Serological test for primary syphilis could be negative the first 5–15 days. The aim of this study was to evaluate the benefit of including dark field microscopy (DFM) in the diagnosis algorythm for primary syphilis.
Materials/methodsPatients attended to a sexual transmission diseases clinic of Madrid, from 2015 to 2019, for a genital ulcer with clinical suspicion of primary syphilis. They were tested for DMF and serological test (EIA/TPPA/RPR).
ResultsOver the total amount of samples (806), 53.2% (429) were positive for DFM. Thus, the 48% of the 429 patients had negative serological test (EIA/RPR) of which the 77.6% were positive at TPPA.
ConclusionsDFM allows primary syphilis early diagnosis, even without serological test. If no direct detection methods are available, for patients without history of syphilis, TPPA could help to diagnose primary syphilis.
La serología luética en la sífilis primaria puede ser negativa los primeros 5–15 días. El objetivo de este trabajo fue evaluar los beneficios de incluir la microscopia de campo oscuro (MCO) en el algoritmo diagnóstico de la sífilis primaria.
MetodologíaSe incluyó a todos los pacientes que acudieron a una clínica de infecciones de transmisión sexual de la Comunidad de Madrid entre 2015 y 2019 que presentaban una úlcera genital sospechosa de sífilis primaria. Se les realizó MCO y serología (EIA/TPPA/RPR).
ResultadosDe las 806 muestras, el 53,2% (429) fueron positivas para MCO. De los 429, el 48% presentaba screening serológico negativo (EIA/RPR) y de ellos en el 77,6% el TPPA fue positivo.
ConclusionesLa MCO permite un diagnóstico de sífilis primaria precoz, incluso sin confirmación serológica. Si no se dispone de técnicas directas, en primoinfección, la TPPA es de gran ayuda en el diagnóstico.
Syphilis is a systemic sexually transmitted infection (STI) caused by the spirochete Treponema pallidum subsp. pallidum (Tp). In Spain in 2017, 4941 cases of syphilis were reported and the incidence has increased in recent years, especially in men who have sex with men (MSM), who represent 66% of cases.1 Treponema penetrates through the mucosa or skin and begins to multiply at the inoculation site after an incubation period that lasts approximately 21 days (between 9 and 90 days). The initial lesion is a papule, which erodes rapidly, forming an indurated and painless chancre, generally anogenital, called primary syphilis.2 Primary syphilis, especially in its atypical manifestations, can lead to misdiagnosis and, therefore, delays in treatment and a high risk of transmission.3
The diagnostic method commonly used as screening for primary syphilis is indirect, via treponemal serological (EIA, TPPA) and non-treponemal (RPR) testing.4–6 The direct diagnosis is based on the identification of Tp and provides an accurate diagnosis. Since 1998, molecular biology techniques have been developed for the detection of Tp, but the old gold standard is dark field microscopy (DFM), which provides immediate diagnosis through visualisation of mobile treponemes.4–7 This technique was applied for the first time in Vienna in 19068 and became the reference technique for the diagnosis of genital syphilitic chancre. Its use in oral or anal samples is not recommended due to the existence of saprophytic spirochetes. Good sampling and rapid visualisation (<30 min) by an expert microscopist are necessary. False negatives may be due to the application of antiseptics, antibiotics or other products to the lesion, so in these cases it could be recommended to repeat the test under optimal conditions.4–6
The objective of this study was to evaluate the benefits of including DFM in the diagnostic algorithm for primary syphilis.
Material and methodsThis was a retrospective descriptive observational study, carried out in a reference clinic for sexually transmitted infections in the Community of Madrid, in the period between January 2015 and December 2019. All patients who presented a genital ulcer with clinical suspicion of syphilitic chancre were included (806 in total). An exudate sample was taken for observation by means of DFM and, to compare the direct and indirect diagnosis, a serological test for syphilis was performed. A structured questionnaire was used to collect sociodemographic, clinical and behavioural data.
The sampling for DFM was done following the recommendations of the clinical laboratory guidelines.5 After cleaning the ulcer with a sterile gauze, the base of the ulcer was pressed and, placing the slide directly on the exudate, the sample was collected. This process was repeated three times in a row. The three samples were then visualised under a dark field microscope (Nikon, fitted with a dark field condenser). It was considered positive if live mobile spirochetes were visualised in any of the three samples.4–6
All patients without a history of syphilis underwent an automated treponemal (TP) test (CAPTIA™ Syphilis Total Antibody EIA, Trinity Biotech, Jamestown, USA) and a manual non-treponemal test (RPR-carbon Linear Chemicals, Barcelona, Spain) with serial dilutions. EIA (−)/non-reactive RPR was considered a negative serological diagnosis. In patients with a history of syphilis, only the RPR titration was performed, considering an increase in two or more dilutions to be a reinfection. A manual agglutination TP test (SERODiA®-TPPA, Fujirebio, Tokyo, Japan) was performed in patients without a history of syphilis, with negative serological tests and positive dark field microscopy.
Statistical analysis was performed using SPSS PASW statistics 18.0. Qualitative variables are given as absolute number and percentage and quantitative variables as median.
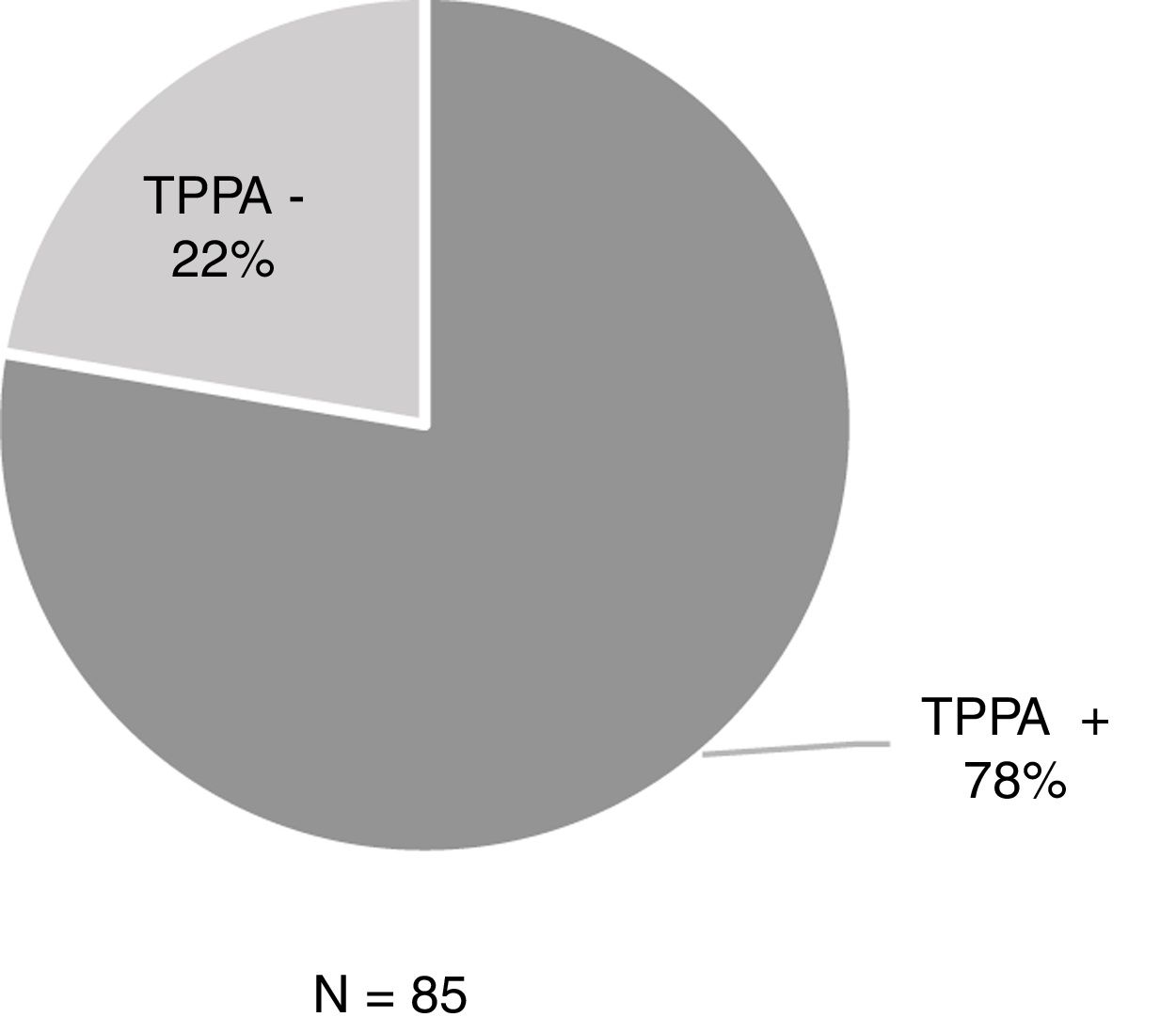
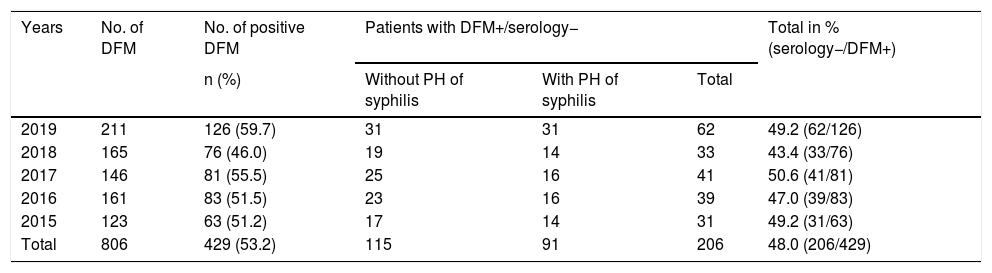
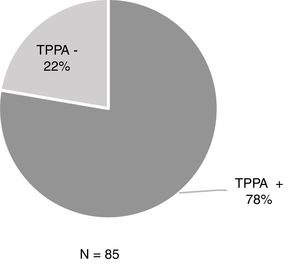
ResultsOf the 806 samples from ulcers suspected of primary syphilis, the DFM was positive in 429 (53.2%) and 48% (206/429) of these were accompanied by a negative serology test. Among the 206 patients, 44.2% (91/206) had previously had syphilis while 55.8% (115/206) had no history of syphilis (Table 1). Of these 115 patients with positive DFM and negative serology, 85 underwent TPPA and 77.6% (66/85) were positive (Fig. 1).
Patients with positive DFM and negative syphilis serology (EIA– and non-reactive RPR) with and without a history.
| Years | No. of DFM | No. of positive DFM | Patients with DFM+/serology− | Total in % (serology−/DFM+) | ||
|---|---|---|---|---|---|---|
| n (%) | Without PH of syphilis | With PH of syphilis | Total | |||
| 2019 | 211 | 126 (59.7) | 31 | 31 | 62 | 49.2 (62/126) |
| 2018 | 165 | 76 (46.0) | 19 | 14 | 33 | 43.4 (33/76) |
| 2017 | 146 | 81 (55.5) | 25 | 16 | 41 | 50.6 (41/81) |
| 2016 | 161 | 83 (51.5) | 23 | 16 | 39 | 47.0 (39/83) |
| 2015 | 123 | 63 (51.2) | 17 | 14 | 31 | 49.2 (31/63) |
| Total | 806 | 429 (53.2) | 115 | 91 | 206 | 48.0 (206/429) |
PH: past history.
Among the patients with positive DFM and negative serology, 98.5% were men and 96.6% of these were MSM. The median age was 39 years (age range: 19–69), the majority were Spanish (78.7%) and 26.7% of the patients were infected with HIV.
DiscussionThe diagnosis of primary syphilis is based on clinical suspicion and confirmation by serological analysis or identification of Tp in the syphilitic chancre, although, during the first 5–15 days, the treponemes are localised in the chancre and serology may be negative.2,5,6 Our results show that 48% of the patients with positive DFM had negative serology. This percentage is higher than that found by Wheeler et al., which was 34%.9 Although this study did not analyse the time elapsed between the onset of symptoms and the performance of the DFM, given that it is a centre without administrative barriers and without the need for a prior appointment, it is possible that the mean time elapsed was less than 10 days. This circumstance could explain the presence of positive DFM with negative serologies.
Primary syphilis is a highly infective phase, with one in three people with exposure becoming infected, so carrying out DFM enables immediate diagnosis and treatment, making it easier to identify sexual contacts and, therefore, stop the chain of transmission.4
Despite the fact that DFM is a very useful tool for the diagnosis of primary syphilis, in a large number of clinics or centres specialised in sexually transmitted infections this technique is not available or it is in disuse. Dowell et al. conducted a survey of infectious disease specialists in which 81% did not have access to DFM and only 11% used it routinely.10 Currently, new molecular biology techniques, such as multiplex-PCR, are replacing DFM, as published by Gayet-Ageron et al., who concluded that Tp-PCR performed on an ulcer sample is a highly recommended method for the diagnosis of primary syphilis.11 In the study by Heymans et al., they report that PCR has a greater sensitivity than DFM for the diagnosis of primary syphilis.12 These conclusions also coincide with those of Arando et al., who consider that these techniques will allow great advances in early diagnosis, especially of non-genital samples.2 Despite it being a very sensitive and highly recommended technique for the diagnosis of primary syphilis, it has a higher cost and is not immediate, so it does not allow diagnosis in the same visit.2,11–13
In the diagnosis of patients with a history of syphilis, performing DFM is especially useful, since the TP tests remain positive once the infection has passed and the RPR results would have to be awaited, which takes longer to become positive.2,4–6 In patients without a history of syphilis who present with both genital and extragenital ulcers, and when DFM or molecular techniques are not available, it would be beneficial to perform a TPPA. In their study, Park et al. showed that TPPA has high sensitivity in the diagnosis of primary syphilis14, as did Manavi et al. in an article in which they concluded that TPPA is the most sensitive TP test for the diagnosis of primary syphilis.15 In our study, 78% of the patients with positive DFM and negative serology (EIA−/non-reactive RPR) had a positive TPPA result.
The main limitation of DFM is the difficulty in performing it correctly, which is why it is not available at many centres. This study was carried out at a clinic with great experience in the use of DFM for the diagnosis of primary syphilis. However, this may be a limitation, as it is a single-centre study based solely on genital ulcers.
DFM has enabled the early diagnosis of primary syphilis even in patients with negative serology. If direct techniques are not available, TPPA in patients without a history of syphilis but with clinical suspicion of primary syphilis can greatly support the microbiological diagnosis.
Conflicts of interestThe authors declare that they have no conflicts of interest.
We thank all the professionals at the Centro Sanitario Sandoval [Sandoval Health Centre] for their participation.
Please cite this article as: Lejarraga-Cañas C, Ayerdi-Aguirrebengoa O, Menéndez-Prieto B, Tello-Romero E, Rodríguez-Martín C, del Romero-Guerrero J. ¿Sigue siendo útil la microscopia de campo oscuro en el diagnóstico de la sífilis primaria en el siglo XXI? Enferm Infecc Microbiol Clin. 2022;40:32–34.