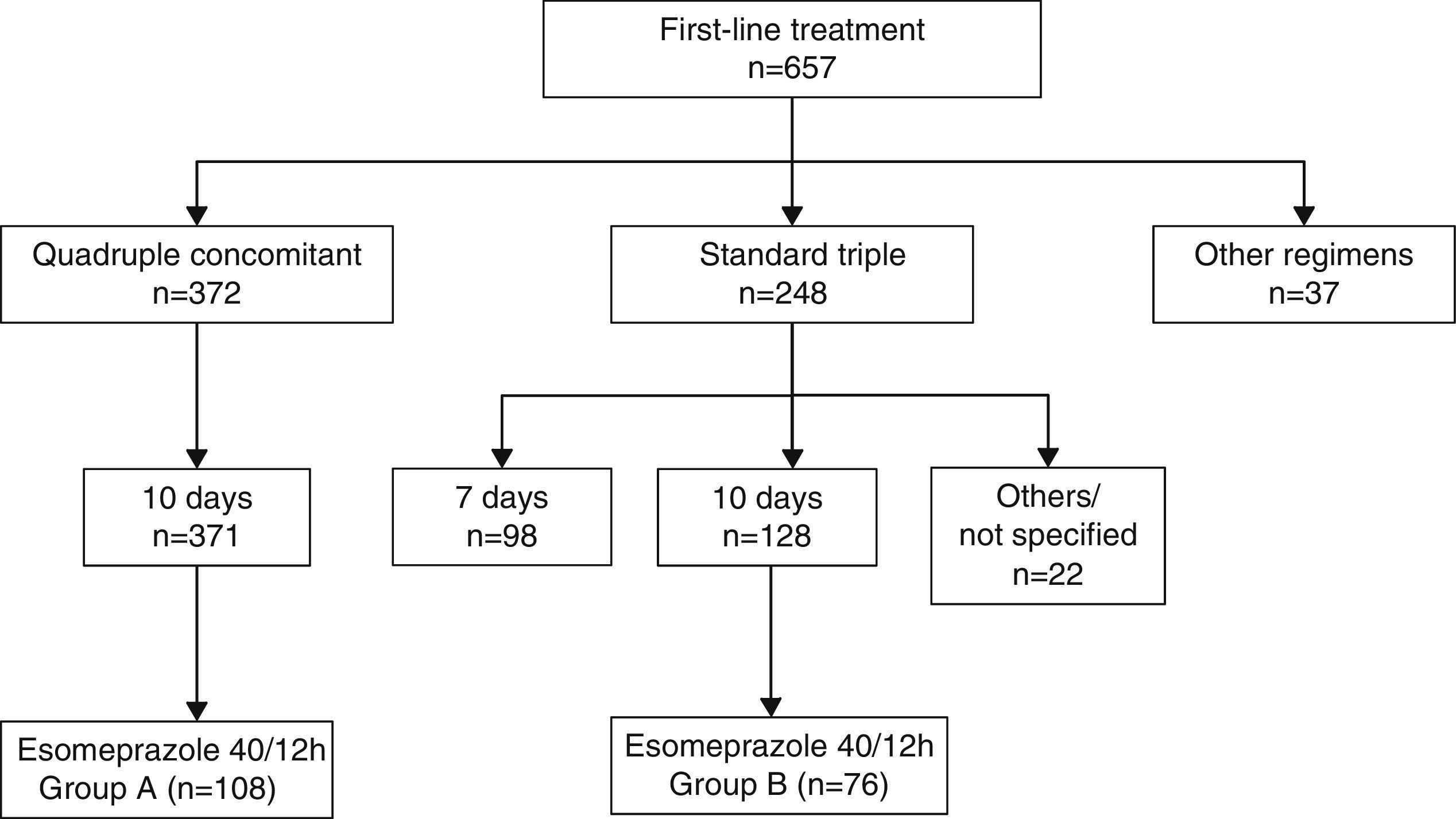
Quadruple concomitant non-bismuth therapy has recently become the most widely prescribed first-line treatment for Helicobacter pylori infection in Spain. Whether optimised conventional triple therapy can achieve comparable efficacy rates remains to be seen.
Materials and methodsRetrospective study comparing the efficacy of triple and quadruple concomitant therapy, and sub-analysis following administration of both for 10 days with esomeprazole 40mg/12h.
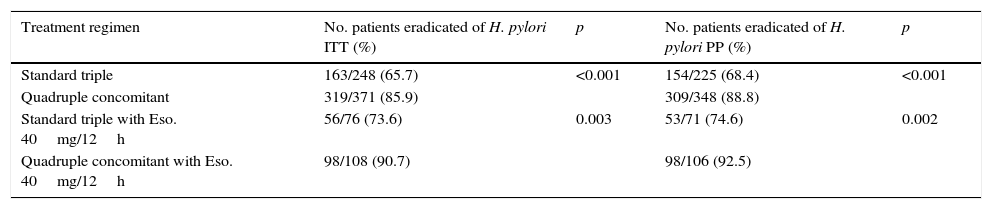
ResultsA first-line therapy was administered to 657 patients from 1st January 2012 to 31st December 2014. Quadruple therapy (n=371) showed higher efficacy than triple therapy (n=248) for both intention-to-treat (85.9% vs. 65.7%; P<.001) and per protocol analysis (92.5% vs. 68.4%; P<.001). When both therapies included esomeprazole 40mg/12h administered for 10 days, quadruple concomitant therapy (n=108) also had higher efficacy than triple therapy (n=76) for intention-to-treat (90.7% vs. 73.6%; P=.003) and per protocol analysis (92.5% vs.74.6%; P=.002).
ConclusionsQuadruple concomitant therapy with high dose proton pump inhibitor (PPI) for 10 days achieves a significantly higher eradication outcome than optimised triple therapy, with rates of over 90% when the PPI prescribed is esomeprazole 40mg/12h.
Recientemente la cuádruple terapia concomitante sin bismuto se ha postulado como tratamiento de primera línea para la infección por Helicobacter pylori en España. Se desconoce si la optimización de la triple terapia clásica puede incrementar su efectividad hasta límites aceptables.
Material y métodosEstudio retrospectivo que compara la eficacia de la triple terapia con la cuádruple concomitante realizando posteriormente un subanálisis cuando ambas se administran durante 10 días empleando esomeprazol 40mg/12h.
ResultadosDesde el 1 de enero de 2012 hasta el 31 de diciembre de 2014 recibieron al menos una primera línea de tratamiento 657 pacientes. La cuádruple combinación (n=371) presentó una eficacia superior a la de la triple terapia clásica (n=248) tanto «por intención de tratar» (85,9% vs. 65,5%; p<0,001) como «por protocolo» (88,8% vs. 68,4%; p<0,001). Cuando ambos tratamientos se administraron durante 10 días empleando esomeprazol 40mg/12h la cuádruple terapia (n=108) también fue más eficaz que la triple terapia clásica «optimizada» (n=76), tanto «por intención de tratar» (90,7% vs. 73,6%; p=0,003) como «por protocolo» (92,5% vs. 74,6%; p=0,002).
ConclusionesLa terapia cuádruple concomitante con dosis altas de IBP durante 10 días consigue tasas de erradicación significativamente superiores a las obtenidos con la triple terapia optimizada y superiores al 90% cuando se utiliza esomeprazol 40mg/12h.
At the first European conference held in Maastricht on the management of Helicobacter pylori (H. pylori) infection, a consensus was reached that only therapies with intention-to-treat eradication rates of over 80% could be recommended in clinical practice.1 However, several studies in the last decade have shown that the most common first-line treatment (amoxicillin 1g/12h+clarithromycin 500mg/12h+standard-dose proton pump inhibitor (PPI)/12h) fails in more than 20% of cases, and that these rates seem to be even higher in clinical practice in Spain.2 In our setting, we were able to corroborate this trend in a retrospective study conducted in 2011, where we observed that in 325 patients treated with the standard triple therapy approach, this regimen presented an intention-to-treat efficacy of 61.6% and per-protocol efficacy of 63.6%.3 It should be noted, though, that 7-day regimens with omeprazole at standard doses (20mg/12h) were mainly used at that time.
The latest consensus guidelines – both European4 and Spanish5 – on the management of H. pylori infection recommend that in areas in which efficacy is acceptable (>80%), standard therapy can continue to be used, although long regimens are recommended (10–14days) with double doses of more potent PPIs, i.e. using “optimised” regimens. However, in those areas where efficacy of the standard regimen is suboptimal (less than 80%), quadruple therapy is recommended, with or without bismuth according to its availability. For this reason, since 2012, the use of optimised triple therapy with PPI, and in particular, non-bismuth quadruple concomitant therapy, has become widespread in our setting.
In order to determine whether optimised triple therapy could achieve acceptable results or whether it should be definitively ruled out in our setting, we undertook this study in order to compare the efficacy of non-bismuth quadruple concomitant therapy with standard triple therapy when both are administered for 10 days with esomeprazole 40mg/12h.
Materials and methodsRetrospective observational study comparing the efficacy of the non-bismuth quadruple concomitant regimen and the standard triple therapy regimen when both are administered for 10days with esomeprazole 40mg/12h.
We searched the register of breath tests performed in our hospital (where all the breath tests for our health area are carried out) to identify all patients who had had this test for the first time between 1 January 2012 and 31 December 2014 as an initial diagnosis of the infection or to confirm eradication after treatment. Endoscopic findings for all these patients were also reviewed. From among these patients, those who had received first-line treatment for H. pylori infection were selected, and then those who had received it in one of the previously indicated forms.
Demographic data (age and sex) were collected from the patients, as well as details of the breath test (department requesting the test and test result), treatment regimen administered (indication, drugs included and duration) and efficacy of the regimen confirmed by breath test at least 5 weeks after having completed treatment.
Data were analysed by descriptive statistics; frequencies were expressed as a percentage in the case of the categorical variables. Frequency and dispersion in the case of quantitative variables were expressed as mean and standard deviation (SD). Statistical significance of the qualitative differences was calculated using the χ2 test. The statistical package SPSS version 19.0 was used. A p value <0.05 was considered significant.
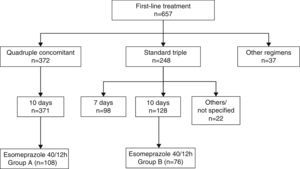
ResultsBetween 1 January 1 2012 and 31 December 2014, the breath test was carried out in our setting in 1123 new patients, 657 of whom received at least 1 first-line treatment (Fig. 1). Non-bismuth quadruple concomitant therapy was the most widely used regimen (administered to 371 patients, 56.6% of those treated) followed by standard triple therapy (n=248, 37.7% of those treated). Among the patients treated with triple therapy, 98 (39.5%) were given a 7-day course of treatment and 128 (51.6%) a 10-day course.
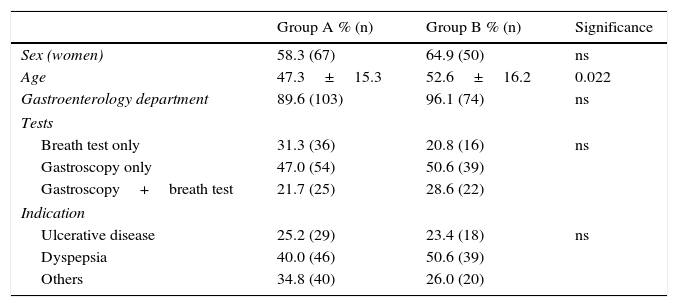
Among the 371 patients treated with non-bismuth quadruple concomitant therapy (all for 10 days), the PPI used in 108 cases was esomeprazole 40mg/12h (group A). A total of 76 patients were treated with the “optimised” standard regimen, also for 10 days and with esomeprazole 40mg/12h (group B). Both patient groups were evenly matched in terms of sex, department where they were followed-up, diagnostic tests performed and indication of eradication (Table 1). The only difference between groups was patient age, a mean of 5 years younger in Group A.
Patient demographic and clinical characteristics.
| Group A % (n) | Group B % (n) | Significance | |
|---|---|---|---|
| Sex (women) | 58.3 (67) | 64.9 (50) | ns |
| Age | 47.3±15.3 | 52.6±16.2 | 0.022 |
| Gastroenterology department | 89.6 (103) | 96.1 (74) | ns |
| Tests | |||
| Breath test only | 31.3 (36) | 20.8 (16) | ns |
| Gastroscopy only | 47.0 (54) | 50.6 (39) | |
| Gastroscopy+breath test | 21.7 (25) | 28.6 (22) | |
| Indication | |||
| Ulcerative disease | 25.2 (29) | 23.4 (18) | ns |
| Dyspepsia | 40.0 (46) | 50.6 (39) | |
| Others | 34.8 (40) | 26.0 (20) | |
The quadruple combination presented higher efficacy than the standard triple therapy, for both “intention-to-treat” (85.9% vs. 65.7%; p<0.001) and “per protocol” analysis (87.4% vs. 68.4%; p<0.001). When both treatments were administered for 10 days using esomeprazole 40mg/12h, quadruple therapy was also more effective than the “optimised” standard triple therapy for both “intention-to-treat” (90.7% vs. 73.6%; p=0.003) and “per protocol” analysis (92.5% vs. 74.6%; p=0.002) (Table 2).
Efficacy of the different treatment regimens.
| Treatment regimen | No. patients eradicated of H. pylori ITT (%) | p | No. patients eradicated of H. pylori PP (%) | p |
|---|---|---|---|---|
| Standard triple | 163/248 (65.7) | <0.001 | 154/225 (68.4) | <0.001 |
| Quadruple concomitant | 319/371 (85.9) | 309/348 (88.8) | ||
| Standard triple with Eso. 40mg/12h | 56/76 (73.6) | 0.003 | 53/71 (74.6) | 0.002 |
| Quadruple concomitant with Eso. 40mg/12h | 98/108 (90.7) | 98/106 (92.5) |
Eso.: esomeprazole; ITT: intention-to-treat; PP: per protocol.
The combination of a PPI together with amoxicillin and clarithromycin has been the most widely used regimen in Spain as first line treatment for H. pylori infection. However, the efficacy of this regimen has decreased in recent years, with the result that non-bismuth quadruple regimens (both sequential and, above all, concomitant) are becoming more widespread. Our hospital is no exception, and after observing the suboptimal efficacy of triple therapy,3 non-bismuth quadruple concomitant therapy (PPI, amoxicillin, clarithromycin and metronidazole, administered together every 12h for 10days) has become the most widely prescribed first-line treatment in recent years, with an “intention-to-treat” efficacy of 85%.
However, as shown in a Spanish multicentre study in 2011,6 the rate of resistance to clarithromycin in Spain is around 12%, so theoretically this regimen could continue to be useful. It should also be noted that when we evaluated the efficacy of triple therapy in 2011 in our setting, it was mainly administered for 7 days using omeprazole at single doses (20mg/12h). Following the publication of the Maastricht IV Consensus Report4 in 2012, “optimised” triple regimens, i.e. longer courses of treatment (for 10–14days) using double doses of second-generation PPIs, became more widespread. This new approach was based on findings suggesting that increasing the length of treatment could increase efficacy by 5%, and prescribing high-dose second-generation PPI instead of omeprazole 20mg/12h could increase the efficacy of triple therapy by 8–12%.
In the present study, in which both regimens were administered for 10days with esomeprazole 40mg/12h, triple therapy continues to present suboptimal outcomes, suggesting that it should no longer be used, at least in our setting. Based on current recommendations, triple therapy must be administered for 14days; despite this, however, its efficacy is not comparable to that of the quadruple therapy, as shown recently in the OPTRICON study,7 in which 16 Spanish centres participated.
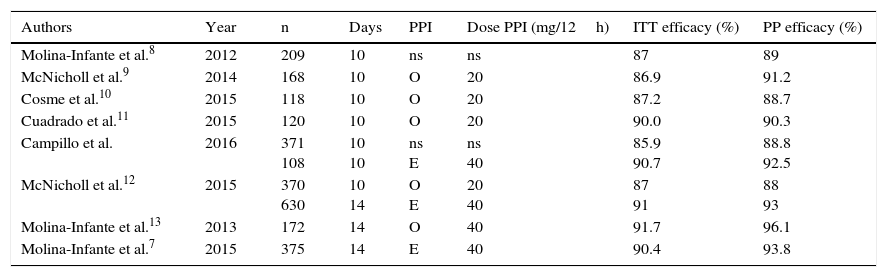
In our series, the 10-day course of quadruple therapy–without specifying whether older or new-generation PPIs were used, or the dosage administered–had an “intention-to-treat” efficacy of 85.9%, but when prescribed with esomeprazole 40mg/12h for the same length of time, the efficacy was 90.7%. It is important to point out that the level of efficacy demanded in H. pylori therapies is increasing, and that eradication rates below 90% are no longer considered wholly satisfactory. In studies conducted in Spain on the efficacy of a 10-day course of non-bismuth quadruple concomitant therapy, the “intention-to-treat” efficacy did not reach 90% when the treatment duration was 10days, except in the subgroup treated with esomeprazole 40mg/12h in the present study (Table 3) and in the series by Cuadrado et al.10 However, eradication rates of over 90% were achieved in studies in which concomitant quadruple therapy was administered for 14days, regardless of the PPI administered, strongly suggesting that prolonging treatment also increases efficacy in the case of quadruple therapy administered for 10 days with omeprazole. Nevertheless, in view of the findings of the foregoing studies, there is no evidence that extending treatment from 10 to 14 days increases efficacy when concomitant therapy with high-dose esomeprazole is administered.
Studies that have evaluated the efficacy of non-bismuth quadruple concomitant therapy in Spain.
| Authors | Year | n | Days | PPI | Dose PPI (mg/12h) | ITT efficacy (%) | PP efficacy (%) |
|---|---|---|---|---|---|---|---|
| Molina-Infante et al.8 | 2012 | 209 | 10 | ns | ns | 87 | 89 |
| McNicholl et al.9 | 2014 | 168 | 10 | O | 20 | 86.9 | 91.2 |
| Cosme et al.10 | 2015 | 118 | 10 | O | 20 | 87.2 | 88.7 |
| Cuadrado et al.11 | 2015 | 120 | 10 | O | 20 | 90.0 | 90.3 |
| Campillo et al. | 2016 | 371 108 | 10 10 | ns E | ns 40 | 85.9 90.7 | 88.8 92.5 |
| McNicholl et al.12 | 2015 | 370 630 | 10 14 | O E | 20 40 | 87 91 | 88 93 |
| Molina-Infante et al.13 | 2013 | 172 | 14 | O | 40 | 91.7 | 96.1 |
| Molina-Infante et al.7 | 2015 | 375 | 14 | E | 40 | 90.4 | 93.8 |
E: esomeprazole; ITT: intention-to-treat; ns: not specified; O: omeprazole; PP: per protocol; PPI: proton pump inhibitor.
With respect to triple therapy, some studies have shown that the use of high-dose second-generation PPIs increases treatment success rates, especially since the publication of a meta-analysis in 2012 that reviewed the role of PPIs in triple regimens.14 This study showed higher efficacy of second-generation PPIs, and in the case of esomeprazole, while the difference between first-generation PPIs was moderate, higher efficacy was achieved especially when used at doses of 40mg/12h. However, few studies are currently available on the possible benefit of using high-dose second generation PPIs in quadruple concomitant treatment. To date, the present study is the first in Spain to evaluate the efficacy of high-dose esomeprazole in a 10-day course of quadruple concomitant therapy. The role of PPIs in concomitant therapy was not the main aim of our study, but it would be interesting to evaluate in larger, preferably prospective studies, whether they can make a difference in this case.
Some recent reviews have examined the relationship between the efficacy of empirically-administered regimens and resistance to the different antibiotics.15,16 As explained in these studies, the efficacy of triple therapies containing PPI, amoxicillin and a third antibiotic is highly dependent on resistance to the third drug. A recent study in the Andalusia region of Spain has highlighted significant variability in resistance to clarithromycin among different centres in the same region.17 Resistance may be less than 15% in some areas, and in these, triple therapy may continue to be useful. We have no data on antibiotic resistance in our setting, but the results obtained suggest rates of resistance to clarithromycin of >20%, since optimised standard triple therapy with PPI fails to achieve an eradication rate of 80%. In the aforementioned study by Graham et al.,15 the authors explained the importance of dual resistance to clarithromycin and metronidazole in non-bismuth quadruple concomitant therapy, since in the case of dual resistance, treatment efficacy depends solely on the amoxicillin and the PPI. Thus, it is estimated that the efficacy of quadruple concomitant therapy will exceed 90% only in areas where dual resistance is less than 15%. This appears to be the case in southern Europe, and corroborates our findings. This theory would also explain the suboptimal eradication rates obtained by quadruple therapy in countries where resistance to metronidazole is greater than 60%18 or there is a risk of high dual resistance (e.g. second-line after triple therapy with metronidazole or clarithromycin).
Our study has a series of notable limitations: it is retrospective and not randomised, with populations that are not completely uniform. Furthermore, neither treatment compliance nor adverse effects were measured. Nevertheless, it has a considerable sample size and has little risk of inclusion bias as there is a single site for performing the breath test in our setting, and gastroscopy reports were also reviewed.
In short, this study highlights that in our setting, standard triple therapy does not reach the efficacy level recommended by guidelines and consensus conferences, even when this treatment is administered for 10days with double doses of esomeprazole. These findings support the use of quadruple concomitant therapy as first-line treatment in the treatment of H. pylori infection.
Conflict of interestThe authors declare that they have no conflict of interest.
Please cite this article as: Campillo A, Amorena E, Ostiz M, Kutz M, LaIglesia M. Triple terapia 10 días con esomeprazol 40mg/12h vs. cuádruple concomitante sin bismuto como tratamiento de primera línea de la infección por Helicobacter pylori. Gastroenterol Hepatol. 2016;39:584–589.