormone secretion |
ACTH: adrenocorticotropic hormone: UFC : urinary free cortisol; DST : dexamethasone suppression test.
Laparoscopic adrenalectomy (LA) is the surgical procedure of choice for most adrenal masses, because it is associated with less postoperative pain and bleeding, a shorter hospital stay, and faster recovery as compared to open adrenalectomy.49 However, open adrenalectomy (OA) is recommended in the case of suspected malignancy with evidence of local invasion or in patients with contraindications for laparoscopy (severe coagulopathy, decompensated heart disease or glaucoma). Likewise, OA may be considered in the presence of large adrenal tumors (> 8−10 cm) where technical difficulties may arise, provided the experience of the surgical team is taken into account. Recently, the group led by Di Buono published its experience with 81 LAs, in which the mean size of the adrenal lesions was 7.5 cm, with some adrenal masses measuring up to 18 cm.50
The surgical decision should be evaluated within a multidisciplinary team, and the patients should be referred to centers experienced in adrenal gland surgery (over 4 adrenalectomies per year) in order to secure better outcomes (lower mortality and fewer complications).51
Surgical treatment in autonomous cortisol secretion- a)
Autonomous cortisol secretion and possible autonomous cortisol secretion.
In the case of AI with possible ACS or confirmed ACS, the decision regarding surgery is controversial and should be made on an individual basis, depending on:
The presence and duration of comorbidities associated with hypercortisolism and their degree of control (arterial hypertension, carbohydrate intolerance/diabetes, obesity, dyslipidemia, cardiovascular or cerebrovascular disease, osteoporosis).
The size of the lesion.
Patient age and preferences.
Although most studies assessing the effect of LA upon ACS are retrospective in design and involve small samples, some findings suggest an improvement in the cardiovascular risk factors - particularly arterial hypertension - after surgery.52,53
The meta-analysis conducted by Elhassan,43 which included 32 studies comprising a total of 3277 patients with AI, revealed a higher prevalence of arterial hypertension (64.0% vs. 58.2%), type 2 diabetes (28.1% vs. 14.4%) and prediabetes (50.0% vs. 11.5%) in AI with ACS versus NFAI. In addition, there was a worsening of pre-existing arterial hypertension (13.4% in ACS vs. 4.8% in NFAI), dyslipidemia (6.8% vs. 4.3%) and glycemic control (9.2% vs. 0.0%), and greater weight gain (21% vs. 8.7%), during follow-up. Likewise, there is growing evidence regarding the rise in cardiovascular risk among patients with ACS, with a two-fold increase in cardiovascular events (12.5% in ACS vs. 6.4% in NFAI)43 and an up to 3–7 fold increase in cerebrovascular events (15.8% vs. 2.3%; p = 0.01).9,54
Taking into account the above, we recommend considering the surgical option in ACS in young patients (under 40–50 years of age) or with at least two comorbidities potentially related to hypercortisolism, with control difficulties or worsening during follow-up.2,3,13
If active monitoring of ACS is decided upon, medical treatment should be provided for the cardiovascular and metabolic risk factors, and for osteoporosis.
- b)
Adrenal hyperplasia with autonomous cortisol secretion
In patients with bilateral adrenal hyperplasia and ACS, we may consider LA of the gland with the largest lesion, taking into account the patient’s age, comorbidities, preferences and the degree of cortisol excess, since there are data showing lesion size and the degree of uptake in norcholesterol scintigraphy to be correlated to the degree of cortisol excess.55,56
- c)
Perioperative corticosteroid therapy
Perioperative treatment with corticosteroids is important in all patients with CS until the hypothalamic-pituitary-adrenal axis has recovered (prevalence of adrenal insufficiency[(AIn] 100%).
With regard to ACS, a systematic literature review of 28 studies recorded a prevalence of AIn after surgery of 65%,57 suggesting that routine corticosteroid replacement therapy may not be required.
Therefore, when choosing LA in ACS or possible ACS, we recommend any of the following options (depending on their availability at each center):
Start glucocorticoid therapy in all patients, with periodic reassessment of the hypothalamic-pituitary-adrenal axis.
Evaluate the morning cortisol levels on the first postoperative day, establishing a diagnosis of AIn when the cortisol values are < 5 µg/dl, and discarding it in the case of >15 µg/dl.41
Perform an ACTH stimulation test (Synacthen test) on the first postoperative day, discarding AIn in the case of cortisol values of ≥15−18 µg/dl.41,58
Special situations- •
Adrenal incidentaloma in young and elderly patients. Urgent evaluation of AI is advised in children, adolescents, young adults and pregnant women, because of the increased likelihood of malignancy. In elderly or frail patients, particularly if they have small lesions, the need for study should be pondered, because the probability of malignancy is very low.
- •
Bilateral AI and adrenal hyperplasia. The recommendations on hormone and radiological monitoring are the same as in the case of unilateral AI. However, it must be taken into account that the risk of developing cortisol hypersecretion is greater,59 as well as the possibility of Ain, though the latter is uncommon in benign lesions, and is mainly found in metastatic and infiltrating adrenal disease.60
- •
Adrenal lesions in patients with extra-adrenal malignancies. Firstly, it should be pointed out that in this context we should not speak of AI in the strict sense. The hormone and radiological studies do not differ from those applicable to AI, though PET with FDG18 is of particular interest in patients with indeterminate lesions, and is even considered before CAT without contrast and/or MRI, due to the high risk of malignancy.2 Nevertheless, in lesions characterized as being benign in the initial study, the same follow-up and/or treatment recommendations as in AI should be followed.
Please cite this article as: Araujo-Castro M, Ituguerri Guevara M, Calatayud Gutiérrez M, Parra Ramírez P, Gracia Gimeno P, Hanzu FA, et al. Guía práctica sobre la evaluación inicial, seguimiento y tratamiento de los incidentalomas adrenales. Grupo de patología adrenal de la Sociedad Española de Endocrinología y Nutrición. Endocrinol Diabetes Nutr. 2020;67:408–419.