Staphylococcus aureus bacteremia (SAB) is still a daily challenge for clinicians. Despite all efforts, the associated mortality and morbidity has not significantly improved in the last 20 years. The available evidence suggests that adherence to some quality-of-care indicators with regard to clinical management is important in improving the outcome of patients, but it is lower than desired in many hospitals; as such, management of patients with SAB by infectious diseases specialists has been demonstrated to contribute in the reduction of the mortality rate of these patients. In this article, the most relevant clinical studies published over the last few years evaluating the efficacy and safety of alternative drugs for the treatment of SAB are reviewed. However, classic drugs are still used in a high proportion of patients because the promising results obtained from in vivo and in vivo studies with these alternative drugs have not translated as frequently as expected into evident superiority in clinical studies. Nevertheless, some data suggest that certain alternatives may offer advantages in specific situations. Overall, an individualised and expert approach is needed in order to decide the best treatment according to the source, severity, complications, patients’ features and microbiological data.
La bacteriemia por Staphylococcus aureus continúa siendo un reto diario para los clínicos. A pesar de todos los esfuerzos realizados, su mortalidad y morbilidad asociadas no han descendido de forma significativa en los últimos 20 años. Existe evidencia de que la adherencia a los indicadores de calidad para su manejo clínico es importante para mejorar el pronóstico de los pacientes, aunque su cumplimiento sigue siendo menor de lo deseado en muchos hospitales; en este sentido, la asistencia por especialistas en enfermedades infecciosas ha demostrado contribuir a reducir la mortalidad de estos pacientes. En este artículo se revisan los estudios clínicos más relevantes realizados en los últimos años con objeto de evaluar la eficacia y la seguridad de los fármacos alternativos a los clásicos. Sin embargo, estos siguen siendo utilizados en un alto porcentaje de pacientes, ya que los prometedores resultados obtenidos por esos fármacos alternativos y determinadas combinaciones en estudios in vitro y modelos animales no se han traducido en una evidente superioridad en los estudios clínicos con la frecuencia que se hubiera esperado. Dicho esto, existen datos que sugieren que determinadas alternativas pueden ofrecer ventajas en situaciones concretas. En general, es necesario un manejo individualizado y experto de los pacientes para decidir la mejor terapia en base al foco, la gravedad y las complicaciones, las características de los pacientes y los datos microbiológicos.
In the industrialised world, the population-based incidence of Staphylococcus aureus bacteremia (SAB) ranges from 10 to 30 per 100,000 person-years and causes 2 to 10 deaths/100,000 population per year.1 SAB attracts the interest of medical scientists: it generates more than 1500 results in PubMed.
S. aureus infections that lead to bacteremia are frequently acquired in the context of healthcare, and preventive measures may reduce S. aureus bacteremia rates, but the population at risk continues to increase. In addition, it must be recognised that we have not been able to significantly and universally improve its prognosis in the last 20 years.2 Beyond mortality, the morbidity and economical costs of SAB continues to be very high, as surgical procedures (sometimes with loss of prosthetic or vascular implants), and additional admission days are frequently needed, and finally patient's quality of life may be severely impaired. Preventive measures for healthcare-associated SAB includes typical infection control actions and decolonisation of patients undergoing high-risk procedures. Curiously, statin use was associated with a dose-dependent lower risk of developing community-acquired SAB in a large Danish population-based study, with adjusted odds ratios (OR) of 0.84 (95% confidence interval [CI], 0.68–1.04) for current users using <20mg/day, 0.71 (95% CI, 0.58–0.87) for 20–39mg/day, and 0.63 (95% CI, 0.49–0.81) for ≥40mg/day.3 The pathophysiological bases of this association are not clear, so these results should be reproduced before any recommendation can be done.
Outcome predictors and potential targets for better clinical managementThe outcome of SAB depends on three aspects: the bacteria, the patient, and the clinical management. Regarding the bacteria, methicillin-resistance have been repeatedly associated with worse outcomes even after controlling for confounders. While some methicillin-resistant S. aureus (MRSA) may be more virulent, the main reason for this increased mortality is probably related to the fact that MRSA are resistant to classic first line anti-staphylococcal drugs and increases the risk of inappropriate empirical therapy. Infections with high bacterial load as shown by shorter time to blood cultures positivity have also been associated with worse outcomes.4 The impact of vancomycin minimum inhibitory concentration (MIC) on the outcome of patients with MRSA bacteraemia attracted considerable attention a few years ago, curiously at the time when some new drugs active against MRSA were commercialised. However, the results from different systematic reviews and meta-analysis are contradictory, probably due to differences in the methodology used in the individual studies and their limitations, because an adequate control of the many host-dependent and treatment-related confounders is complex.5,6 Unexpectedly, vancomycin MIC was also associated to worse prognosis in patients with methicillin-susceptible S. aureus (MSSA) not treated with this antibiotic in some studies, suggesting that reduced vancomycin susceptibility could itself be a marker for some other intrinsic property of these isolates that would cause a poorer outcome.7,8 However, some later studies did not find any association between reduced vancomycin susceptibility and higher mortality, rate of complicated SAB or severity at presentation.9 Therefore, whether this should be considered for therapeutic decisions is controversial. In addition, the impact of several bacterial factors that might serve as therapeutic targets has been studied. So far, clonality, different virulence factors (alpha-toxin, TSST-1, PVL, etc.) or agr type and its dysfunction have not been consistently associated with worse outcomes.10,11 Therefore, more studies are needed to identify potential therapeutic bacterial targets.
Regarding the host, different putative outcome biomarkers have also been studied. Again, many of the studies performed are flawed by an inadequate control of confounders. Interleukin IL-8 (a neutrophil-recruiting chemokine) and CCL2 (a myeloid cell-recruiting chemokine) showed the strongest association with mortality among 13 studied proteins, and baseline IL-17A levels were higher in patients with persistent SAB or those with endovascular and metastatic tissue infections in a study.12 Data from a recent trial failed to demonstrate an association of IL-10 serum levels and outcome.13 Again, the numerous circumstances that determine the prognosis of SAB makes it difficult to assess the real relevance of each of these biomarkers individually. Regarding phenotypic features of the patients, worse outcomes are consistently associated with older age, a higher Charlson comorbidity index or Pitt score, community acquisition (probably as a marker for endocarditis) or liver cirrhosis.14,15 Male gender might be associated with increased risk of SAB, with male-to-female ratios of 1.5 and a adjusted hazard ratio for 30-day mortality of 1.30 (95% CI, 1.11–1.53).16,17 The basis for this increased risk is not well understood.
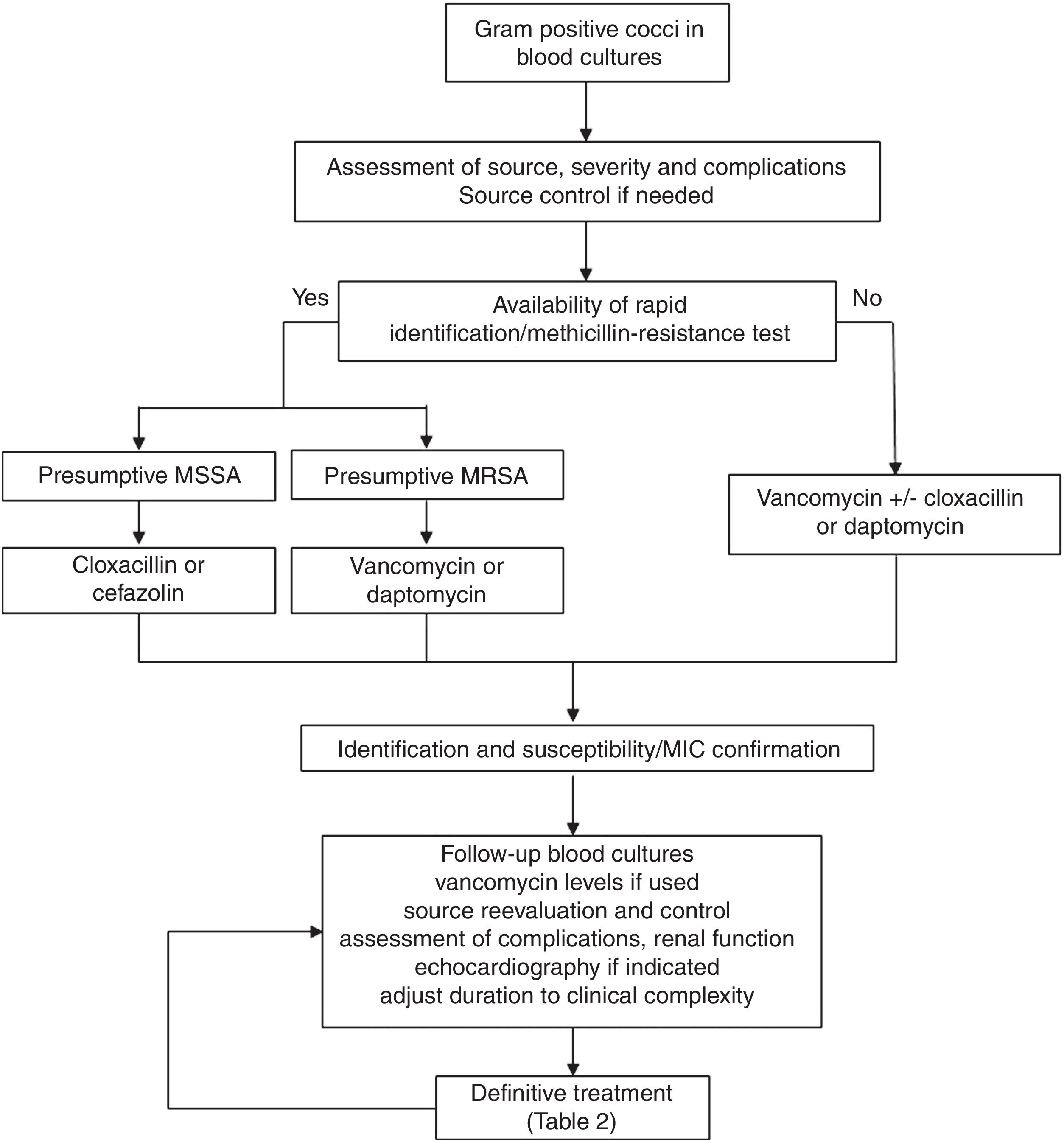
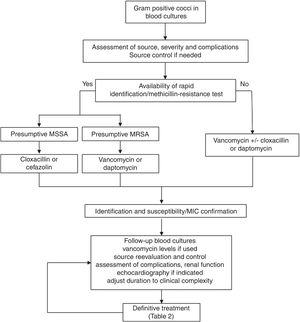
Regarding the clinical management, a key aspect is the concept of “complicated bacteraemia”. Since its definition in 1998,18 some consensus has been reached for its criteria, including the presence of a foreign indwelling implant or intravascular device, positive follow-up blood cultures or fever lasting more than 72h while on active treatment, evidence of metastatic sites of infection or deep-seated focus, and infectious endocarditis.19 At present, the duration of antimicrobial treatment in uncomplicated cases is 10–14 days, while it is at least 28 days since the first negative follow-up blood culture in case of complicated SAB; also, echocardiography must be done in these cases (see Fig. 1). Transoesophagic echocardiography (TOE) may be reserved for some patients, such as those at high-risk of endocarditis (particularly, in case of predisposing valve diseases, prosthetic valves and intracardiac devices) or with a high clinical suspicion and poor echocardiographic window. A scoring system to guide the indications for TEE has been developed based on a cohort of 678 patients from a single hospital.20 The score included community acquisition, presence of a cardiac device and persistent bacteremia for at least 72h, and showed a high specificity (96%) but low sensitivity (21%) for the day-1 model, with a high sensitivity (98.8%) and negative predictive value (98.5%) for the day-5 model. Another score for day 2 of the bacteremia included similar variables and showed a negative predictive value of 98.8% (95% CI 98.4–99.4) and a sensitivity of 95.8% (95% CI 94.3–97.8) for a score value ≤2.21 Unfortunately, none of these scores have been so far validated in external cohorts. Anyhow, the best moment to perform an echocardiography is not well established, probably because it is essential to individualise the decision on the bases of the type of clinical presentation and characteristics of the patients, once the follow-up cultures results are available.
Consultation or direct management by infectious diseases specialists has been associated with better prognosis in different studies and meta-analysis.22 However, standardisation of management is still a problem. Some quality-of-care indicators for the management of SAB have been identified; unfortunately, adherence to these indicators in clinical practice is lower than desirable as shown by the results of two recent published surveys carried out in United States and Canada.23 The application of a bundle including six of these evidence-based indicators (early treatment with cloxacillin for MSSA, early source control, follow-up blood cultures in all cases, echocardiography if indicated, vancomycin dose based on trough levels if this drug is used, and duration of treatment of at least 14 days in uncomplicated SAB and 4 weeks in complicated cases) using a structured intervention was shown to increase the adherence to the indicators and was associated with lower mortality in a quasi-experimental multicentre study24; this approach has been validated in later studies.25,26 A proposal for the clinical management of S. aureus bacteremia is included in Fig. 1.
Antimicrobial therapyUntil recently, the preferred options for the treatment of SAB had remained unchanged for decades. The fact that SAB-associated morbidity and mortality continue to be high had triggered the search for new alternatives; in fact, there are many published studies with in vitro and in vivo evaluation of the activity of new drugs and new combinations. However, the promising results of these models did not always translate to improved outcomes in clinical studies. Therefore, in this review we will mainly discuss the results of clinical studies. The different drugs used for SAB are specified in Table 1, and a summary of the recommended regimens in Table 2. The published studies included in this review are included in Table 3.
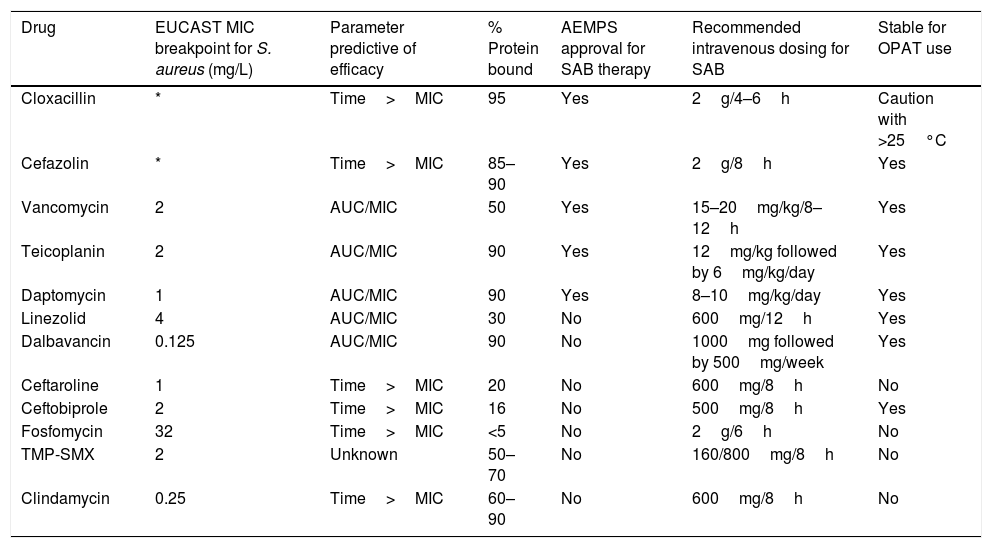
Antimicrobials used for the treatment of S. aureus bacteremia.
| Drug | EUCAST MIC breakpoint for S. aureus (mg/L) | Parameter predictive of efficacy | % Protein bound | AEMPS approval for SAB therapy | Recommended intravenous dosing for SAB | Stable for OPAT use |
|---|---|---|---|---|---|---|
| Cloxacillin | * | Time>MIC | 95 | Yes | 2g/4–6h | Caution with >25°C |
| Cefazolin | * | Time>MIC | 85–90 | Yes | 2g/8h | Yes |
| Vancomycin | 2 | AUC/MIC | 50 | Yes | 15–20mg/kg/8–12h | Yes |
| Teicoplanin | 2 | AUC/MIC | 90 | Yes | 12mg/kg followed by 6mg/kg/day | Yes |
| Daptomycin | 1 | AUC/MIC | 90 | Yes | 8–10mg/kg/day | Yes |
| Linezolid | 4 | AUC/MIC | 30 | No | 600mg/12h | Yes |
| Dalbavancin | 0.125 | AUC/MIC | 90 | No | 1000mg followed by 500mg/week | Yes |
| Ceftaroline | 1 | Time>MIC | 20 | No | 600mg/8h | No |
| Ceftobiprole | 2 | Time>MIC | 16 | No | 500mg/8h | Yes |
| Fosfomycin | 32 | Time>MIC | <5 | No | 2g/6h | No |
| TMP-SMX | 2 | Unknown | 50–70 | No | 160/800mg/8h | No |
| Clindamycin | 0.25 | Time>MIC | 60–90 | No | 600mg/8h | No |
SAB: S. aureus bacteremia; OPAT: outpatient antimicrobial therapy; TMP-SMX: trimethoprim sulfamethoxazole; AUC: area under the curve; MIC: minimum inhibitory concentration; AEMSP: Spanish Agency of Medicines and Health Products; (*): Susceptibility of staphylococci to cephalosporins is inferred from cefoxitin.
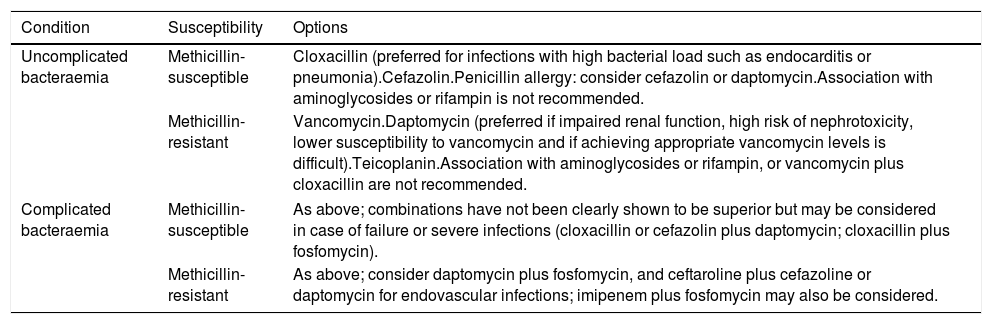
Summary of recommended drugs for the definitive treatment of S. aureus bacteraemia.
| Condition | Susceptibility | Options |
|---|---|---|
| Uncomplicated bacteraemia | Methicillin-susceptible | Cloxacillin (preferred for infections with high bacterial load such as endocarditis or pneumonia).Cefazolin.Penicillin allergy: consider cefazolin or daptomycin.Association with aminoglycosides or rifampin is not recommended. |
| Methicillin-resistant | Vancomycin.Daptomycin (preferred if impaired renal function, high risk of nephrotoxicity, lower susceptibility to vancomycin and if achieving appropriate vancomycin levels is difficult).Teicoplanin.Association with aminoglycosides or rifampin, or vancomycin plus cloxacillin are not recommended. | |
| Complicated bacteraemia | Methicillin-susceptible | As above; combinations have not been clearly shown to be superior but may be considered in case of failure or severe infections (cloxacillin or cefazolin plus daptomycin; cloxacillin plus fosfomycin). |
| Methicillin-resistant | As above; consider daptomycin plus fosfomycin, and ceftaroline plus cefazoline or daptomycin for endovascular infections; imipenem plus fosfomycin may also be considered. | |
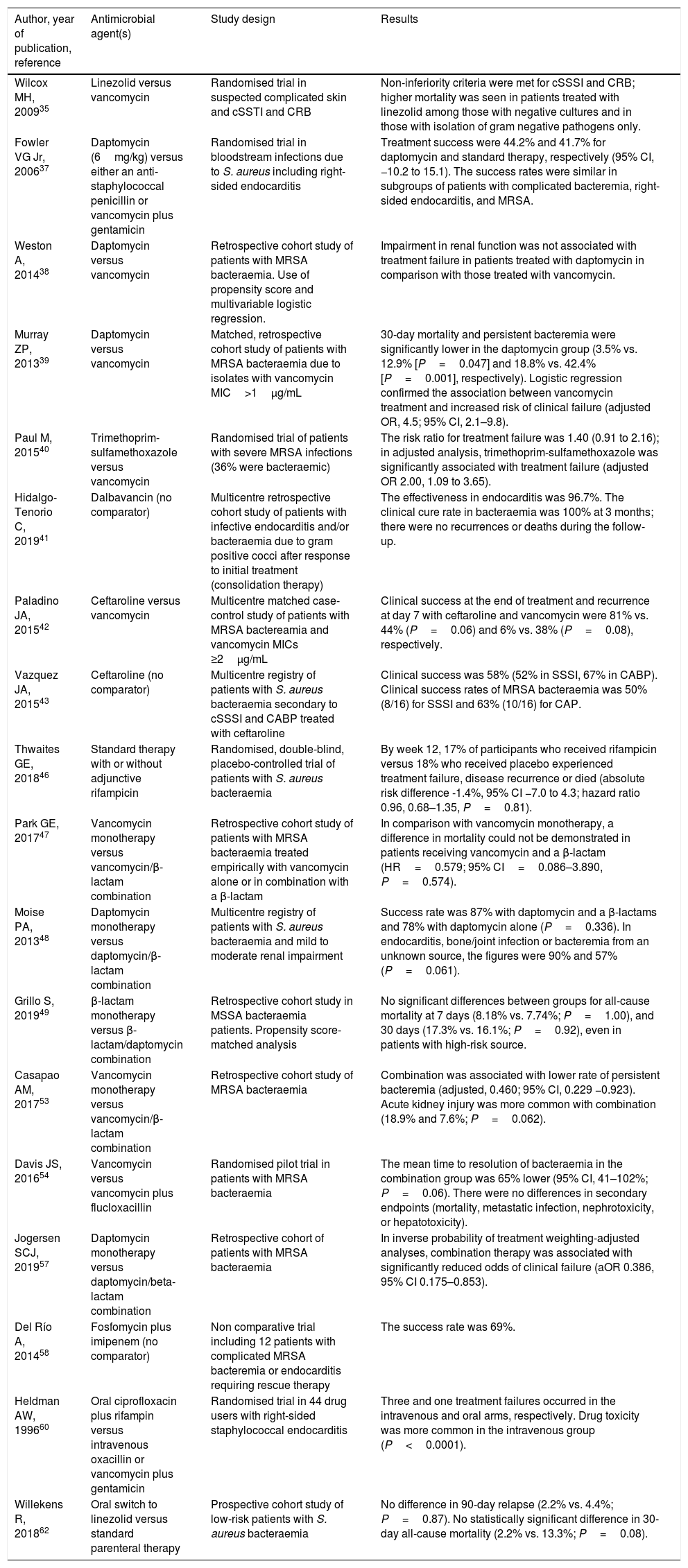
Summary of clinical studies on antimicrobial therapy for S. aureus bacteraemia included in this review.
| Author, year of publication, reference | Antimicrobial agent(s) | Study design | Results |
|---|---|---|---|
| Wilcox MH, 200935 | Linezolid versus vancomycin | Randomised trial in suspected complicated skin and cSSTI and CRB | Non-inferiority criteria were met for cSSSI and CRB; higher mortality was seen in patients treated with linezolid among those with negative cultures and in those with isolation of gram negative pathogens only. |
| Fowler VG Jr, 200637 | Daptomycin (6mg/kg) versus either an anti-staphylococcal penicillin or vancomycin plus gentamicin | Randomised trial in bloodstream infections due to S. aureus including right-sided endocarditis | Treatment success were 44.2% and 41.7% for daptomycin and standard therapy, respectively (95% CI, −10.2 to 15.1). The success rates were similar in subgroups of patients with complicated bacteremia, right-sided endocarditis, and MRSA. |
| Weston A, 201438 | Daptomycin versus vancomycin | Retrospective cohort study of patients with MRSA bacteraemia. Use of propensity score and multivariable logistic regression. | Impairment in renal function was not associated with treatment failure in patients treated with daptomycin in comparison with those treated with vancomycin. |
| Murray ZP, 201339 | Daptomycin versus vancomycin | Matched, retrospective cohort study of patients with MRSA bacteraemia due to isolates with vancomycin MIC>1μg/mL | 30-day mortality and persistent bacteremia were significantly lower in the daptomycin group (3.5% vs. 12.9% [P=0.047] and 18.8% vs. 42.4% [P=0.001], respectively). Logistic regression confirmed the association between vancomycin treatment and increased risk of clinical failure (adjusted OR, 4.5; 95% CI, 2.1–9.8). |
| Paul M, 201540 | Trimethoprim-sulfamethoxazole versus vancomycin | Randomised trial of patients with severe MRSA infections (36% were bacteraemic) | The risk ratio for treatment failure was 1.40 (0.91 to 2.16); in adjusted analysis, trimethoprim-sulfamethoxazole was significantly associated with treatment failure (adjusted OR 2.00, 1.09 to 3.65). |
| Hidalgo-Tenorio C, 201941 | Dalbavancin (no comparator) | Multicentre retrospective cohort study of patients with infective endocarditis and/or bacteraemia due to gram positive cocci after response to initial treatment (consolidation therapy) | The effectiveness in endocarditis was 96.7%. The clinical cure rate in bacteraemia was 100% at 3 months; there were no recurrences or deaths during the follow-up. |
| Paladino JA, 201542 | Ceftaroline versus vancomycin | Multicentre matched case-control study of patients with MRSA bactereamia and vancomycin MICs ≥2μg/mL | Clinical success at the end of treatment and recurrence at day 7 with ceftaroline and vancomycin were 81% vs. 44% (P=0.06) and 6% vs. 38% (P=0.08), respectively. |
| Vazquez JA, 201543 | Ceftaroline (no comparator) | Multicentre registry of patients with S. aureus bacteraemia secondary to cSSSI and CABP treated with ceftaroline | Clinical success was 58% (52% in SSSI, 67% in CABP). Clinical success rates of MRSA bacteraemia was 50% (8/16) for SSSI and 63% (10/16) for CAP. |
| Thwaites GE, 201846 | Standard therapy with or without adjunctive rifampicin | Randomised, double-blind, placebo-controlled trial of patients with S. aureus bacteraemia | By week 12, 17% of participants who received rifampicin versus 18% who received placebo experienced treatment failure, disease recurrence or died (absolute risk difference -1.4%, 95% CI −7.0 to 4.3; hazard ratio 0.96, 0.68–1.35, P=0.81). |
| Park GE, 201747 | Vancomycin monotherapy versus vancomycin/β-lactam combination | Retrospective cohort study of patients with MRSA bacteraemia treated empirically with vancomycin alone or in combination with a β-lactam | In comparison with vancomycin monotherapy, a difference in mortality could not be demonstrated in patients receiving vancomycin and a β-lactam (HR=0.579; 95% CI=0.086–3.890, P=0.574). |
| Moise PA, 201348 | Daptomycin monotherapy versus daptomycin/β-lactam combination | Multicentre registry of patients with S. aureus bacteraemia and mild to moderate renal impairment | Success rate was 87% with daptomycin and a β-lactams and 78% with daptomycin alone (P=0.336). In endocarditis, bone/joint infection or bacteremia from an unknown source, the figures were 90% and 57% (P=0.061). |
| Grillo S, 201949 | β-lactam monotherapy versus β-lactam/daptomycin combination | Retrospective cohort study in MSSA bacteraemia patients. Propensity score-matched analysis | No significant differences between groups for all-cause mortality at 7 days (8.18% vs. 7.74%; P=1.00), and 30 days (17.3% vs. 16.1%; P=0.92), even in patients with high-risk source. |
| Casapao AM, 201753 | Vancomycin monotherapy versus vancomycin/β-lactam combination | Retrospective cohort study of MRSA bacteraemia | Combination was associated with lower rate of persistent bacteremia (adjusted, 0.460; 95% CI, 0.229 −0.923). Acute kidney injury was more common with combination (18.9% and 7.6%; P=0.062). |
| Davis JS, 201654 | Vancomycin versus vancomycin plus flucloxacillin | Randomised pilot trial in patients with MRSA bacteraemia | The mean time to resolution of bacteraemia in the combination group was 65% lower (95% CI, 41–102%; P=0.06). There were no differences in secondary endpoints (mortality, metastatic infection, nephrotoxicity, or hepatotoxicity). |
| Jogersen SCJ, 201957 | Daptomycin monotherapy versus daptomycin/beta-lactam combination | Retrospective cohort of patients with MRSA bacteraemia | In inverse probability of treatment weighting-adjusted analyses, combination therapy was associated with significantly reduced odds of clinical failure (aOR 0.386, 95% CI 0.175–0.853). |
| Del Río A, 201458 | Fosfomycin plus imipenem (no comparator) | Non comparative trial including 12 patients with complicated MRSA bacteremia or endocarditis requiring rescue therapy | The success rate was 69%. |
| Heldman AW, 199660 | Oral ciprofloxacin plus rifampin versus intravenous oxacillin or vancomycin plus gentamicin | Randomised trial in 44 drug users with right-sided staphylococcal endocarditis | Three and one treatment failures occurred in the intravenous and oral arms, respectively. Drug toxicity was more common in the intravenous group (P<0.0001). |
| Willekens R, 201862 | Oral switch to linezolid versus standard parenteral therapy | Prospective cohort study of low-risk patients with S. aureus bacteraemia | No difference in 90-day relapse (2.2% vs. 4.4%; P=0.87). No statistically significant difference in 30-day all-cause mortality (2.2% vs. 13.3%; P=0.08). |
cSSSI: complicated skin and skin structure infection; CRB: Catheter-related bacteremia; OR: Odds ratio; CI: confidence interval; MRSA: methicillin resistant Staphylococcus aureus; CABP: community-acquired bacterial pneumonia.
Penicillinase-resistant penicillins (cloxacillin, oxacillin) are usually considered the drugs of choice for MSSA bacteremia. However, cefazolin, a first generation cephalosporin, has several potential advantages: available data suggest it may have lower liver and renal toxicity, lower rate of phlebitis, and better stability at room temperature, making it more convenient for outpatient parenteral antimicrobial therapy (OPAT).27 In case of patients under hemodialysis, the longer half-life of cefazolin (1g/day or 2 gr after the dialysis session) allows to reduce the manipulation of the dialysis catheter. Despite it was traditionally considered a less efficacious drug because of showing lower in vitro activity when exposed to higher bacterial concentrations (the so-called inoculum effect), the results of several recent meta-analysis have challenged that idea by showing similar efficacy and lower renal toxicity.28–30 The inoculum effect seems to be related to the inactivation of the drug by a type A beta-lactamase codified by blaZ gene. An observational study including 185 isolates from 5 centres in USA found the blaZ gene in 77% of them; 27% had a >4-fold increase in the cefazolin MIC when exposed to a high inoculum, and 4% were not-susceptible to cefazolin, all of which harboured blaZ genes.31 Some data support a clinical impact of the inoculum effect30; if this is confirmed in future studies, it might be useful to develop a rapid technique to detect such isolates. At this moment and until the results of an ongoing randomised trial are available,32 it seems prudent to avoid the use of cefazolin in severe infections with high bacterial load such as endocarditis or pneumonia. The use of other cephalosporins or penicillins do not seem to provide any advantage over cefazolin. For penicillin-allergic patients, and contrary to what is frequently believed, there is no evidence for an increased risk of anaphylaxis when exposed to cefazolin.33 Also, switching to cefazolin in patients with non-IgE, immune mediated reactions to nafcillin seems to be safe.34 It should be reminded that vancomycin is less efficacious than antistaphylococcal penicillins. Whenever cefazolin is not considered suitable in penicillin-allergic patients, the therapeutic options are similar to those in case of MRSA (see below).
In the case of MRSA bacteraemia, vancomycin has been the reference drug for years. The issue of vancomycin dosing has been extensively reviewed before and will not be discussed here. Two reasons triggered the investigation of alternatives to vancomycin: the fact that has been shown to be less efficacious than penicillins when used for MSSA and the emergence of MRSA isolates with low susceptibility to vancomycin. Some advantages of vancomycin are the long experience gained with its use, and the possibility of measuring blood levels in most centres.
Teicoplanin and linezolid were early candidates for becoming alternatives to vancomycin. Teicoplanin is also a glycopeptide active against MRSA; the accumulated experience in SAB is lower than with vancomycin, but it seems to have lower vascular and renal toxicity than vancomycin. Due to its difficulty to rapidly achieve adequate serum levels, administration of a loading dose of 6mg/kg followed by 3mg/kg once daily is recommended. Measurement of blood levels is not available in most centres. In a randomised trial of patients with suspected catheter-related bacteraemia and skin-structure infections (SSTI), linezolid was non-inferior to vancomycin overall, but was associated with higher mortality in the subgroups of patients with negative cultures and in those with gram negative pathogens only35; in a previous pooled analysis of five randomised studies including 53 patients with MRSA bacteraemia, no significant differences in clinical or microbiological cure rates versus vancomycin could be found (OR, 1.47; 95% CI, 0.50–4.34; and 0.83; 95% CI, 0.37–1.87, respectively).36 Linezolid might be preferred over vancomycin in cases of bacteraemic pneumonia due to MRSA, but its superiority is controversial.
Daptomycin was shown to be non-inferior to standard therapy in MSSA and MRSA bacteraemia in a randomised trial,37 which was criticised because it included right-side endocarditis cases and allowed treating patients with MSSA with vancomycin. Despite the theoretical advantages of daptomycin over vancomycin (faster bactericidal and anti-biofilm activity, favourable pharmacokinetic profile or lower nephrotoxicity), comparative studies performed so far are flawed by important limitations and therefore, whether it is truly superior is controversial. The recommended dose of daptomycin for SAB have been increased since its approval from 6 to 8–10mg/kg/d in order to avoid the emergence of resistance during treatment. Most experts consider that daptomycin would be the drug of choice for MRSA bacteraemia in patients with impaired renal function, high risk of nephrotoxicity, reduced susceptibility to vancomycin (defined as high MIC within the range of susceptibility), and whenever achieving appropriate vancomycin trough levels is difficult.37–39 Daptomycin should not be used for S. aureus pneumonia since it is inactivated by pulmonary surfactant. Of note, whether there is an association of worse outcomes in patients treated with vancomycin when their isolates show reduced susceptibility to vancomycin is debatable (see above), since the determination of MIC is not highly reproducible, particularly when gradient strips are used. In other circumstances, there is not a clear consensus: while some experts consider that vancomycin should be abandoned in all or most circumstances, we think that it still has a role in many patients, particularly in those with uncomplicated bacteremia caused by fully susceptible isolates. Trimetoprim-sulfamethoxazole is frequently active against MRSA isolates; however, it did not achieve non-inferiority to vancomycin in a randomised trial of serious MRSA infection including bacteraemia.40 Data on clindamycin in bacteraemia are scarce.
Some newer drugs active against MRSA such as the lipoglycopeptides dalbavancin and oritavancin, or the anti-MRSA cephalosporins ceftaroline and ceftobiprole, are now available. Curiously, none of them have been specifically approved for MRSA bacteraemia despite it is probably the indication where new drugs are more needed. Due to its intrinsic characteristics (one weekly intravenous dose), dalbavancin may be particularly useful as sequential treatment allowing early discharge of patients in the absence of OPAT programmes. It showed promising results in multicentre, observational, retrospective study including 83 patients with bacteraemia caused by gram positive bacteria (59%) and infective endocarditis (49%), as sequential therapy; crude mortality at 12 months was 8.8%, and the rate of therapeutic failure was 2.9%.41 However, adequate comparative studies are needed. Regarding ceftaroline, a multicentre, case-control matched study compared the outcomes of patients receiving ceftaroline as rescue therapy after vancomycin failure with those treated with vancomycin; patients treated with ceftaroline cleared the bacteremia more rapidly and had a higher rate of clinical success.42 An analysis of the registry of patients treated with ceftaroline included 48 patients with SAB from SSTI or community-acquired pneumonia (CAP).43 The clinical success was 58% (52% for SSTI, and 67% for CAP); in those with MRSA bacteremia, the figures were 50% and 63%, respectively. The published experience with ceftobiprole as monotherapy in SAB is limited; a recent report provided a post hoc analysis of 95 patients with bacteraemia included in four randomised trials44; 30-day mortality were similar to the different comparators; the number of patients with MRSA bacteremia (18) is too low to draw any conclusion. Therefore, more data are needed for these new drugs.
Combination therapyThe combination of cloxacillin and vancomycin has been recommended for patients in whom S. aureus is isolated from blood cultures, pending susceptibility results, particularly if risk factors for MRSA are present.45 Initially, this recommendation was based on the higher efficacy of cloxacillin for MSSA, and in order to avoid a delay in administering an active drug in the case of MRSA. Beyond that, definitive combination therapy has been investigated as a potential way to improve the outcome of SAB, both for MSSA and MRSA, particularly in the case of complicated SAB or failure of monotherapy.45
The use of aminoglycosides in combination with cloxacillin or vancomycin has been abandoned as they increase toxicity without any evident benefit. Rifampin has long been considered a good candidate for combination due to its in vitro synergy, antibiofilm activity, intracellular penetration and rapid bactericidal action. However, a recent landmark double-blind randomised trial evaluated the benefits of adding rifampin (600mg or 900mg per day according to weight, oral or intravenous) for 2 weeks to standard therapy, and found no differences in the rate of treatment failure, disease recurrence, or mortality.46 However, rifampin might still be considered in patients with cases of valve, endovascular or joint prosthesis, who were underrepresented in the trial.
For MSSA, different combinations have shown synergy in vitro and in vivo; however, data from clinical studies are scarce or limited so far, and have not shown a consistent benefit. The combination of beta-lactams with vancomycin did not show lower mortality than monotherapy with vancomycin for bacteraemia due to MSSA (in any case, vancomycin is not the drug of choice for MSSA), in a retrospective cohort study.47 An analysis of the registry of patients treated with daptomycin including patients with moderate renal impairment suggested that the combination of daptomycin and a beta-lactam might be beneficial in patients with SAB with endocarditis or a bone/joint infection or unknown source.48 However, a later propensity score–matched analysis could not find significant difference in mortality between patients treated with a beta-lactam (cloxacillin or cefazolin) in monotherapy or in combination with daptomycin at 10mg/kg/day, even in the subset of patients with high risk source of infection.49 Fosfomycin is active against most S. aureus isolates, and is an attractive drug for combinations according to in vivo studies and animal models; a randomised trial comparing cloxacillin and fosfomycin versus cloxacillin monotherapy will soon start recruitment.50
Also, different combinations are being studied for MRSA bacteremia. The combination of beta-lactams, trimethoprim-sulfamethoxazole or fosfomycin, mostly with vancomycin, daptomycin or dalvabancin have been studied in vitro and in vivo models, frequently with good results.51 In fact, the results of a few retrospective cohort studies suggested that combination of vancomycin with some beta-lactams might be associated with improvement but only in “secondary” outcomes such as microbiologic or clinical failure or persistent bacteremia, but reduction in mortality could not be shown.52,53 A pilot randomised trial also found shorter duration of MRSA bacteremia in patients treated with vancomycin and flucloxacillin compared to vancomycin alone54; however, this pilot trial led to a bigger randomised study, in which vancomycin or daptomycin alone were compared with combination with flucloxacillin or cefazolin.55 The vast majority of patients received with vancomycin. Unfortunately, the trial had to be prematurely stopped because of higher rate of acute renal injury in the combination arm, which was more frequent among patients receiving vancomycin and flucloxacillin. It should be noted that the combination of piperacillin-tazobactam with vancomycin has also been associated with increased risk of acute renal injury compared to vancomycin monotherapy, which reinforce the concept that penicillin derivatives particularly when combined with vancomycin, may be nephrotoxic.56 Probably the combination of vancomycin and cefazolin still merits to be clinically evaluated.
The combination of daptomycin and beta-lactams has also been found to be synergistic in vitro and in animal studies. A retrospective cohort study found that patients treated with daptomycin and a beta-lactam (mostly, cefepime and cefazoline) had a lower adjusted probability of failure than patients treated with daptomycin in monotherapy.57 A randomised trial comparing daptomycin and ceftaroline vs. vancomycin or daptomycin in monotherapy was prematurely stopped because there were 7 deaths among 23 patients treated with monotherapy (all had intravascular infections, and 6 of them had been treated with vancomycin) while no deaths occurred in the combination arm (17 patients).13 The consequence is somehow paradoxical; despite being stopped because of a mortality difference, the small sample size in this study may preclude a change clinical practice since it is difficult to know which patients would benefit from this more expensive regimen, or if any safety issue would arise if more patients are treated. At this point and until more data are available, it seems reasonable to consider this combination for patients with MRSA bacteraemia from endovascular sources. The combination should be compared to daptomycin alone and/or daptomycin in combination with a beta-lactam in patients with MRSA bacteraemia.
Combinations including intravenous fosfomycin are also being investigated. It has the potential limitation of its high sodium content, which may be a problem for patients with heart failure, mostly if used in infective endocarditis. Curiously, fosfomycin and imipenem showed to be highly active in vitro against MRSA; a small randomised trial in complicated MRSA bacteraemia or endocarditis compared 8 patients treated with intravenous fosfomycin (2g/6h) plus imipenem (1g/6h) with 7 treated with vancomycin.58 None of the cases receiving combination therapy presented persistent bacteremia after 72h of therapy, and success rate was similar between the two arms. A trial comparing daptomycin with daptomycin plus fosfomycin alone has completed recruitment; a preliminary report of the results suggested a higher treatment success with the combination.59
Therefore, while combination therapy in MSSA has not proved to be superior to monotherapy so far, the results of several studies suggest that some combinations are promising for MRSA and might improve the results of vancomycin monotherapy.
Switch to oral therapyBecause the duration of treatment of SAB must be at least 10–14 days in non-complicated SAB and 4 weeks in complicated cases, patients with SAB have traditionally needed prolonged hospitalisation in order to receive intravenous antibiotic treatment. OPAT programmes allow a significant reduction in the hospital stay, but such programmes are not available in all centres, and still the patients need a vascular catheter. Therefore, the possibility of switching from intravenous to oral treatment in selected patients with SAB, as is done in many other infections, is being investigated. Some previous experience with oral therapy with ciprofloxacin and rifampin for patients with right-sided S. aureus endocarditis was encouraging.60 An ongoing randomised trial will try to demonstrate the non-inferiority of switching to oral vs continuing with intravenous treatment in patients with non-complicated SAB after clinical stability is reached. Oral treatment in the trial may be done with trimethoprim-sulfamethoxazole, clindamycin or linezolid.61 Data from a recent propensity score-matched cohort study with limited statistical power suggested that switching to oral linezolid may be a good alternative to maintaining intravenous therapy in selected patients (90-day relapse rates, 2.2% vs. 4.4%; P=0.87).62 If oral therapy is demonstrated to be effective and safe, the best oral options would need to be studied, depending on the susceptibility of the isolates and features of the patients. Meanwhile, the role of infectious diseases specialists in these decisions is also crucial.
ConclusionsSAB is still associated with a considerable mortality and morbidity, and therefore there is room for improving the outcome of patients with SAB. Clinical management of SAB is complex; the results of recent randomised, quasi-experimental and observational studies have provided useful information, and the results of some ongoing trials are awaited. In uncomplicated cases caused by MSSA, cloxacillin is the drug of choice; however, in low-inoculum infections, cefazolin may provide some advantages. For MRSA bacteraemia, vancomycin should be considered in uncomplicated cases due to fully susceptible isolates; in other circumstances, daptomycin may be preferred, except in pneumonia. In the case of clinical or microbiological failure, and in severe or complex infections, different combination regimens should be considered. Overall and so far, the newer drugs and combination regimens show some promising results but must still consistently demonstrate their superiority over the traditional drugs, and importantly, in which subgroups of patients they might be particularly beneficial. Meanwhile, management based on quality-of-care indicators by infectious diseases specialist is mandatory to guarantee the best results.
Conflict of interestsLELC has served as scientific advisor for Novartis, speaker for MSD, Pfizer, Angelini, and ViiV, and has served as trainer for MSD and ViiV. JGA has received grants for attending meetings from Angelini, Pfizer and Gilead, and has participated in accredited educational activities funded by MSD. JRB has participated in unrestricted accredited educational activities funded by MSD.
FundingThe authors received funds for research from Plan Nacional de I+D+i 2013–2016 and Instituto de Salud Carlos III, Subdirección General de Redes y Centros de Investigación Cooperativa, Ministerio de Ciencia, Innovación y Universidades, Spanish Network for Research in Infectious Diseases (REIPI RD16/0016/0001) – co-financed by European Development Regional Fund “A way to achieve Europe”, Operative programme Intelligent Growth 2014–2020.