The patient living with HIV (PLWH) profile has changed and with it, the importance of patient-oriented pharmaceutical care (PC) has been highlighted, for which the stratification tool of the Capacity-Motivation-Opportunity (CMO) PC model helps us which adapts to the needs of each patient. To assess the true relevance, our main objective is to evaluate the differences of one-year mortality among PLWH stratified according to this model.
MethodsA single-center observational analytical survival research study including adult PLWH on antiretroviral therapy (ART) from January-2021 to January-2022 treated at hospital pharmacy outpatient service according to CMO pharmaceutical care model.
ResultsA total of 428 patients were included, a median age of 51 years (interquartile range 42–57 year). Overall, the number of patients stratified according to the CMO PC model was 86.2% at level 3, 9.8% at level 2, and 4.0% at level 1. Cox proportional hazard model that included the stratification level was associated with a higher mortality, whose level 1 patients had a 99.7% higher mortality (Hazard ratio=0.0003; 95%CI: 0.001–0.027).
ConclusionsTo sum up, mortality of-one year differs when comparing the PC strata of level 1 and non-level 1, although being similar in age and other clinical conditions. This result suggests that the multidimensional stratification tool, included in the CMO PC model, could be used to modulate the patients intensity follow-up and design interventions more tailored to their needs.
El perfil del paciente que vive con virus de inmunodeficiencia humana (PVVIH) ha cambiado y con ello se ha puesto de manifiesto la importancia de la atención farmacéutica (AF) orientada al paciente, para lo que nos ayuda la herramienta de estratificación del modelo de AF capacidad-motivación-oportunidad (CMO) que se adapta a las necesidades de cada paciente. Para determinar la relevancia, nuestro principal objetivo fue evaluar las diferencias de mortalidad a un año entre las PVVIH estratificadas según este modelo.
MétodosEstudio de supervivencia analítica observacional unicéntrico que incluyó PVVIH adultos en terapia antirretroviral (TAR) de enero de 2021 a enero de 2022 atendidos en el servicio de farmacia hospitalaria acorde con el modelo de atención farmacéutica de CMO.
ResultadosSe incluyeron un total de 428 pacientes, con una mediana de edad de 51 años (rango intercuartílico: 42-57). En general, el número de pacientes estratificados según el modelo AF CMO fue de 86,2% en nivel 3, de 9,8% en nivel 2 y de 4% en nivel 1. El modelo de riesgos proporcionales de Cox que incluía el nivel de estratificación se asoció con una mayor mortalidad, donde los pacientes de nivel 1 tuvieron una mortalidad de 99,7% mayor (OR = 0,0003; IC 95%: 0,001-0,027).
ConclusionesLa mortalidad al año difiere al comparar los estratos, aunque son similares en edad y otras condiciones clínicas. Este resultado sugiere que la herramienta de estratificación multidimensional, incluida en el modelo de AF CMO, podría utilizarse para diseñar intervenciones más adaptadas a las necesidades de cada paciente.
Currently, HIV infection is considered a chronic disease. The success of highly active antiretroviral therapy (ART), together with the arrival of new, more powerful drugs with better dosage guidelines, has allowed people living with HIV (PLWH) to substantially reduce the risk of HIV transmission and have a near normal life expectancy. This confronts us with new challenges such as the development of age-related comorbidities in these patients. In fact, analysis of HIV cohorts indicates that medical conditions such as hypertriglyceridemia, hypercholesterolemia, arterial hypertension, or diabetes mellitus are very common among PLWH.1 All of this makes it necessary to start concomitant treatments with ART for the management of comorbidities, which can be challenging given the increased risk of adverse events and drug interactions, adherence problems, and increased risk of hospitalization.2
Proper management of PLWH requires a multidisciplinary healthcare team, including an HIV-specialized clinical pharmacist.3 Traditionally, the pharmaceutical care (PC) model relied mainly on the medication, not considering the individual characteristics of the patients.4 However, multiple studies have shown that alternatives to the classical medicine-focused design were more effective in improving health outcomes.5,6 Patient demographic, educational and cognitive factors, as well as the use of health resources should be previously evaluated to provide the best care for patients. Furthermore, improving patient empowerment should also be considered a priority intervention to increase their self-efficacy in medication management.
Taking into account all the above, five years ago the Spanish Society of Hospital Pharmacy coordinated by Ramón Morillo Ph.D. developed a new redefined a redefined PC model based on three differential aspects.7 First, the capacity pillar, understood as the stratification of patients. This constitutes a fundamental activity within the PC model that allows the provision of individualized pharmaceutical care adapted to each patient according to their specific needs. Therefore, we speak of stratification as a model for approaching chronic diseases, making it possible to identify those patients with the greatest risk or need for care and, consequently, interventions can be better designed to achieve the proposed objectives. Secondly, a motivational interview with the objective of setting and defining individualized pharmacotherapeutic objectives. And third, real-time monitoring of patients using the new technological tools available.
The capacity-motivation-opportunity (CMO) pharmaceutical care model intervention has previously been tested in PLWH, showing successful results in improving patient adherence to ART, reducing, for example, cardiovascular risk and increasing patient activation.8–10 Currently, there is data on the specific effect of this kind of program on other clinical outcomes of health that are frequently altered in PLWH, even readmissions.11–13
Different tools and predictive models, based on clinical or analytical parameters, have been used to predict clinical worsening or death at different analysis periods in PLWH.14,15 However, it is necessary to develop new tools that allow modulating the interventions and predicting the subsequent evolution among those who are in routine follow-up.
To better assess the true relevance and usefulness in the clinical course of pharmaceutical care stratification, based on the CMO model, we performed a retrospective analysis evaluating differences in overall one-year mortality between patients classified in different levels of care in a real clinical cohort of PLWH.
MethodsStudy setting and populationWe completed a single-center observational analytical survival study in Seville, Spain. The study population included persons ≥18 years attending at hospital pharmacy outpatient service from January-2021 to January-2022. We established as exclusion criteria the loss of follow-up of the patients due to abandonment of care or change of hospital center.
Those patients received the pharmacotherapeutic follow-up already routinely applied to ambulatory care patients according to a Capacity-Motivation-Opportunity (CMO) pharmaceutical care model.8,9 We stratified the patients according to the model risk-stratification of patients for pharmaceutical care in patients with HIV of the Spanish Society of Hospital Pharmacy. The stratification model is made up of 4 blocks of variables that allow this classification of patients.12 These blocks are demographic variables, medication-related variables, socio-sanitary variables and of the cognitive-functional state and, finally, Clinical variables and of use of services sanitary. The result of the stratification allows us to classify the patients into 3 categories based on the score obtained. All patients with any chronic pathology are classified as N3 (15 or less scoring points), patients stratified as N2 (27 to 16 points) are those who present some risk related to medication or social health and, finally, patients classified as N1 (28 points or more total score) who present a global high with greater needs in pharmaceutical care. The complete description of the variables included in each block and their weight in the stratification model can be found in digital format to facilitate their access and encourage their use.16 In addition, the description of the different variables that make up each block and their scores in the stratification model have been included in supplementary material 1.
DefinitionsTo describe the patterns of multimorbidity, the classification proposed by De Francesco et al. was used, including cardiovascular pathology, chronic obstructive pulmonary disease (COPD)-Liver pathology, events related to HIV/AIDS, neurological-psychiatric diseases, sexually transmitted diseases, and general health.17
Polypharmacy was defined as the use of 6 or more different drugs, including antiretroviral medication. To describe the patterns of polypharmacy, we employed the categorization proposed by Calderón-Larrañaga et al.18 who classified the patterns depending on the type of disease. They were intended to treat cardiovascular, depression-anxiety, acute respiratory infection, chronic pulmonary disease, rhinitis-asthma, pain and menopause. A patient was categorized to a specific pattern when at least three drugs included in the pattern were dispensed.
The Medication Regimen Complexity Index (MRCI) is a validated 65-item tool that evaluates treatment regimen complexity based on the number of medications, dosage form, dosage frequency, and additional or special instructions.19 This index score ranges from 1.5 (for someone taking a single tablet or capsule taken once a day) to an undefined maximum since the score increases with the number of medications; greater scores indicate higher complexity.20 Additionally, according to Morillo-Verdugo et al. a cut-off value of 11.25 for MRCI index score was employed for considering complex patients.21
Statistical analysisThe following variables were analyzed: demographic (age, sex); analytical data, plasma viral load (copies/mL), CD4 cell count (cells/μL); clinical variables (comorbidities, CDC classification, mortality and time until death) and pharmacotherapeutics. Only those patients with all variables completed were included in the analysis.
We arrange descriptive statistics for baseline characteristics, including frequencies and proportions for categorical variables. In the case of continuous variables, they were described by means of medians and ranges. To evaluate and determine the differences in mortality based on the inclusion characteristics of the patient, the χ2 tests, Fisher's exact test and the T-Student test were used.
We identified a priori factors that were previously suspected or suspected to be associated with mortality, such as age, level of stratification, presence of comorbidities, polypharmacy, pharmacotherapeutic complexity index and CDC classification, together with the level of Stratification will be analyzed with Kaplan–Meier curves and the Log-Rank test to compare the differences in survival between the different variables. Subsequently, a multivariate Cox regression model will be carried out.
Data analysis is performed with the statistical package IBM SPSS 28.0 for iOS.
Ethical issuesThe study fulfilled all the ethical requirements and was approved by the Clinical Research Ethics Committee of Sevilla-Sur (C.I. 0983-N-22). This study has been carried out according to the guidelines of the Declaration of Helsinki for biomedical research.
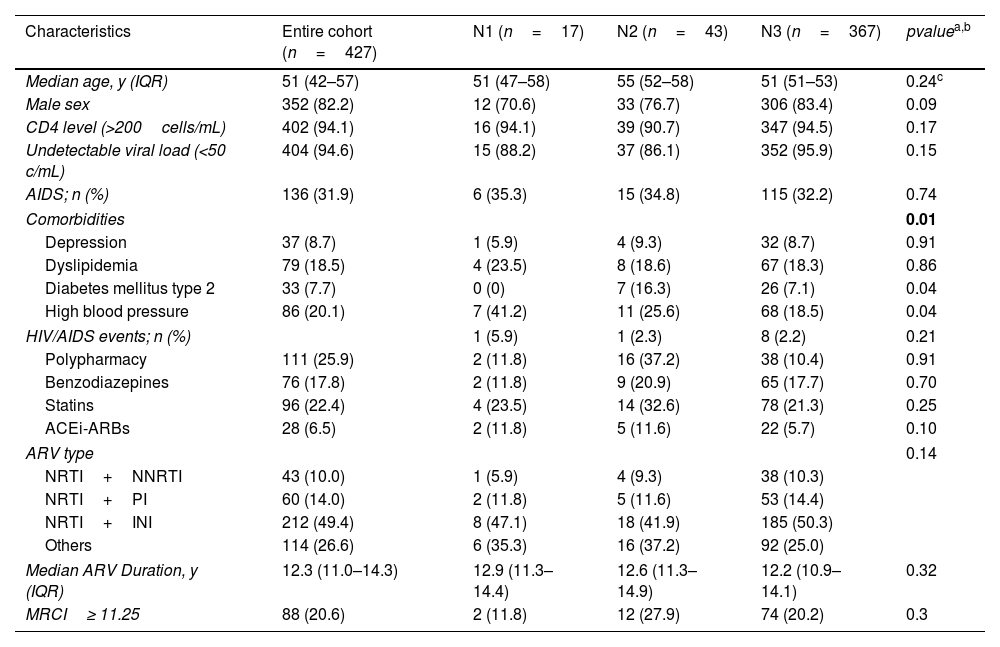
ResultsWe included 427 patients between 1 January and 31 March 2021, 352 (82.2%) males. Three PLWH were lost to follow-up, 2 of them due to a change in hospital center and 1 due to abandonment of follow-up by both the doctor and the pharmacist. The median age of the cohort was 51 years (interquartile range (IQR), 42–57 years). At baseline 396 patients presented an undetectable viral load (<20cop/mL) (96.5%) and a CD4+ >200cell/mm3 count (97.4%). Globally, the number of patients stratified according to the CMO pharmaceutical care model were 369 in level 3 (86.2%), 42 in level 2 (9.8%) and 17 in level 1 (4.0%). Baseline characteristics of patients are shown in Table 1.
Baseline characteristics PLWH.
| Characteristics | Entire cohort (n=427) | N1 (n=17) | N2 (n=43) | N3 (n=367) | pvaluea,b |
|---|---|---|---|---|---|
| Median age, y (IQR) | 51 (42–57) | 51 (47–58) | 55 (52–58) | 51 (51–53) | 0.24c |
| Male sex | 352 (82.2) | 12 (70.6) | 33 (76.7) | 306 (83.4) | 0.09 |
| CD4 level (>200cells/mL) | 402 (94.1) | 16 (94.1) | 39 (90.7) | 347 (94.5) | 0.17 |
| Undetectable viral load (<50 c/mL) | 404 (94.6) | 15 (88.2) | 37 (86.1) | 352 (95.9) | 0.15 |
| AIDS; n (%) | 136 (31.9) | 6 (35.3) | 15 (34.8) | 115 (32.2) | 0.74 |
| Comorbidities | 0.01 | ||||
| Depression | 37 (8.7) | 1 (5.9) | 4 (9.3) | 32 (8.7) | 0.91 |
| Dyslipidemia | 79 (18.5) | 4 (23.5) | 8 (18.6) | 67 (18.3) | 0.86 |
| Diabetes mellitus type 2 | 33 (7.7) | 0 (0) | 7 (16.3) | 26 (7.1) | 0.04 |
| High blood pressure | 86 (20.1) | 7 (41.2) | 11 (25.6) | 68 (18.5) | 0.04 |
| HIV/AIDS events; n (%) | 1 (5.9) | 1 (2.3) | 8 (2.2) | 0.21 | |
| Polypharmacy | 111 (25.9) | 2 (11.8) | 16 (37.2) | 38 (10.4) | 0.91 |
| Benzodiazepines | 76 (17.8) | 2 (11.8) | 9 (20.9) | 65 (17.7) | 0.70 |
| Statins | 96 (22.4) | 4 (23.5) | 14 (32.6) | 78 (21.3) | 0.25 |
| ACEi-ARBs | 28 (6.5) | 2 (11.8) | 5 (11.6) | 22 (5.7) | 0.10 |
| ARV type | 0.14 | ||||
| NRTI+NNRTI | 43 (10.0) | 1 (5.9) | 4 (9.3) | 38 (10.3) | |
| NRTI+PI | 60 (14.0) | 2 (11.8) | 5 (11.6) | 53 (14.4) | |
| NRTI+INI | 212 (49.4) | 8 (47.1) | 18 (41.9) | 185 (50.3) | |
| Others | 114 (26.6) | 6 (35.3) | 16 (37.2) | 92 (25.0) | |
| Median ARV Duration, y (IQR) | 12.3 (11.0–14.3) | 12.9 (11.3–14.4) | 12.6 (11.3–14.9) | 12.2 (10.9–14.1) | 0.32 |
| MRCI≥ 11.25 | 88 (20.6) | 2 (11.8) | 12 (27.9) | 74 (20.2) | 0.3 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: ACEi, angiotensin-converting enzyme inhibitors; ARBs, angiotensin receptor blockers; NRTI, nucleoside analog reverse-transcriptase inhibitors; NNRTI, non-nucleoside reverse-transcriptase inhibitors; PI, protease inhibitors; INI, integrase inhibitors; MRCI, Medication Regimen Complexity Index.
Consistent with comorbidities, the polypharmacy patterns more prevalent were cardiovascular (11.2%) and anxious-depressive (4.0%).
Regarding the complexity of the patients, MRCI index mean was 6.9±5.5. Moreover, MRCI index was higher in patients stratified as N1 (12.4±8.4) compared to N2 (9.2±5.9) and N3 (4.7±4.8) (p=0.001). A MRCI value greater than 11.25 (complex patients) was observed in 88 patients (20.6%).
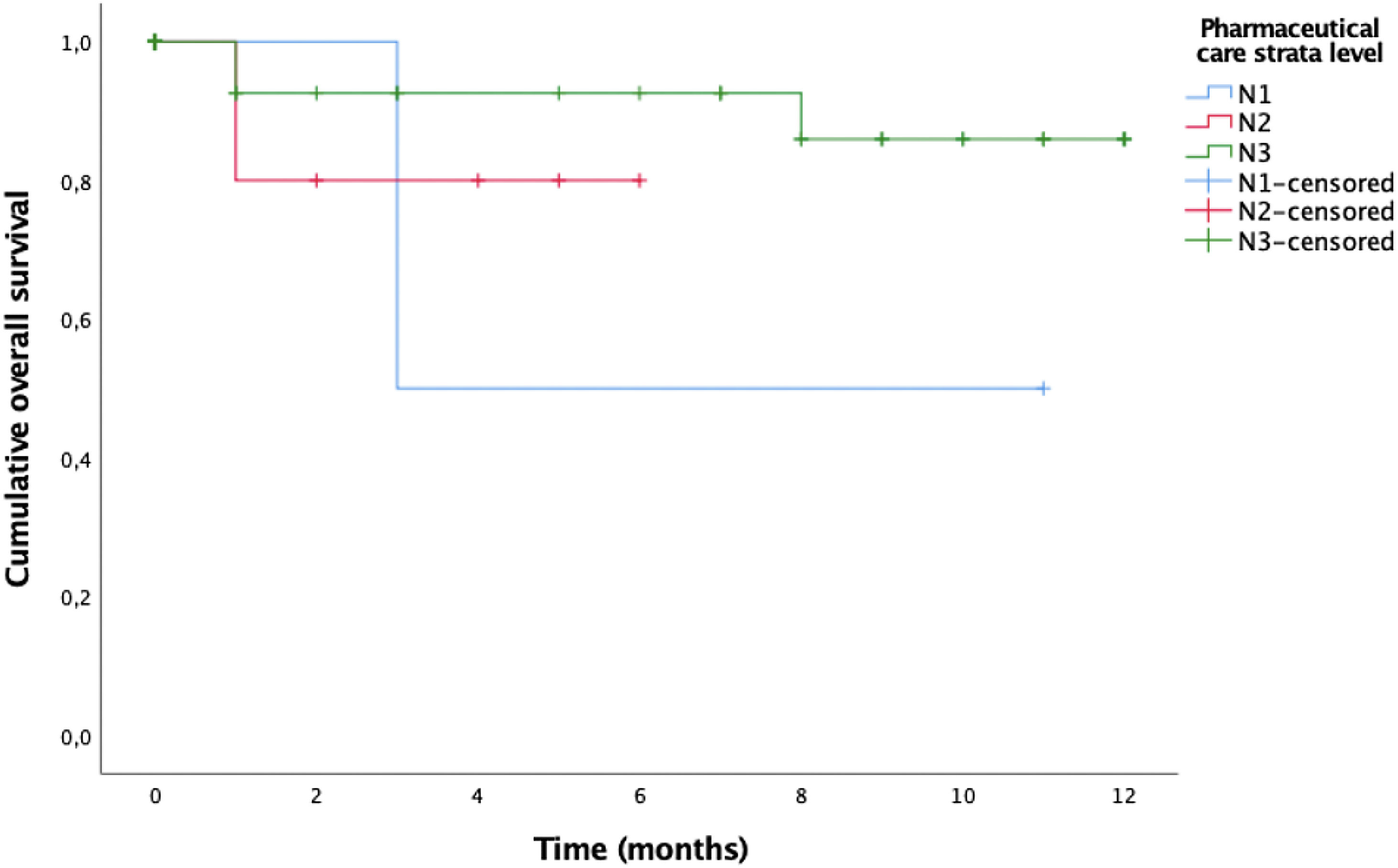
The overall mortality rate from any cause in our population was 11.7 per 1000 PLWH. Causes of death in PLWH were cardiovascular disease (n=2), cancer (n=1), pneumonia (n=1), and acute respiratory failure (n=1). If we focus on the 5 patients in the cohort who died, they had a median age of 61 years (IQR: 58–67 years). According to the level of stratification, these were classified as 3 patients in level 1 and one person in both level 2 and level 3. The clinical variables prior to the death of the patients who suffered the event show that 80% had burden undetectable viral load, only the patient stratified as N1 had a detectable viral load, and 60% had a CD4+ count>200cells/mm3, except for the patient categorized as N1 and one patient stratified as N3. In addition, 60% of the patients were being treated with regimens based on Integrase inhibitors and 20% with regimens containing boosted Protease inhibitors. The median duration of antiretroviral treatment was 13.6 (12.3–14.7). In relation to comorbidities related to HIV infection, no patient suffered AIDS-related events during their clinical course. Overall survival curve based on the level of stratification shown in Fig. 1.
If we focus on clinical variables, the Long-Rank test found significant differences in those patients who suffered from type 2 diabetes mellitus (p=0.04) and arterial hypertension (p=0.04) compared to those without a diagnosis of these diseases, which may be related to the patterns of polypharmacy more prevalent in the patients included in the study. However, this difference was not found in those patients who suffered from multimorbidity patterns, for which the patient must suffer from at least two of the diseases from the groups established by De Francesco et al.17 (p=0.3).
Another relevant aspect to analyze due to its implication in the death of patients is that associated with the pharmacotherapeutic variable of patient stratification. When using the Long-Rank test to compare the level of stratification, we found significant differences in the proportion of deaths (p=0.02).
A multivariable Cox proportional hazards model was performed including the level of stratification to analyze its influence. The results showed an association with higher mortality whose level 1 patients had 99.7% higher mortality (Hazard ratio [HR], 0.0003 [95% CI, 0.001–0.027], p<0.01) and level 2 patients had a 22% higher mortality ([HR], 0.078 [95% CI, 0.01–0.58], p=0.02) than patients classified in level 3.
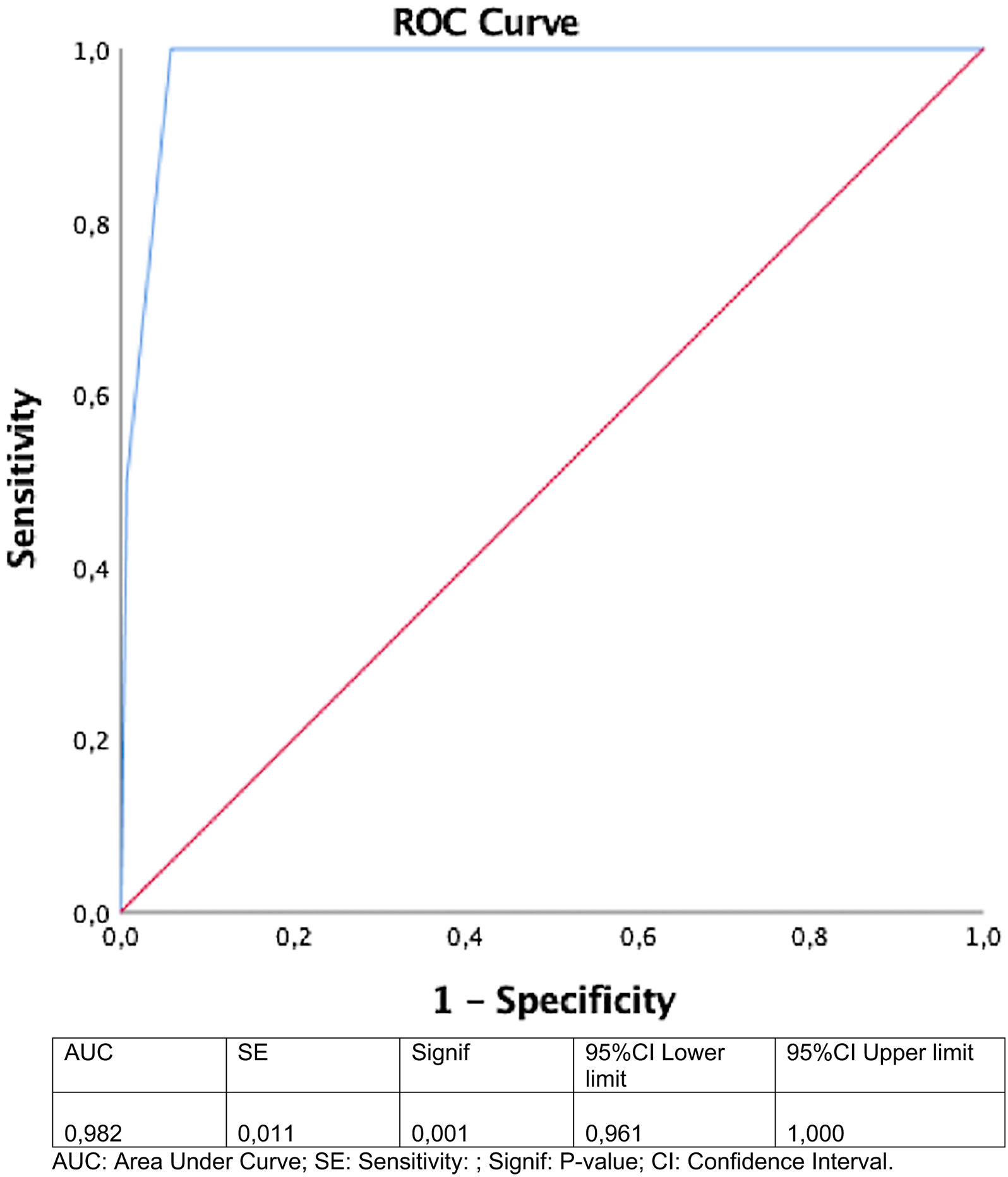
The ROC curve was constructed, and this demonstrated that the model predicted mortality in HIV infected patients according to stratification level (area under curve=0.98 [95% CI, 0.96–1.0) (Fig. 2).
DiscussionAt one year, there is a higher risk of mortality in those patients classified as level 1 risk compared to the rest of the patients included in the other pharmaceutical care strata.
The prevalence of patients who report multiple affected dimensions underlines the urgency in developing interventions that will alleviate these barriers and attenuate mortality. Urgent stratification, both naive and pretreated, can help us identify patients for targeted interventions that can help them to care, improving adherence to all pharmacotherapy (antiretroviral and non-antiretroviral) subsequently reducing their likelihood of death.22 Targeted structural, multidisciplinary, and overall multidimensional interventions can improve outcomes for high- risk patients.
A potential explanation of higher mortality in patients who report high level may be related to adherence to HIV care (i.e., ART and follow-up appointments). Previous studies have established associations between suboptimal adherence and perceived poor service delivery, a lack of financial means for transport, patient perception that the individual is feeling well, responsibilities to care for dependents, and inconvenient clinic hours. Suboptimal adherence to HIV care is directly linked to poor virologic outcomes and mortality.23
Thus, it is possible that participants who reported barriers did not adhere to HIV care, as a mechanism leading to an increased risk of death.24 In our study, the included patients were in routine follow-up in medical and pharmaceutical consultations in the last year, and if they had been admitted or visited the emergency room, the stratification model itself scored this circumstance, placing them in higher strata.25
There are other widely contrasted and validated predictive tools and models that help identify which patients are at risk and the severity of the evolution of HIV patients, based on indicators of HIV infection (CD4 count and viral load), indicators of organic functions such as hemoglobin, platelets, transaminases, and co-infections by other viruses, such as the VACS index.26 However, limitations have also been observed, such as for example, in assessing the importance of frailty or cognitive aspects in older patients.27 Our model, which integrates the VACS index, including other relevant assessment aspects in the current patient, could also help to better identify people at risk.
One of the barriers to implementing multidisciplinary and collaborative guide and protocols is the lack of information on the risk assessment of PLWH. The easily implemented and validated CMO pharmaceutical care model, including the stratification tool, may overcome this barrier.11 To help pharmacist define the appropriate intervention to patients identified as ‘at risk’, our team will develop and implement an electronic clinical evaluation protocol in PLWH. The new stratification tool will automatically produce recommendations for pharmacists and clinicians based on their risk level and will inform clinical decision-making as part of the person-centered care planning process to fill the gap of multidisciplinary, multidimensional and shared decision model. There are already some studies that have shown how the CMO pharmaceutical care model, where these tools are included, have improved health outcomes, and have reduced the rate of readmission in PLWH.28,29
Several limitations of currently used pharmaceutical care stratification measures have been noted. First, it is a retrospective cohort study limited to our hospital and the results may not be generalizable. Second, although most approaches generally consider stratification cross-sectionally, it is an evolving process, with people often moving in or out of care as their personal circumstances and health change. Finally, we could consider the small number of deceased patients a possible limitation, but we must bear in mind that thanks to improvements in antiretroviral medication, patients have better immunovirological control and their life expectancy has increased. In addition, the follow-up of the patients has been only one year and since the causes of mortality have been associated with age-related comorbidities and non-AIDS events, the need for future studies with a longer follow-up in this population would be justified to analyze the influence on the survival of this population.
Furthermore, clinic monitoring policies are also changing, in response to improved ART outcomes and increasing patient numbers. Our proposed approach to defining stratification and re-estratification reflects such changes at both an individual and clinic level. Moreover, this study was carried out by applying the stratification model published in 2017. A new simplified version has recently been published and has been adapted to the variables and characteristics of patients today.30 All this is part of the CMO model, which also presents the motivation and opportunity cornerstones that allow us to longitudinally monitor the patient, adapting to their needs and with the incorporation of new technologies that help PC beyond face-to-face care, as stated the new definition of PC.7
Future studies will allow us to confirm whether the new model can show the same results as the initial version, within the CMO methodology.
Thanks to the own dynamism of the stratification and re-estratification, it is possible to significantly adjust the clinical efforts, with greater intensity to those patients with greater complexity. However, it is necessary to carry out studies with rigorous designs that allow the highest possible number of patients to lower their risk, thus favoring their survival and quality of life.
Furthermore, future studies will allow us to know the value of the tool to predict the use of health resources, at all levels, in PLWH that are at the highest risk level compared to others.
ConclusionsIn this cohort of PLWH outpatients, overall mortality of one year differs when comparing the pharmaceutical care strata of level 1 and non-level 1, despite being similar in age and other clinical conditions.
This suggests that the multidimensional stratification tool, included in the CMO pharmaceutical care model, could be used to modulate the intensity of patient follow-up and design interventions more tailored to their needs, in order to improve their health status and quality of life.
FundingNone to declare.
Conflicts of interestThe authors declare that they have no conflict of interest.