Acute respiratory infections (ARI) are a common cause of inappropriate antibiotic prescription (ATB) in pediatrics. FebriDx® is a rapid diagnostic test that differentiates between viral and bacterial infections. The objective is to analyse the impact of FebriDx® on ATB prescription when managing febrile ARI.
MethodsProspective study carried out in patients aged 1–<18 years with febrile ARI in the emergency department. FebriDx® was performed and the impact on management was evaluated at follow-up.
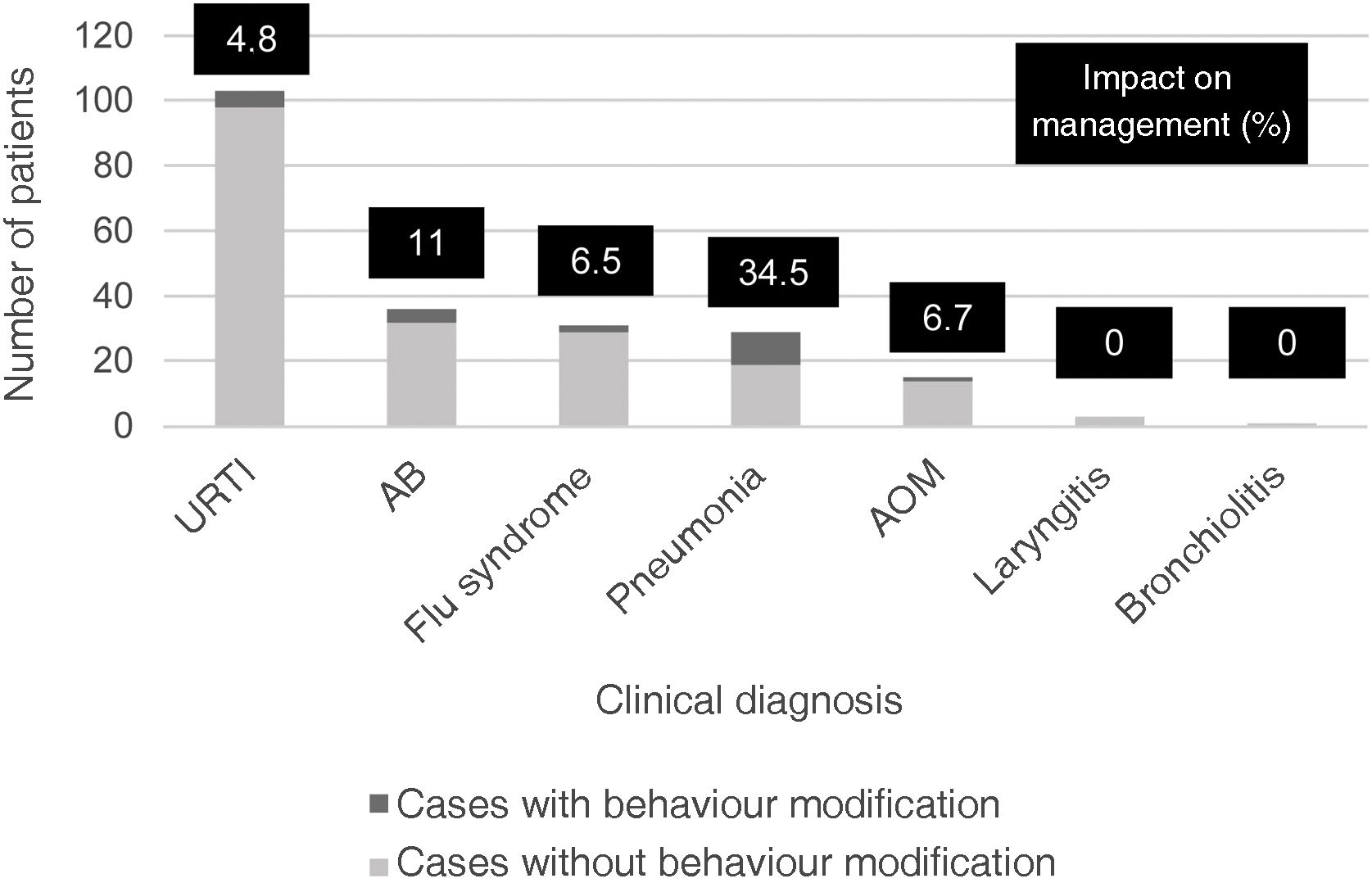
ResultsA total of 216 patients were included. Clinical assessment and FebriDx® result coincided coincided in 174 (80.5%) cases. A modification of the initial therapeutic plan was made in 22 (52.4%) of the 42 discordant ones (10.2% of the overall patients). In pneumonia the impact was 34.5%; in all cases it involved not prescribing ATB.
ConclusionsFebriDx® could be a useful tool in the management of pediatric patients with febrile ARI to optimize ATB prescription.
Las infecciones respiratorias agudas (IRA) son una causa común de prescripción inadecuada de antibióticos (ATB) en pediatría. FebriDx® es una prueba de diagnóstico rápido que diferencia infecciones virales y bacterianas. El objetivo es analizar su impacto en el manejo de niños con IRA febril.
MétodosEstudio prospectivo realizado en pacientes de 1-<18 años con IRA febril en Urgencias. Se realizó FebriDx® y se evaluó su impacto en el manejo en relación con la prescripción ATB.
ResultadosSe incluyeron 216 pacientes. La orientación médica y el resultado de FebriDx® coincidieron en 174 (80,5%) casos. Se realizó modificación del plan terapéutico inicial en 22(52,4%) de los 42 discordantes (10,2% del global de los pacientes). En neumonías el impacto fue del 34,5%; en todos los casos implicó el no prescribir ATB.
ConclusiónFebriDx® podría ser una herramienta útil en el manejo de pacientes pediátricos con IRA febril para optimizar la prescripción ATB.
Acute respiratory infections (ARI) are the most common infectious diseases in paediatric practice, generating numerous medical consultations in hospital Accident and Emergency departments (A&E). Their high prevalence, together with the difficulty in differentiating between viral and bacterial aetiology, due to the scarcity of rapid microbiological diagnostic tests and limited patient follow-up, contributes to inappropriate and excessive antibiotic (ATB) prescribing. This represents a global public health problem, associated with the emergence of bacterial resistance with possible side effects, as well as unnecessary healthcare spending.1
FebriDx® is a diagnostic test that allows the differential diagnosis of viral and bacterial ARI in 10–15 min using a capillary blood sample. It is based on the visual detection of elevated levels of myxovirus resistance protein A and C-reactive protein by immunoassay. Various studies carried out in hospital and out-of-hospital A&E, which include paediatric and adult populations, have shown its high diagnostic validity for this purpose, with high specificity and negative predictive value. Furthermore, it has demonstrated a significant impact on decision-making, reducing the unnecessary use of ATB in a high percentage of cases, especially in the adult population.2–8
The aim of this study was to analyse the impact of FebriDx® in the management of paediatric patients with febrile ARI in relation to the prescribing of ATB in a hospital A&E.
MethodsProspective and quasi-experimental study carried out in the A&E of a tertiary maternity and children's hospital. It is the referral centre for an area with a population of 1,800,000 and attends to around 110,000 consultations a year.
We included patients from one to 18 years of age with febrile ARI (upper respiratory tract infection [URTI], bronchospasm, pharyngotonsillitis, laryngitis, bronchiolitis, pneumonia, acute sinusitis, acute otitis media and flu syndrome) for less than seven days, treated from November 2022 to August 2023.
Patients with immunosuppression, underlying disease that required the prescribing of ATB, immunisation, ATB or antiviral treatment in the previous 14 days, other rapid aetiological diagnostic tests performed during the A&E visit and indeterminate FebriDx® test result were excluded, as well as those with no informed consent.
During the study period, two researchers attended the A&E twice a week to recruit patients. Previously trained nursing staff performed the FebriDx® test on patients from a 5-μl capillary blood sample. Said sample was obtained directly using the test, an integrated lancet-cassette device with a nitrocellulose test strip. Results are available in 10–15 min and are displayed categorically, presented in coloured lines:
- -
Black line: bacterial aetiology.
- -
Red line: viral aetiology.
- -
Blue line (control): undetermined aetiology.
For the test to be valid, the blue control line must appear in the result window.
The A&E doctors who treated these patients expressed to the researchers their intention to prescribe ATB before and after knowing the test result. The change in the initial treatment plan in relation to the prescribing of ATB was considered an impact on case management. One week after the consultation in the A&E, a telephone follow-up was performed for all cases to determine their clinical progress.
Data were analysed with the statistical software SPSS® v25.0 for Windows. Descriptive statistics were presented as counts (percentages) for categorical variables and median (interquartile range) for quantitative variables.
The study was approved by the Ethics Committee of the study hospital (code PS-19-22). Written informed consent was obtained from the parents or legal guardians of all patients, as well as from patients over 12 years of age.
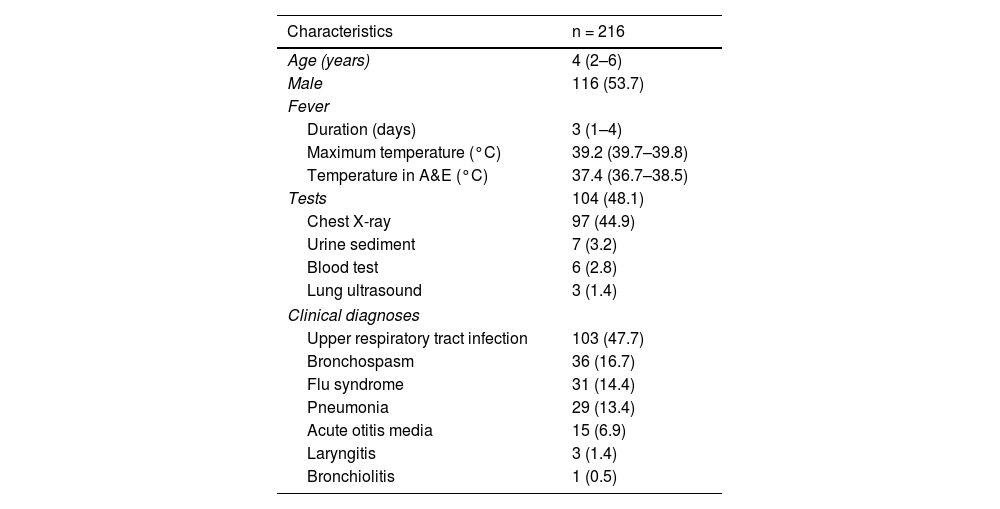
ResultsA total of 216 patients were included. The demographic and clinical characteristics are shown in Table 1. In all, 104 (48.1%) patients underwent one or more investigations, the most common being a chest X-ray (97; 44.9%). The main clinical diagnosis was URTI (103 patients; 47.7%), with a chest X-ray performed in 36 (35.0%) of these cases.
Demographic and clinical characteristics of the 216 patients included.
| Characteristics | n = 216 |
|---|---|
| Age (years) | 4 (2–6) |
| Male | 116 (53.7) |
| Fever | |
| Duration (days) | 3 (1–4) |
| Maximum temperature (°C) | 39.2 (39.7–39.8) |
| Temperature in A&E (°C) | 37.4 (36.7–38.5) |
| Tests | 104 (48.1) |
| Chest X-ray | 97 (44.9) |
| Urine sediment | 7 (3.2) |
| Blood test | 6 (2.8) |
| Lung ultrasound | 3 (1.4) |
| Clinical diagnoses | |
| Upper respiratory tract infection | 103 (47.7) |
| Bronchospasm | 36 (16.7) |
| Flu syndrome | 31 (14.4) |
| Pneumonia | 29 (13.4) |
| Acute otitis media | 15 (6.9) |
| Laryngitis | 3 (1.4) |
| Bronchiolitis | 1 (0.5) |
Categorical variables are reported in absolute frequencies with percentages and continuous variables in median with interquartile range.
In 39 (18.1%) cases, the paediatrician expressed a prior intention to treat with ATB due to suspected bacterial aetiology. The initially suspected aetiology and the FebriDx® test result coincided in 174 (80.5%) patients. In the 42 cases in which there was discordance, FebriDx® diagnosed 28 (71.8%) of the 39 cases initially suspected to be bacterial as viral, and 14 (8.0%) of the 174 cases initially suspected to be viral as bacterial.
Modification of the initial treatment plan was made in 22 (52.4%) of the 42 discordant cases, representing an impact on case management in 10.2% of all patients. Eleven (39.3%) of the 28 cases with initial bacterial orientation did not receive ATB (10 pneumonia and one bronchospasm) and 11 (78.6%) of the 14 cases initially suspected to be bacterial received ATB (5 URTI, 3 bronchospasms, 2 flu syndromes and one acute otitis media). The specific impact of FebriDx® according to clinical diagnosis is shown in Fig. 1. Pneumonia was the condition with the greatest impact (34.5%).
In clinical follow-up, in three of the 11 cases in which the initial treatment plan was modified and ATB was not prescribed, the viral aetiological diagnosis was confirmed (two cases of influenza A and one of enterovirus infection). One of the patients with influenza A was prescribed ATB five days after the A&E consultation due to suspicion of bacterial superinfection. No other patients were prescribed ATB, and all of them made a good recovery.
DiscussionTo our knowledge, this is the study with the largest sample size to evaluate the utility of FebriDx® in the management of paediatric patients with febrile ARI in routine clinical practice. The results obtained support the idea that FebriDx® could be a useful tool in A&E to optimise ATB prescribing in these patients.
In our series, FebriDx® was found to be especially useful in patients with suspected ARI of bacterial aetiology, changing the aetiology in almost three out of four cases. This result is in line with that described in the pilot studies by Davidson4 and Onrubia and González,7 in which this proportion is around 80%. Pneumonia was the clinical condition in which the greatest impact was observed, with ATB being stopped in one in every three cases and these patients making a good recovery with symptomatic treatment. Various studies have analysed different biomarkers for the aetiological differentiation of pneumonia, with conflicting results,9 so FebriDx® could be especially useful in the evaluation of this disease.
In patients initially suspected to be viral, the utility of FebriDx® is much more limited, not reaching 10% of the patients tested. Overall, these results would imply a theoretical impact on the management of patients with febrile ARI close to 20%, a much lower percentage than that described in the two studies cited (48% and 87.5% respectively). This discrepancy could be explained by the selection of the sample. The inclusion of an adult population in Davidson’s study4 and exclusively patients with suspected bacterial aetiology in the Onrubia and González study7 meant that the prior intention to treat with ATB in both studies (57% and 100% respectively) was much higher than that found in ours (18.1%). This rate also contrasts with higher rates observed in A&E in different European countries, with antibiotic prescription rates of 15–67% for upper respiratory tract ARI and 24–87% for lower respiratory tract ARI,10 as well as in other healthcare areas.11,12
It should be noted that a chest X-ray was performed on almost half of the patients, although only 30% were abnormal. This result would support the utility of FebriDx® not only for the optimisation of ATB treatment, but also for the reduction of unnecessary irradiation, a finding in line with other studies which have evaluated the impact of different rapid viral diagnostic tests in the management of patients with febrile ARI.13–15
This study has a series of limitations. It is a single-centre study carried out in the A&E of a tertiary hospital, so it may not be possible to extrapolate the results to medical centres with different profiles. The inclusion of a high number of patients with URTI may have reduced the overall impact percentage, as it is a condition of presumably viral aetiology in most cases. Also, in half of the cases in which the aetiological orientation was discordant, the prior treatment intention expressed by the paediatrician prevailed, which could lead to bias in the interpretation of the results. However, the majority of these cases were initially suspected to be bacterial, so if they had been managed according to the FebriDx® test result, the real impact would have been notably greater than that found.
In conclusion, FebriDx® could be a useful tool in the management of paediatric patients with febrile ARI to optimise antibiotic prescribing, especially with conditions, such as pneumonia, in which the aetiology is more difficult to determine. It could also be useful in reducing unnecessary chest x-rays.
FundingThis work was funded by Lumos Diagnostics Inc.
Conflicts of interestNone.