Data concerning the use of peripherally inserted central catheters (PICC) for the administration of intravenous (IV) antimicrobials in the acute care setting is scarce.
MethodsWe performed a single-center retrospective case–control study (1:1). Case subjects were defined as patients who received IV antimicrobial treatment through a PICC line placed and maintained by specifically trained nurses (PICC group). Control subjects were defined as patients who received antimicrobial therapy by a peripheral or a central venous catheter (CVC) (control group). Control subjects were matched by type of antimicrobial, causative microorganism of the infection that was being treated and duration of treatment. An event leading to undesired catheter removal (ELUCR) was defined as any circumstance which lead to the removal of the indwelling catheter other than the completion of the scheduled course of antimicrobial therapy.
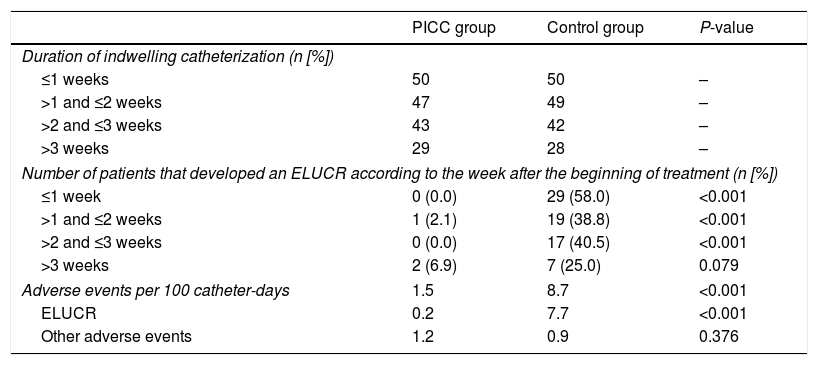
ResultsThe study included 50 patients in each group. The total follow-up time was 1376 catheter-days for the PICC group and 1362 catheter-days for the control group. We observed a significantly lower incidence of ELUCR in the PICC group (0.2 versus 7.7 events per 100 catheter-days; P<0.001). When the incidence of ELUCR was analyzed according to the duration of indwelling catheterisation for each type of catheter (divided into one-week intervals), differences between both groups were also significant (P-values≤0.001 for the first three weeks of treatment). During the second week of IV treatment, only one patient in the PICC group (2.1%) developed an ELUCR compared to 19 (38.8%) in the control group (P<0.001).
ConclusionsA PICC placed and maintained by a dedicated nursing team is an excellent alternative to peripheral venous catheters or CVCs for administrating antimicrobial therapy for both short and long periods of treatment.
Existe escasa información disponible sobre el empleo de catéteres venosos centrales de inserción periférica (PICC en sus siglas en inglés) para la administración de antimicrobianos por vía intravenosa (iv) en la atención a pacientes con procesos agudos.
MétodosRealizamos un estudio unicéntrico retrospectivo de casos y controles (1:1). Los casos estaban constituidos por pacientes que recibieron tratamiento antimicrobiano iv a través de un catéter tipo PICC que fue insertado y cuidado por un equipo de enfermería especialmente entrenado a tal efecto (grupo PICC). Los controles estaban constituidos por pacientes que recibieron el tratamiento antimicrobiano a través de un catéter venoso periférico o a través de un catéter venoso central (CVC) (grupo control). Los controles fueron emparejados con los casos considerando el tipo de antimicrobiano administrado, el microorganismo causal de la infección que se estaba tratando y la duración del tratamiento. Se definió como un evento que condujo a la retirada no deseada del catéter (ECRDC) a cualquier circunstancia que obligara a la retirada del catéter insertado antes del tiempo programado para completar el tratamiento antimicrobiano establecido.
ResultadosEl estudio incluyó 50 pacientes en cada grupo. El tiempo total de seguimiento fue de 1.376 días de catéter en el grupo PICC y de 1.362 días de catéter en el grupo control. Se observó una incidencia de ECRDC significativamente menor en el grupo PICC que en el grupo control (0,2 versus 7,7 eventos por cada 100 días de catéter; P < 0,001). Cuando la incidencia de ECRDC se analizó según la duración del tiempo de inserción de cada tipo de catéter (dividido en intervalos de una semana), se pudo constatar que las diferencias entre ambos grupos también eran significativas (P ≤ 0.001 para las tres primeras semanas de tratamiento). Durante la segunda semana de tratamiento iv, solamente un paciente en el grupo PICC (2,1%) desarrolló un ECRDC en comparación con 19 (38,8%) en el grupo control (P (P<0,001).
ConclusionesUn catéter tipo PICC insertado y cuidado por un equipo de enfermería entrenado es una excelente alternativa a los catéteres venosos periféricos o a los CVC para la administración de antimicrobianos tanto para periodos cortos como para periodos largos de tiempo.
The use of peripherally inserted central catheters (PICCs) for the intravenous (IV) administration of antimicrobials has significantly grown in recent years. PICC lines avoid much of the complications associated with central venous catheters (CVC) or peripheral venous catheters.1 The use of PICCs allows an earlier hospital discharge, since IV treatment can be administered in the outpatient environment. PICCs are associated to a risk of infection and thrombosis.1–4 Most of the experiences about the use of PICCs proceed from studies of oncological or pediatric patients. There is scarce available information about PICCs specifically used for the administration of IV antimicrobials in the acute care setting. The safety of PICCs in contrast to other types of catheters has been scarcely studied.5 Some guidelines and expert reports consider that a PICC should be used instead of a peripheral venous catheter when the proposed duration of IV treatment is six or more days,6,7 but there is a very low level of evidence for this recommendation as it is based on a single study that established an arbitrary span of treatment of at least five days.8 To the best of our knowledge, this is the first study to compare the safety of a PICC versus other types of vascular accesses specifically used for antimicrobials and considering length of administration.
Patients and methodsStudy design and settingWe performed a single center retrospective case–control study (1:1) in the University Hospital 12 de Octubre (Madrid, Spain). The inclusion period spanned from December 2015 to May 2017. Case subjects were defined as patients to whom IV antimicrobial treatment was administered through a PICC line placed and maintained by specifically trained nurses belonging to the staff of the Department of Oncology of our institution (PICC group). Consecutive patients were included according to the data obtained from the database provided by this nursing team. Control subjects were defined as patients who received antimicrobial therapy by a peripheral or a CVC or patients who received therapy through a PICC line that was not inserted and maintained by the aforementioned dedicated nursing team (control group). These patients’ data were obtained from the historical registry provided by the Department of Pharmacy of the same institution, which controls all the antimicrobials administered in our center. Control subjects were then matched by type of IV antimicrobial therapy, causative microorganism and duration of treatment.
Collected variables included baseline demographic data, clinical data (cause for antimicrobial treatment, isolated microorganism, antibiotic prescribed and duration of treatment) and catheter-related complications.
Institutional PICC placement and care protocolTrained nurses from the Department of Oncology are in charge of placing and caring for the PICCs for the whole hospital. Prior to the PICC insertion, all patients receive oral and written information about the insertion procedure and possible complications that might occur during its placement. All the patients sign an informed consent document authorizing the procedure. The patient is placed in the supine position with the dominant arm extended in a 90-degree angle from the torso. The dominant arm is usually the first choice. Vessels with phlebitis or signs of thrombosis are avoided. The vein that is going to be catheterized is identified by ultrasonography. The basilic vein is considered the best option due to its location, course and simplicity to isolate. The brachial vein and the radial vein are considered as second and third options, respectively. Once the vessel is located, the point of insertion is marked. The length of the catheter is calculated by adding the distance between the middle third of the arm and the middle clavicular point to the distance between this point and the third intercostal space. A surgical handwashing is always performed before the procedure. A wide sterile field surrounding the catheter insertion area is prepared and the skin is disinfected with 5% chlorhexidine alcohol-based solution. Local anesthesia is also administered. The ultrasound-guided modified Seldinger technique is used for inserting the catheter. Before fixing the PICC, a novel localization system called Sherlock 3CG® Tip Confirmation System (C.R. Bard Ltd, New Jersey, USA) is employed in order to assess that the PICC line is correctly placed in the superior vena cava. Using the patient's own cardiac electrical activity, this system confirms the position of the catheter's tip in real time by connecting an intravascular electrode within the tip of the catheter to an electrocardiographic monitor. This eliminates the need for a chest X-ray to confirm that the PICC line is properly positioned. After confirming that the PICC line is correctly placed, the external end is fixed and covered with a sterile dressing. All patients receive a guide specifying the care that the PICC will require and the signs of alarm indicating the need for urgent revision. This guide also includes a section with the appointment dates for the line's follow-up. The PICC line is revised and redressed within the first 48–72h by the inserting team and, thereafter, by the primary nursing team of each patient. The inserting nursing team is also available on demand for any issue related to the PICC line as long as it is in use.
Study definitionsAn event leading to undesired catheter removal (ELUCR) was defined as any circumstance which leaded to the removal of the indwelling catheter other than the completion of the scheduled course of therapy. Phlebitis and catheter-related bloodstream infection (CRBSI) were defined according to the updated clinical guidelines for the diagnosis and management of intravascular catheter-related infection proposed by the Infectious Diseases Society of America.9 We have also differentiated between partial and complete catheter occlusion.10 The main outcome of the study was the incidence of ELUCRs per 100 catheter-days.
Statistical analysisContinuous variables were summarized by the mean±standard deviation (SD) or the median with interquartile ranges (IQR). Categorical variables were summarized using absolute counts and percentages. Categorical variables were compared using the McNemar test, whereas the Student's t-test for repeated measures or the Wilcoxon signed-ranks test were applied for continuous variables. All results were considered statistically significant at a P-value≤0.05. Statistical analysis was performed with SPSS version 20.0 (IBM Corp., Armonk, NY) and EPIDAT version 3.1 (Conselleria de Sanidade, Xunta de Galicia, Spain).
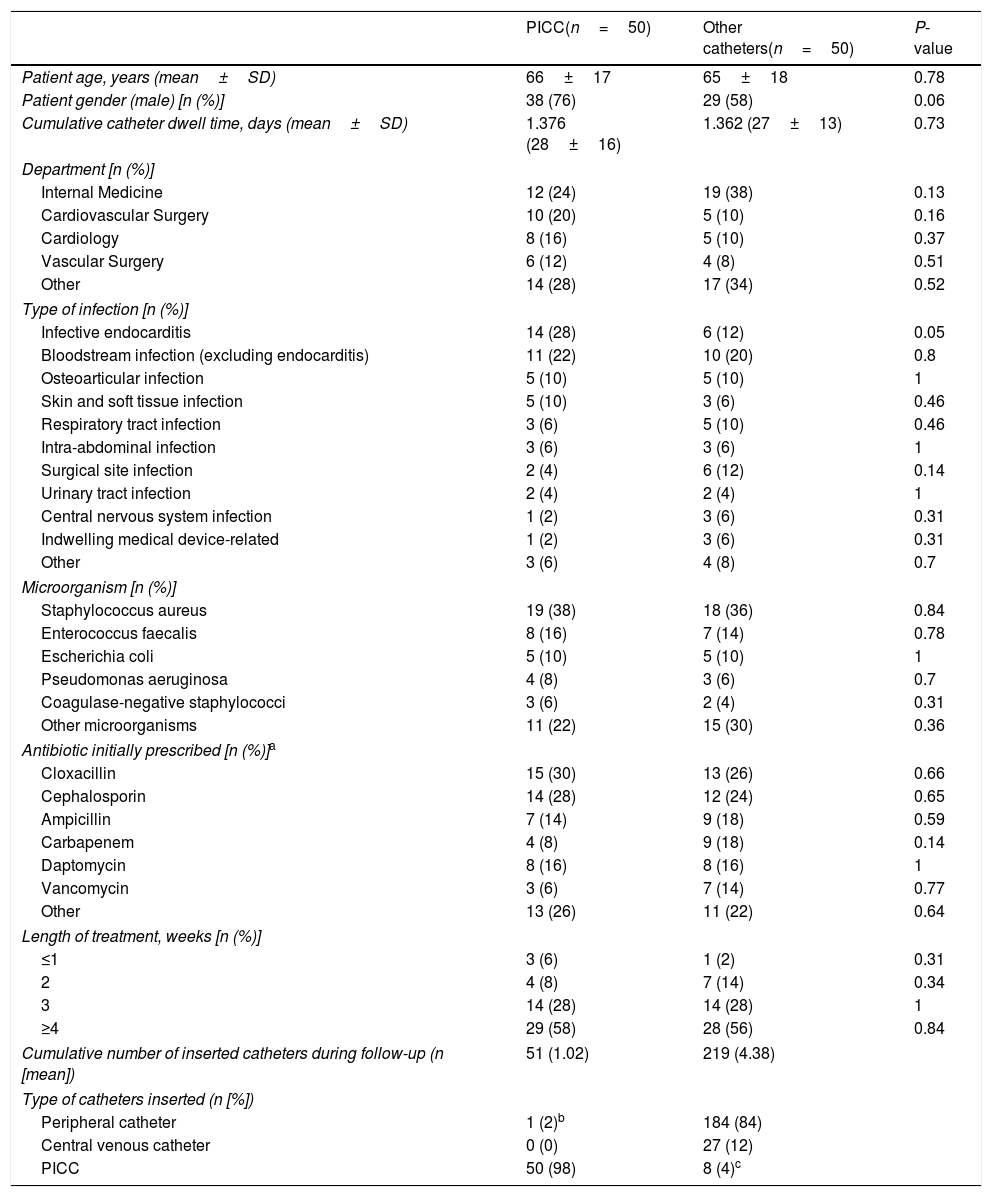
ResultsStudy population and baseline characteristicsThe study included 50 patients each in the PICC group and in the control group. Demographics and clinical characteristics are depicted in Table 1. The total follow-up time was 1376 catheter-days for the PICC group and 1362 days for the control group. In the control group, IV therapy was administered through a peripheral line in most cases, although 8 patients (4.0%) received treatment through a PICC line placed by a different team than the Oncology Nursing Staff after the removal of a previous intravenous catheter.
Demographics and baseline clinical characteristics according to group.
| PICC(n=50) | Other catheters(n=50) | P-value | |
|---|---|---|---|
| Patient age, years (mean±SD) | 66±17 | 65±18 | 0.78 |
| Patient gender (male) [n (%)] | 38 (76) | 29 (58) | 0.06 |
| Cumulative catheter dwell time, days (mean±SD) | 1.376 (28±16) | 1.362 (27±13) | 0.73 |
| Department [n (%)] | |||
| Internal Medicine | 12 (24) | 19 (38) | 0.13 |
| Cardiovascular Surgery | 10 (20) | 5 (10) | 0.16 |
| Cardiology | 8 (16) | 5 (10) | 0.37 |
| Vascular Surgery | 6 (12) | 4 (8) | 0.51 |
| Other | 14 (28) | 17 (34) | 0.52 |
| Type of infection [n (%)] | |||
| Infective endocarditis | 14 (28) | 6 (12) | 0.05 |
| Bloodstream infection (excluding endocarditis) | 11 (22) | 10 (20) | 0.8 |
| Osteoarticular infection | 5 (10) | 5 (10) | 1 |
| Skin and soft tissue infection | 5 (10) | 3 (6) | 0.46 |
| Respiratory tract infection | 3 (6) | 5 (10) | 0.46 |
| Intra-abdominal infection | 3 (6) | 3 (6) | 1 |
| Surgical site infection | 2 (4) | 6 (12) | 0.14 |
| Urinary tract infection | 2 (4) | 2 (4) | 1 |
| Central nervous system infection | 1 (2) | 3 (6) | 0.31 |
| Indwelling medical device-related | 1 (2) | 3 (6) | 0.31 |
| Other | 3 (6) | 4 (8) | 0.7 |
| Microorganism [n (%)] | |||
| Staphylococcus aureus | 19 (38) | 18 (36) | 0.84 |
| Enterococcus faecalis | 8 (16) | 7 (14) | 0.78 |
| Escherichia coli | 5 (10) | 5 (10) | 1 |
| Pseudomonas aeruginosa | 4 (8) | 3 (6) | 0.7 |
| Coagulase-negative staphylococci | 3 (6) | 2 (4) | 0.31 |
| Other microorganisms | 11 (22) | 15 (30) | 0.36 |
| Antibiotic initially prescribed [n (%)]a | |||
| Cloxacillin | 15 (30) | 13 (26) | 0.66 |
| Cephalosporin | 14 (28) | 12 (24) | 0.65 |
| Ampicillin | 7 (14) | 9 (18) | 0.59 |
| Carbapenem | 4 (8) | 9 (18) | 0.14 |
| Daptomycin | 8 (16) | 8 (16) | 1 |
| Vancomycin | 3 (6) | 7 (14) | 0.77 |
| Other | 13 (26) | 11 (22) | 0.64 |
| Length of treatment, weeks [n (%)] | |||
| ≤1 | 3 (6) | 1 (2) | 0.31 |
| 2 | 4 (8) | 7 (14) | 0.34 |
| 3 | 14 (28) | 14 (28) | 1 |
| ≥4 | 29 (58) | 28 (56) | 0.84 |
| Cumulative number of inserted catheters during follow-up (n [mean]) | 51 (1.02) | 219 (4.38) | |
| Type of catheters inserted (n [%]) | |||
| Peripheral catheter | 1 (2)b | 184 (84) | |
| Central venous catheter | 0 (0) | 27 (12) | |
| PICC | 50 (98) | 8 (4)c | |
The most frequent isolated microorganism was Staphylococcus aureus. Most patients in the PICC group received antimicrobial therapy due to infective endocarditis (28%), while in the control group most patients were treated for BSI (20%). Cloxacillin and cephalosporins were the most frequent administered antibiotics in both groups. Antimicrobial therapy was administered for more than 4 weeks in more than half of the included patients in both the case and control groups (58.0% and 56.0%, respectively).
A total of 51 catheters were placed in the PICC group (50 PICC and 1 peripheral catheter), whereas 219 catheters were used in the control group (Table 1).
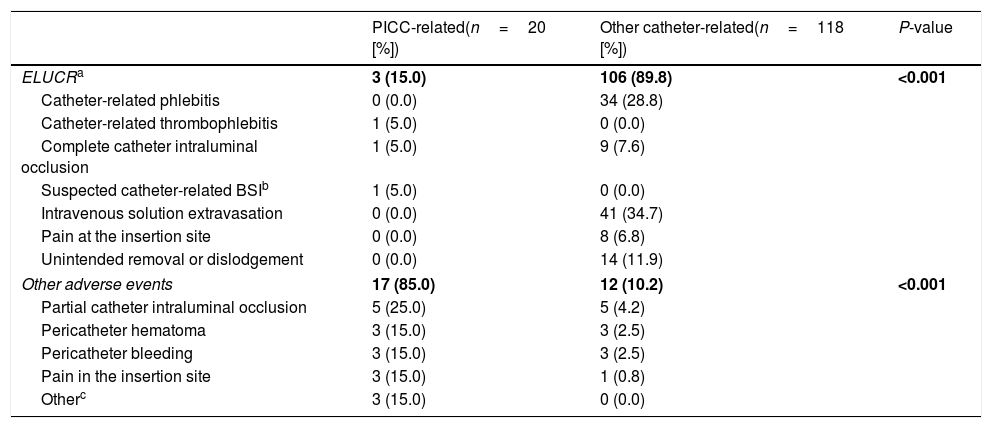
Catheter-related complicationsA total of 20 PICC-related adverse events were diagnosed, including three ELUCRs (detailed in Table 2) that occurred 13, 32 and 35 days after the placing of the PICC line. In two cases, an intravenous-to-oral therapy switch was decided, whereas in another one IV treatment was subsequently continued through a peripheral venous catheter without further complications. No cases of CRBSI occurred in this group.
PICC-related and other catheter-related adverse events.
| PICC-related(n=20 [%]) | Other catheter-related(n=118 [%]) | P-value | |
|---|---|---|---|
| ELUCRa | 3 (15.0) | 106 (89.8) | <0.001 |
| Catheter-related phlebitis | 0 (0.0) | 34 (28.8) | |
| Catheter-related thrombophlebitis | 1 (5.0) | 0 (0.0) | |
| Complete catheter intraluminal occlusion | 1 (5.0) | 9 (7.6) | |
| Suspected catheter-related BSIb | 1 (5.0) | 0 (0.0) | |
| Intravenous solution extravasation | 0 (0.0) | 41 (34.7) | |
| Pain at the insertion site | 0 (0.0) | 8 (6.8) | |
| Unintended removal or dislodgement | 0 (0.0) | 14 (11.9) | |
| Other adverse events | 17 (85.0) | 12 (10.2) | <0.001 |
| Partial catheter intraluminal occlusion | 5 (25.0) | 5 (4.2) | |
| Pericatheter hematoma | 3 (15.0) | 3 (2.5) | |
| Pericatheter bleeding | 3 (15.0) | 3 (2.5) | |
| Pain in the insertion site | 3 (15.0) | 1 (0.8) | |
| Otherc | 3 (15.0) | 0 (0.0) | |
In the control group, a total of 118 catheter-related adverse events were diagnosed (detailed in Table 2), including 106 (89.8%) ELUCRs. Most of them consisted in solution extravasation (34.7%) and phlebitis (28.8%). Almost 12.0% of events were considered unintentional catheter removal or dislodgment. Two out of eight patients in the control group with a PICC (25.0%) required the catheter to be prematurely removed (one of them due to lack of lumen permeability and the other because of a CRBSI). Moreover, another three patients (37.5%) of the control group with a PICC were diagnosed with a catheter-related adverse event (pericatheter hematoma, pericatheter bleeding and partial obstruction, respectively).
The incidence of ELUCRs was significantly lower in the PICC group compared to the control group (0.2 versus 7.7 events per 100 catheter-days, respectively; P-value≤0.001) (Table 3). When the incidence of ELUCR was separately analyzed according to the duration of indwelling catheterization (divided into one-week intervals), differences between both groups were also significant (P-values≤0.001 in the first three weeks of treatment) (Table 3).
Comparison of catheter-related events in both groups.
| PICC group | Control group | P-value | |
|---|---|---|---|
| Duration of indwelling catheterization (n [%]) | |||
| ≤1 weeks | 50 | 50 | – |
| >1 and ≤2 weeks | 47 | 49 | – |
| >2 and ≤3 weeks | 43 | 42 | – |
| >3 weeks | 29 | 28 | – |
| Number of patients that developed an ELUCR according to the week after the beginning of treatment (n [%]) | |||
| ≤1 week | 0 (0.0) | 29 (58.0) | <0.001 |
| >1 and ≤2 weeks | 1 (2.1) | 19 (38.8) | <0.001 |
| >2 and ≤3 weeks | 0 (0.0) | 17 (40.5) | <0.001 |
| >3 weeks | 2 (6.9) | 7 (25.0) | 0.079 |
| Adverse events per 100 catheter-days | 1.5 | 8.7 | <0.001 |
| ELUCR | 0.2 | 7.7 | <0.001 |
| Other adverse events | 1.2 | 0.9 | 0.376 |
ELUCR,: event leading to undesired catheter removal.
We carried out a single-center retrospective study comparing the outcomes of PICCs placed and maintained by a dedicated and specifically trained nursing team with other modalities for IV administration of antimicrobial therapy across different durations of catheterization. In our experience, while only 50 lines were inserted in the PICC group (one per patient), a total of 219 catheters of different types had to be used in the control group. The use of a PICC avoids the patients’ pain associated with the punctures due to repeated catheters exchanges. Moreover, some patients require more than one attempt to achieve a successful catheter insertion, increasing the patients’ discomfort.
According to the results of our study, PICCs are generally safe for administering antimicrobial therapy, even for courses of therapy of 4 weeks or longer. Most of the documented adverse events among patients with a PICC were mild and local (pericatheter bleeding or hematoma and non-specific pain). Only three PICCs had to be removed before the completion of the scheduled course, while in the control group a total of 219 catheters were used for the treatment of the same number of patients and with an equivalent time of IV antimicrobial administration.
To the best of our knowledge, this is the first study to confirm with a comparative design the benefit of using a PICC placed and maintained by a trained team instead of other type of venous catheter in terms of the development of catheter-related adverse events, even for shorts periods of times. No PICC was removed in the first seven days after placement compared to 29 patients who required catheter exchange in the control group, and only one PICC was removed in the second week after placement. Our study is the first to confirm the arbitrarily established previous recommendation for inserting a PICC when the predicted use of a catheter is more than five days.8 It also paves the way for the consideration to use a PICC for even predicted shorter periods of time.
There are some limitations for our study. Due to its retrospective design, we cannot exclude some bias in the comparison of the groups, albeit we considered the type of antibiotic and the length of administration for the selection of controls. Nonetheless, there was an important number of peripheral intravenous catheters used for the administration of antimicrobial treatment in the control group, in spite of the fact that up to 43 patients received at least 14 days of therapy.
Another limitation could be the cost of the PICC line. It has been communicated that the cost of six exchangings of peripheral intravenous catheters is almost the same as the cost of using a PICC once.11 According to the data provided by the Materials Management Department of our center, a PICC line costs 147.29 euros, a CVC 10 euros and a peripheral intravenous catheter 0.57 euros. As such, the entire cost of the PICC group would be as much as 7365 euros while the control group would only reach 1553 euros (374.88 euros if we exclude the 8 PICC inserted in the control group). Nevertheless, PICC would most likely be cost-effective in patients who require long-term therapy by reducing the length of hospitalization as they facilitate the treatment in an outpatient setting. This fact, associated to the less catheter-related complications when the PICC line is placed and maintained by a trained team, would surely be associated with a significative increase of the patient's quality of life.
It is important to mention that this study strengthens the evidence that the incidence of complications associated with PICC is dramatically reduced when a trained team is responsible for placing and maintaining the catheter. A team-based multidisciplinary approach to managing PICC appears to reduce the rate of complications and avoids much of the prematurely PICC removals due to an unconfirmed complication.12–14
On the other hand, it is also important to highlight the higher rates of catheter-related phlebitis and intravenous solution extravasation diagnosed in the control group, which points out the importance of periodic, educational sessions concerning the care of intravenous catheters in order to reduce the burden catheter-related complications.
In conclusion, a PICC placed and maintained by a specifically trained and dedicated nursing team is an excellent alternative to peripheral venous catheters or CVCs for administrating antimicrobial therapy for both short and long periods of treatment.
Ethical approvalApproval was obtained from the local ethical committee.
Informed consentSince the study has a retrospective design, the requirement for obtaining informed consent was waived.
FundingNo external funding was received.
M.F.R. holds a clinical research contract “Miguel Servet” (CP 18/00073) from the Instituto de Salud Carlos III, Spanish Ministry of Economy and Competitiveness.
Conflict of interestThe authors have no conflict of interest to disclose.