Nontuberculous mycobacteria are a heterogeneous group of microorganisms that can often cause human infection, although they may also be considered to be contaminants or colonizers on occasions. The management of these infections must necessarily take into account the identification of isolated species and their in vitro susceptibility testing (although not for all of them), as well as the characteristics of the patient, because these treatments are usually prolonged and must be carried out by experts in the management of these infections. Classically divided into slowly growing mycobacteria and rapidly growing mycobacteria, the treatment regimens and the antibiotics used are different for both groups. In addition, in certain circumstances, this treatment must necessarily be linked to other measures (removal of foreign bodies, surgery) in order to maximize the likelihood of curing the patient.
Las micobacterias no tuberculosas forman un grupo heterogéneo de microorganismos que en numerosas ocasiones son causa de infección en humanos, si bien también pueden considerarse en ocasiones como contaminantes o colonizadores. El manejo de estas infecciones debe necesariamente tener en cuenta la especie aislada y su sensibilidad in vitro (aunque no en todas ellas), así como las características del propio paciente, ya que estos tratamientos suelen ser prolongados y, necesariamente, deben ser llevados a cabo por expertos en el manejo de estas infecciones. Clásicamente divididas en micobacterias de crecimiento lento y micobacterias de crecimiento rápido, los esquemas de tratamiento y los antibióticos empleados son diferentes en ambos casos. Además, en determinadas circunstancias este tratamiento deberá necesariamente ir unido a otras medidas (retirada de cuerpos extraños, cirugía) con el objetivo de tener las máximas posibilidades de conseguir la curación del paciente.
Today, the genus Mycobacterium includes over 150 different species.1,2 The group of mycobacteria different from Mycobacterium tuberculosis complex (M. bovis, M. africanum, M. microti, M. canetti, M. caprae, M. pinnipedii, M. suricattae and M. mungi) and the group of mycobacteria causing leprosy (M. leprae and M. lepromatosis) are usually referred to as atypical, environmental, or nontuberculous mycobacteria (NTM); these are microorganisms widely distributed in the environment with a non-uniform distribution and regional variations that are possibly due to not very well known environmental factors.
NTM are traditionally classified based on their phenotypical characteristics into two (2) different groups: slowly growing mycobacteria, and rapidly growing mycobacteria. Today's molecular biology techniques have greater discrimination capabilities are at the base of the taxonomic classification and epidemiological typing of NTM, and the advances made on this area are the reason why, during the last few years, a great deal of species and subspecies of mycobacteria have been added, and reclassified.
Unlike M. tuberculosis and M. leprae, the association of NTM with human pathology is occasional and opportunistic; as a matter of fact, most species have never be categorized as human pathogens, and only a relatively small group of them are common human pathogens, mostly in patients with predisposing factors, since the capacity of these mycobacteria to cause disease depends not only on intrinsic pathogenicity factors of the different species, but also on host factors such as the integrity of the immune system, or the presence of loco-regional factors (surgical incision; prior tissue pathology; foreign body; etc.).3 The larger number of patients with this type of risk factors such as HIV-co-infection, the higher survival rates of onco-hematological patients and transplant receivers, or with other chronic conditions such as COPD and cystic fibrosis, and the growing use of biomedical devices explain the increased infection rate reported during the last few years.
Within the wide group of possible syndromes called mycobacteriosis we find respiratory infections, commonly associated with prior pulmonary conditions such as cystic fibrosis, COPD, bronchiectasias, etc.; disseminated infections, usually associated with immunodeficiencies; skin and soft tissue infections including lymphadenitis; and surgical bed infections associated, or not, with the implantation of biomaterials.
Disseminated infections are usually related to immunosuppression and they have been reported, above all, in patients with infection due to HIV, onco-hematological patients, transplant receivers, and patients treated with anti-TNF-α; biological drugs; the natural immunity against mycobacteria is based on the interferon-gamma and interleukin 12 pathway—responsible for the control of monocytes, macrophages, and dendritic cells with T-lymphocytes and NK (natural-killers); also, localized and/or disseminated infections due to NTM have been reported both in congenital immunodeficiencies due to mutations in receptors or ligands of this pathway, and in late immunosuppressions due to the development of anti-interferon-gamma bodies.4
The microbiological diagnosis of mycobacteriosis is not very different from the diagnosis of tuberculosis from the microbiological standpoint; most NTM grow in culture media common to mycobacteria incubated at 95–98.6°F; however, the culture media of skin and osteo-joint samples use incubation temperatures of 82.4–86°F—optimal temperature for the growth of some species such as M. abscessus, M. ulcerans, or M. marinum. Other species such as M. xenopi grow better at temperatures of 113°F, and others require additional culture media (M. genavense and M. haemophilum), or need prolonged incubation (M. ulcerans, M. genavense, M. malmoense).
Although, for the moment, we do not have rapid direct diagnostic techniques, the sensitivity of the different culture techniques is excellent for most part of the most relevant NTM in the clinical practice and, also, we have rapid and precise identification techniques and one standardized methodology for the study of antimicrobial sensitivity.5
However, in the clinical practice, the main problem is establishing the meaning of isolation of these organisms in the clinical samples since the NTM can be pathogens, but also contaminating or colonizing. Identification is very important since species such as M. gordonae, M. terrae, or M. lentiflavum are usually water contaminants and an exceptional cause of pulmonary disease, while species such as M. kansasii, or M. szulgai are pathogens in most cases.
The management of these patients is usually complex and can require not only extended and different antibiotic therapies—based on the mycobacterium that has been isolated, but also the removal of affected devices, or even the use of surgery to eliminate the source of infection in some cases.
Biofilms and therapeutic complicationsOne especially important aspect in the management of patients with infection due to NTM is the involvement of many of these infections (in particular, pulmonary and material-related infections) in the development of biofilms. These structures are a very important mechanism of antimicrobial resistance in all microorganisms, including mycobacteria. It has been confirmed that different species are capable of developing these structures,6,7 and that this development increases antimicrobial resistance with MIC peaks ≥1000 times when the mycobacterium is in sessile form.8 This increased resistance is probably due to numerous factors such as the penetration capacities of the antibiotic (not very effective), and the bacteria metabolic state that seems to be the essential mechanism, yet others cannot be ruled out, such as the existence of persisters or the activation of resistance genes.9
The implications of these facts are very important. In biomaterial-induced infections, the development of biofilms in inert, nonvascularized areas would not allow its eradication through the use of antibiotics only, which means that it is essential to remove the foreign body if we want to cure the patient. In the case of respiratory infections, the involvement of biofilms has recently been reported in clinical cases, meaning that the problems would be similar to the aforementioned case.10 In these infections, however, there is no foreign body to remove, and all there is to do is adjust the treatment for greater effectiveness against biofilms, and maybe even include in the therapeutic scheme the surgical removal of damaged tissues whenever possible on the technical level.
The search for new strategies in the management of biofilm-induced infections is a developing field and results are still non-applicable to the clinical practice. However, it is possible that in a near future we will have therapeutic patterns specifically designed to treat biofilms that when combined with conventional antibiotic therapies will improve the prognosis of patients.11
Slowly growing mycobacteriaMycobacterium avium complexThe Mycobacterium avium complex (MAC) is one heterogeneous group of mycobacteria that traditionally includes two (2) different species: M. intracellulare, and M. avium with three (3) subspecies: M. avium subsp. avium, M. avium subsp. Paratuberculosis, and M. avium subsp. silvaticum. Recently, new species have been recognized such as M. chimera—involved in infections acquired after cardiovascular surgery due to water contamination and aerosolization, M. colombiense, M. vulneris, M. marseillense, M. bouchedurhonense, M. yongonense, M. arosiense, M. indicus pranii, and M. timonense.12
The MAC is the most common cause of NTM-induced pulmonary infection. Some cases affect patients with chronic obstructive pulmonary disease (COPD, emphysema, asthma, bronchiectasis, prior tuberculosis, etc.), or gastroesophageal reflux, but others may be de novo cases occurring in patients without any preexisting pulmonary conditions or known immunodeficiencies; a part of these cases affects non-smoking slim women, with chronic pulmonary affectation in the form of localized bronchiectasis, which in the medical literature is referred to as the Lady Windermere syndrome. In some of these cases, genetic alterations of mutations in the CFTR gene involved in cystic fibrosis are found to affect the immune system, the ciliary function, or heterozygotes.
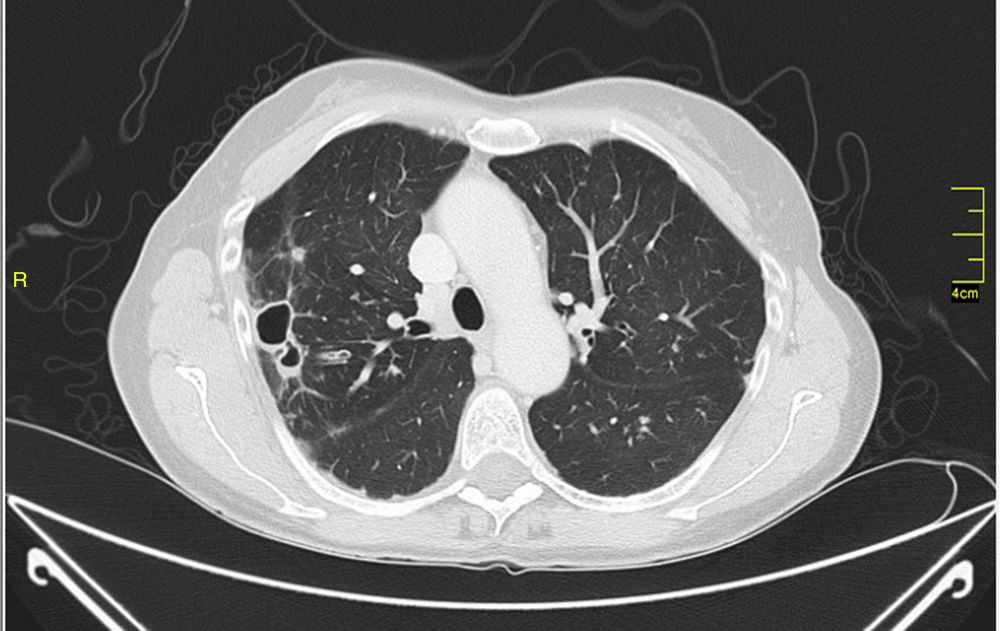
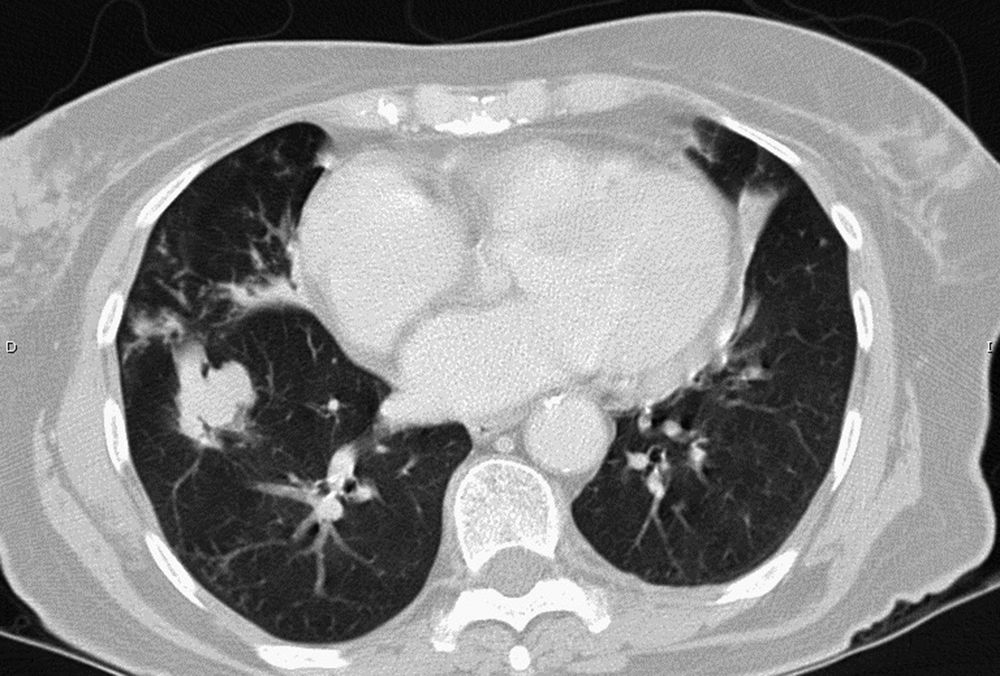
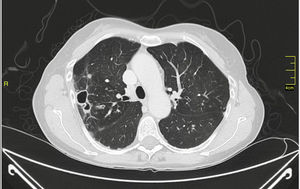
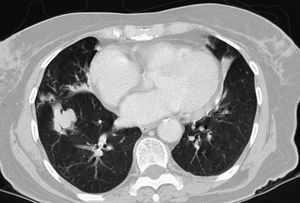
MAC-induced and, overall, NTM-induced pulmonary infections have been categorized as fibrocavitary and multibacillary types with a destructive pattern similar to the classic cavitating pulmonary tuberculosis with usually positive staining for acid-alcohol resistant bacilli and nodular bronchiectasic, paucibacillary, and more indolent types that pose differential diagnosis inquiries of slowly progressive pulmonary disease versus the transient airway colonization by NTM (Figs. 1 and 2).
MAC is also the leading cause of lymphadenitis in kids under 5 years old, ahead of M. scrofulaceum in developed countries,13 and in HIV-positive patients and in patients with other immunodeficiencies is cause for extrapulmonary and disseminated affectation.
The introduction of macrolides (clarithromycin and azithromycin) has been a huge advancement in the management of MAC-induced infections improving the rates of response compared to the classic course based on rifampicin, isoniazid and ethambutol both for the management of pulmonary infections and HIV infection-related disseminated disease. The combination of one macrolide with ethambutol and one rifamycin (rifampicin or rifabutin) is the basis of the treatment of MAC-induced infections; fluoroquinolones such as amikacin and clofazimine are also active in vitro, although the clinical response and the in vitro resistance only have a good correlation with macrolides in which high MICs to clarithromycin (MIC≥32μg/ml) are clearly associated with therapeutic failure. There is a preliminary experience with the use of inhaled amikacin, especially in advanced cases that are refractory to conventional therapy with somehow clinical and biological benefits. The effectiveness of the use of quinolones (ciprofloxacin, levofloxacin or moxifloxacin) in combination with patterns based on macrolides is dubious and increases the risk of suffering from arrhythmias as a consequence of a prolonged QT interval.
Although there is some controversy on the dose and choice of the macrolide, clarithromycin 1000mg/day (15mg/kg if <50kg), and azithromycin 250mg/day, or 500mg 3 t.i.d. seem equally effective.14 The same thing happens with rifamycin, being rifampicin 600mg/day, or rifabutin 150–300mg/day probably the same.
Disseminated infection in HIV-positive patientsMAC-induced disseminated infections affect patients with CD4 counts below 50cells/μl; the treatment of choice is the combination of clarithromycin 500mg every 12h, and ethambutol 15mg/kg/day.
Azithromycin has been used as a substitute for clarithromycin for its better digestive tolerance and better profile of pharmacokinetics interactions, and its effectiveness is not inferior to clarithromycin.
The role of rifampicin or rifabutin plus clarithromycin-ethambutol for an early management is not well defined and both pose problems of interaction with antiretroviral drugs. Amikacin is suggested in severe cases and re-treatment courses. In one clinical trial on MAC-induced disseminated infections,15 adding clofazimine to the standard pattern of clarithromycin-ethambutol was associated with higher mortality rates, and did not improve the clinical response or microbiology.
The treatment of MAC-induced disseminated infections should be kept for at least 12 months, and it can be withdrawn in patients on antiretroviral therapy who reach virological suppression and CD4 counts over 100cells/μl for more than 3–6months.
Pulmonary infection due to Mycobacterium avium complexThe early management of a MAC-induced pulmonary infection should include a combination of one macrolide, ethambutol, and one rifamycin.
The clinical guidelines published by the ATS back in 200716 recommend the use of rifampicin preferably over rifabutin, while differentiating the courses of treatment based on whether we are treating fibrocavitary multibacillary or nodular-bronchiectasic types. For the management of the first ones, the recommendation includes using daily doses of oral drugs plus one aminoglycoside (amikacin or streptomycin), whereas for the management of nodular paucibacillary types, the recommendation includes using the drugs three (3) times a week while getting rid of the aminoglycoside. The duration of the treatment should be 18–24months, for at least 12months ever since the culture results test negative. The microbiological cure occurs in no more than 50–60 per cent of the cases, with relapses with isolates showing macrolide-resistance that are often interpreted through molecular typing as re-infections due to new clones.17
Lymphadenitis due to Mycobacterium avium complexNTM-induced cervical lymphadenitis in children occurs in kids between one (1) and four (4) years old and it is due to the MAC, and less frequently to M. scrofulaceum and M. haemophilum. It can resolve spontaneously or cause skin fistulae. The best results are obtained with surgical resection of the affected nodes, but it has not been established yet whether it is necessary to add pharmacological therapy with the usual patterns (macrolides, ethambutol, rifamycines).13
Mycobacterium kansasiiM. kansasii is one photochromogenous mycobacterium that causes pulmonary infections with a fibrocavitary pattern similar to that of tuberculosis, and with less focal or disseminated infections in patients with HIV infections or with other causes of immunodeficiency.
M. kansasii is sensitive in vitro to rifamycins, isoniazid, macrolides, ethambutol, quinolones, streptomycin, linezolid, and cotrimoxazole. The in vitro activity of isoniazid against M. kansasii is lower than the vitro activity against M. tuberculosis, with MIC ranges between 0.5 and 5μg/ml, that is, usually above the critical concentrations used against M. tuberculosis (0.2 and 1μg/ml). Yet despite this fact, courses of treatment with rifampicin-isoniazid, and ethambutol are usually effective, being the primary rifampicin-resistance the reason that explains most therapeutic failures. As a matter of fact, it is recommended to conduct one rifampicin-sensitivity study in primary isolates of M. kansasii and extend the study of sensitivity to other drugs with MICs above 1μg/ml.
The most commonly used pattern is rifampicin-isoniazid-ethambutol for at least 12months ever since the culture results test negative. Optionally, one fourth drug may be used in the early stage of extensive disease, or until confirming sensitivity to rifampicin (streptomycin, clarithromycin, or quinolone). Some authors suggest substituting isoniazid for clarithromycin as the initial empirical pattern.18
Other slowly growing mycobacteriaMycobacterium xenopiSame as it happens with the MAC, M. xenopi is responsible for the fibrocavitary pulmonary or nodular disease and for the disseminated disease in immunodeficient patients.
It can be contaminating—pseudoepidemias due to water contamination after bronchoscope cleaning procedures have been reported. Sensitivity studies are difficult to interpret due to its slow growth, although it is usually considered sensitive to rifampicin, ethambutol, clarithromycin and high concentrations of isoniazid. A combination of these drugs is recommended for 18–24months, for at least 12months ever since the culture results test negative for pulmonary disease.19
Mycobacterium malmoenseIt is responsible for pulmonary infections, lymphadenitis and tenosynovitis. It is treated with similar schemes than the ones used against the MAC, since with the difficulties and interpretation of in vitro sensitivity studies, it is considered sensitive to rifampicin, ethambutol, and clarithromycin.20
Mycobacterium szulgaiIt is responsible for pulmonary infections with fibrocavitary pattern, especially in patients with preexistent predisposing pulmonary conditions, and immunodeficient patients of disseminated and extrapulmonary infections.
It is usually sensitive to rifampicin, isoniazid, ethambutol, quinolones, and macrolides. The treatment recommended includes, at least, three (3) active drugs for at least 12months ever since the culture results test negative for pulmonary disease.21
Mycobacterium ulceransM. ulcerans is responsible for Buruli ulcer—one very prevalent condition in the tropics of devastating consequences if misdiagnosed and not treated adequately. The primary culture of skin lesions is poorly sensitive; M. ulcerans grows slowly and needs additional media such as incubation at low temperatures (82.4–91.4°F), and prolonged incubation. It is a condition basically managed surgically based on wide debridement and grafting. However, several studies suggest better results with an early course of pharmacological treatment with patterns including rifampicin, clarithromycin, and streptomycin.22
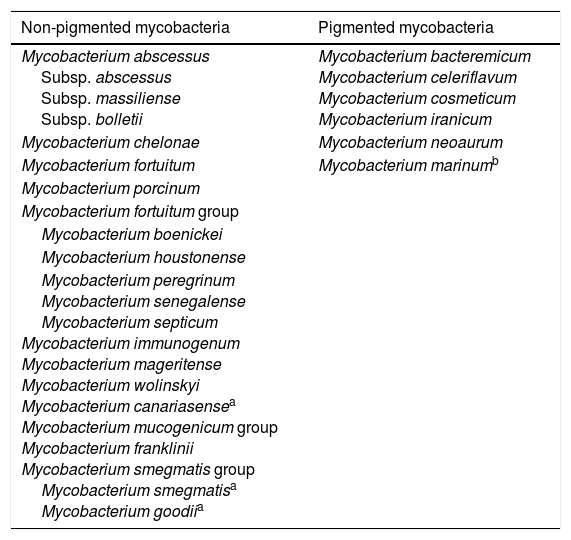
Rapidly growing mycobacteriaWithin the genus Mycobacterium, rapidly growing mycobacteria (RGM) represent, approximately, half of the species reported23; most of them are environmental mycobacteria that have never been confirmed to be disease-causing mycobacteria. However, some species have been said to be human pathogens, especially the group made up of different non-pigmented species (Table 1). Aside from these species, there are other occasional cases of infections due to other rapidly growing mycobacteria,23,24 but most of these are exceptional cases.
Species of rapidly growing mycobacteria important to human pathology.
| Non-pigmented mycobacteria | Pigmented mycobacteria |
|---|---|
| Mycobacterium abscessus Subsp. abscessus Subsp. massiliense Subsp. bolletii | Mycobacterium bacteremicum Mycobacterium celeriflavum Mycobacterium cosmeticum Mycobacterium iranicum |
| Mycobacterium chelonae | Mycobacterium neoaurum |
| Mycobacterium fortuitum | Mycobacterium marinumb |
| Mycobacterium porcinum | |
| Mycobacterium fortuitum group | |
| Mycobacterium boenickei | |
| Mycobacterium houstonense | |
| Mycobacterium peregrinum Mycobacterium senegalense Mycobacterium septicum Mycobacterium immunogenum Mycobacterium mageritense Mycobacterium wolinskyi Mycobacterium canariasensea Mycobacterium mucogenicum group Mycobacterium franklinii Mycobacterium smegmatis group Mycobacterium smegmatisa Mycobacterium goodiia |
The spectrum of infections due to RGM is wide and includes numerous clinical syndromes.5,23,25,26 However, among them there are three (3) groups that are especially significant: respiratory infections, skin and soft tissue infections, and biomaterial-related infections. Each and every one of them with specific therapeutic issues.
RGM-induced respiratory infections are usually chronic clinical manifestations associated with the presence of preexistent pulmonary conditions such as cystic fibrosis. These manifestations can affect preexistent pulmonary cavities such as bullae and scarring lesions of previous infections, especially tuberculosis, in which the mycobacterium will initially colonize the lesion and then proceed to invade the tissues, or appear as a bronchiectasis infection similar to the clinical manifestations due to slowly growing mycobacteria. One especially relevant aspect in this case is to identify the causing species, since the meaning of the isolates in the clinical samples is not the same in all species.27 Also, as we will see below, the antimicrobial sensitivity is also variable, which is why this is a very important thing to take into consideration. In these clinical manifestations, the M. abscessus species is especially relevant since this mycobacterium leads to respiratory infections whose therapeutic complexity is such that these infections are very hard to cure. Also, the presence of clones of special pathogenicity capable of being transmitted across different countries has been reported,28 meaning that it is very important to know different aspects such as epidemiology, underlying conditions, species and subspecies that cause the clinical manifestations, etc. before planning the optimal course of treatment.
In the case of skin and soft tissue infections,29 many of them are associated with cosmetic procedures such as mesotherapy, hair removal, or tattoos that cause chronic clinical manifestations which, although they are not life-threatening for the patient, do cause important esthetic complications even after these clinical manifestations have gone away.
Biomaterial-related infections have been more and more relevant during the last few years, and these mycobacteria cause very different clinical manifestations within this group such as prosthetic osteojoint infections, intravascular catheter-related infections, prosthetic valve endocarditis, etc.23 Today, all of these clinical presentations share one characteristic in common—the removal of the infected material as an indispensable condition to cure the patient, since the microbacterium usually found in this material creates some sort of biofilm on the surface of such material with the corresponding therapeutic difficulties that we will discuss below.
Antimicrobial sensitivityOne particular characteristic of RGM, especially the non-pigmentated strains, is its microbial sensitivity which is very different from that of slowly growing strains. Generally speaking, most non-pigmented RGM species are resistant to the usual anti-tuberculosis drugs such as isoniazid, rifampicin, ethambutol, pyrazinamide, and streptomycin, but they are sensitive to other antibiotics commonly used in the management of different bacterial infections such as macrolides, quinolones, cotrimoxazole, tetracyclines, aminoglycosides, linezolid, some beta-lactam antibiotics (cefoxitin, imipenem), or tigecycline.9,30 Recently, the standardization of the sensitivity studies for these organisms has been published as the SEIMC protocol published back in 2017 shows, where microdilution is established as the go-to technique for these sensitivity studies. One especially significant piece of information is that there can be a significant variation in the sensitivity patterns, not only among different species, but also among strains within the same species, which is why it is recommended to conduct individualized sensitivity studies in isolates considered relevant.
In general, most strains and species are sensitive to amikacin and have low MICs to tigecycline, even though the specific cut-off point for this antibiotic has not been established yet. M. abscessus and M. chelonae are usually the most resistant species of all, which is why the most useful courses of treatment in these cases are macrolides (except for strains with an inducible type of methylase), cefoxitin, and aminoglycosides (especially tobramycin for the management of M.chelonae). The species of M.fortuitum complex are usually sensitive to quinolones, aminoglycosides, cotrimoxazole, and linezolid, but they can be resistant to macrolides in many cases. Other species such as M.peregrinum, M.mucogenicum, or M.mageritense are usually sensitive to numerous antibiotics.5,9,23,30,31
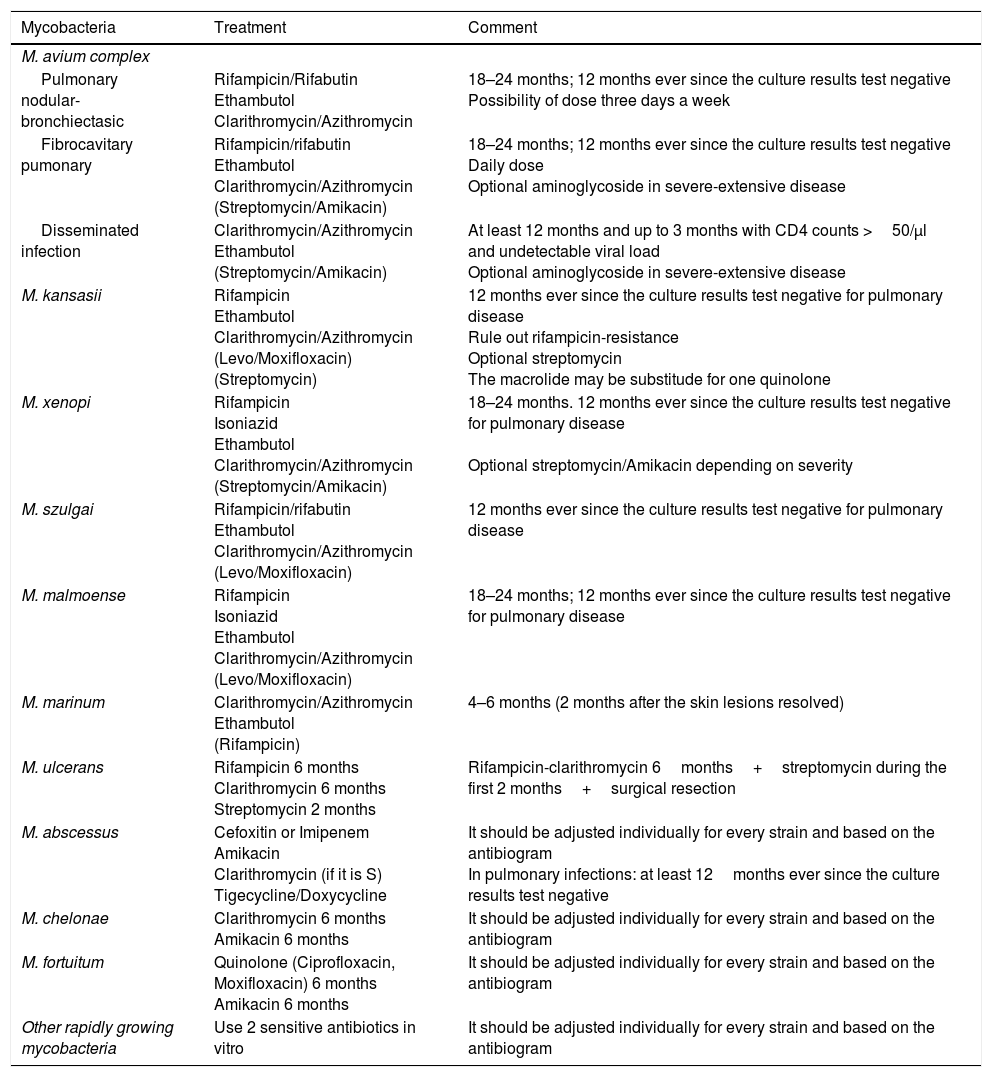
Treatment recommendationsThe actual recommendations for the management of these infections are based on the clinical manifestations and type of isolated mycobacterium (Table 2).
Early management of nontuberculous mycobacteria (individualize if the treatment fails, when in re-treatment, and drug-resistance).
| Mycobacteria | Treatment | Comment |
|---|---|---|
| M. avium complex | ||
| Pulmonary nodular-bronchiectasic | Rifampicin/Rifabutin Ethambutol Clarithromycin/Azithromycin | 18–24 months; 12 months ever since the culture results test negative Possibility of dose three days a week |
| Fibrocavitary pumonary | Rifampicin/rifabutin Ethambutol Clarithromycin/Azithromycin (Streptomycin/Amikacin) | 18–24 months; 12 months ever since the culture results test negative Daily dose Optional aminoglycoside in severe-extensive disease |
| Disseminated infection | Clarithromycin/Azithromycin Ethambutol (Streptomycin/Amikacin) | At least 12 months and up to 3 months with CD4 counts >50/μl and undetectable viral load Optional aminoglycoside in severe-extensive disease |
| M. kansasii | Rifampicin Ethambutol Clarithromycin/Azithromycin (Levo/Moxifloxacin) (Streptomycin) | 12 months ever since the culture results test negative for pulmonary disease Rule out rifampicin-resistance Optional streptomycin The macrolide may be substitude for one quinolone |
| M. xenopi | Rifampicin Isoniazid Ethambutol Clarithromycin/Azithromycin (Streptomycin/Amikacin) | 18–24 months. 12 months ever since the culture results test negative for pulmonary disease Optional streptomycin/Amikacin depending on severity |
| M. szulgai | Rifampicin/rifabutin Ethambutol Clarithromycin/Azithromycin (Levo/Moxifloxacin) | 12 months ever since the culture results test negative for pulmonary disease |
| M. malmoense | Rifampicin Isoniazid Ethambutol Clarithromycin/Azithromycin (Levo/Moxifloxacin) | 18–24 months; 12 months ever since the culture results test negative for pulmonary disease |
| M. marinum | Clarithromycin/Azithromycin Ethambutol (Rifampicin) | 4–6 months (2 months after the skin lesions resolved) |
| M. ulcerans | Rifampicin 6 months Clarithromycin 6 months Streptomycin 2 months | Rifampicin-clarithromycin 6months+streptomycin during the first 2 months+surgical resection |
| M. abscessus | Cefoxitin or Imipenem Amikacin Clarithromycin (if it is S) Tigecycline/Doxycycline | It should be adjusted individually for every strain and based on the antibiogram In pulmonary infections: at least 12months ever since the culture results test negative |
| M. chelonae | Clarithromycin 6 months Amikacin 6 months | It should be adjusted individually for every strain and based on the antibiogram |
| M. fortuitum | Quinolone (Ciprofloxacin, Moxifloxacin) 6 months Amikacin 6 months | It should be adjusted individually for every strain and based on the antibiogram |
| Other rapidly growing mycobacteria | Use 2 sensitive antibiotics in vitro | It should be adjusted individually for every strain and based on the antibiogram |
Dose:
Rifampicin 600mg/day; Rifabutin 150–300mg.
Isoniazid 300–600mg/day.
Ethambutol 15mg/kg/day.
Clarithromycin 500mg every 12h; Azithromycin 250mg/day or 500mg/t.i.d.
Streptomycin-Amikacin 10–15mg/kg/day, or t.i.d.
Levofloxacin 500mg/day, or moxifloxacin 400mg/day.
Skin and soft tissue infections with poor clinical expression are usually paucibacillary, which means that they could be successfully treated with monotherapy using one active antimicrobial agent against the isolated strain. Usually, one macrolide (normally, clarithromycin) is used against the strains of the M.abscessus-chelonae in cases where the mycobacterium is sensitive and there is no inducible methylase, and one quinolone is used against the strains of the M.fortuitum complex.9,23,29 As alternatives, cefoxitin, amikacin, cotrimoxazole, or tetracyclines can be used as long as the strain is sensitive in vitro. Although linezolid or tigecycline are usually active, there is not much clinical experience on this regard. One of the problems with these antibiotics is that they need a parenteral route of administration, with the corresponding longer hospital stays, since it is recommended that the duration of the treatment should not be shorter than 4–6 months; also, a second antibiotic can be administered during the first few weeks if the case is more severe.9,23,29
If the bacillary load is significant (oversized abscesses, for example) we would need combination therapy in order to avoid the development of resistances following the chromosome mutation. These mutations have been reported in M.chelonae, and monotherapy with clarithromycin, in which one mutation in the 23S rDNA gene provides the strains with high level resistance against this antibiotic.23 In these cases, the dose limitations are the same as in the former case, yet it has been reported that it is possible to use amikacin in alternative patterns of treatment (3–4days a week) aimed at facilitating the extrahospitalary management of these patients. In these cases, it can be desirable and even necessary to proceed with the surgical drainage of the oversized abscesses in order to reduce the bacillary load.
The management of RGM-induced respiratory infections is a real challenge these days. Most infections are due to M.abscessus (subespecies abscessus and massiliense), and in these cases there are not too many therapeutic options especially when it comes to administering oral treatment, above all, in cases due to strains with the functional erm(41) gene and, therefore, macrolide-resistant. In these cases, it is advisable to use 2–3 in vitro sensitive drugs, such as cefoxitin, amikacin, tigecycline, or imipenem, initially through parenteral rout of administration, and oral combination therapy with clarithromycin, if possible, if the isolate is sensitive to it, for at least 12months ever since the culture results test negative for respiratory samples. However, both the drug dose and toxicity limitations and the difficulty trying to eradicate the mycobacterium from the pulmonary tissue when it has created a biofilm makes the complete clinical-microbiological cure of these patients impossible,32 which is why, in these cases, we will alleviate the symptom and limit the infection-induced pulmonary damage through successive cycles of treatment.23 Nevertheless, in those cases where the strain is macrolide-sensitive, adding these drugs is a game changer in the prognosis of these patients, because it is feasible to cure them.33 In the case of other species, the treatment will follow the same principles (use of 2–3drugs the mycobacterium is sensitive to for at least 12months ever since the culture results test negative).
When it comes to the management of biomaterial-related infections, it is essential to remove the infected material in order to cure these patients. In the case of intravascular catheter-related bacteremias,34 or breast implant-related infections23 it may not be that hard, but in other infections such as prosthetic joint infections,35 or prosthetic valve endocarditis26 it is much more complicated. In these cases, we should also use combination therapy based on the individualized study of the isolate-sensitivity in each case. Also, the course of treatment should last, at least 6months, especially in the case of severe infections. We should also bear in mind that, yet despite the ominous prognosis of some of these infections, such as endocarditis, the cure has been reported in some cases, even without the removal of the prosthesis infected with M.fortuitum.36,37 The infection-causing species is probably something very important too when it comes to establishing the prognosis of the patient.
Mycobacterium marinumThis mycobacterium has been described both as a rapidly and a slowly growing mycobacterium, since this characteristic depends on the temperature of incubation. The disease it causes is known as pool or fish tank granuloma, and it is usually described as one single granulomatous lesion that has an epidemiological precedent of contact with water from these places.38
In the case of M.marinum conducing systematic sensitivity tests is not advisable since, on many occasions, surgical excision of the lesion can be curative. However, it is usually sensitive to some conventional anti-tuberculosis drugs such as rifampicin or ethambutol (both used clinically with good results), and tetracyclines, cotrimoxazole, or clarithromycin.38,39 Generally, empirical treatment with ethambutol plus clarithromycin and, on some occasions, rifampicin is administered for at least 2 months from the moment of clinical recovery (total: 3–6months). Monotherapy, especially quinolones, is not recommended since there is a risk of developing resistant mutants with the corresponding therapeutic failure.29
ConclusionsThere is a wide spectrum of infections due to nontuberculous mycobacteria, and the number of infections is growing since the number of susceptible patients is growing as well. Although the management of these infections has not been studied as much as M.tuberculosis, there is enough evidence to study it based on the actual knowledge on the medical literature and the advances made in microbiological techniques. However, the basis for the management of these patients is the correct interpretation of the isolates in order to differentiate genuine infections from colonizations or contaminations. Once the diagnosis of infection has been achieved, the management of the patient should be adjusted to the location of both the infection and the causing mycobacterium. These can be very long courses of treatment and, in some cases, they can have poor outcomes because of the highly specific nature of these conditions. This is why it is essential that the management of these patients is conducted by expert multidisciplinary teams that are savvy on this type of infections so that the patients can have the best possible results.
Conflict of interestsThere are no conflicts of interest related to this manuscript whatsoever.
Please cite this article as: Esteban J, Navas E. Tratamiento de las infecciones producidas por micobacterias no tuberculosas. Enferm Infecc Microbiol Clin. 2018;36:586–592.