Variceal upper gastrointestinal bleeding (UGIB) can trigger acute hypoxic hepatitis (AHH). The aim of this study was to analyse the incidence, associated risk factors and mortality of AHH after variceal UGIB.
Patients and methodsRetrospective study of cirrhotic patients with variceal UGIB, classified into two groups according to the development of AHH. AHH was diagnosed when AST and ALT reached levels 10 times above the upper limit of normal, after ruling out other causes of hepatitis. The standard initial treatment consisted of haemodynamic support, emergency endoscopy with rubber band ligation, somatostatin and antibiotics. In the case of failure of primary haemostasis, a transjugular intrahepatic portosystemic shunt (TIPS) was implanted. Both groups (AHH and non-AHH) were compared.
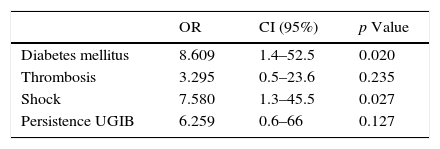
ResultsSixty-eight cirrhotic patients with variceal UGIB admitted to the gastroenterology department of Hospital Ramón y Cajal between January 2007 and March 2012 were analysed. Eleven of these patients (16.2%) developed AHH. Univariate analysis showed the following items as risk factors: diabetes (OR: 7.5; CI: 1.9–29), shock (OR: 8.5; CI: 2.06–34) and persistent bleeding (OR: 9.0, CI: 1.6–49, p=0.03). However, multivariate analysis confirmed only diabetes (OR: 8.61; CI: 1.4–52.5) and shock (OR: 7.58; CI: 1.26–45.51) as risk factors. Mortality rate in the AHH group was 45%, compared to 10.5% in the non-HAA group (p=0.012).
ConclusionsAHH after variceal UGIB occurred in 16.2% of cirrhotic patients and was associated with a poorer prognosis, with a mortality rate of 45%. Our findings suggest that diabetes and shock are risk factors for the development of AHH. Early identification of at-risk patients could therefore help prevent AHH.
La hemorragia digestiva alta por varices esofagogástricas (HDA por VEG) puede desencadenar una isquemia hepática aguda (IHA). El objetivo de este estudio fue analizar la incidencia de IHA tras una HDA por VEG, los factores de riesgo y su mortalidad.
Pacientes y métodosEstudio retrospectivo sobre pacientes cirróticos con HDA por VEG. Se clasificaron en 2 grupos, determinados por el desarrollo o no de una IHA. Definimos IHA como AST y ALT por encima de 10 veces el valour basal, descartando otras causas de hepatitis aguda. El tratamiento inicial estándar fue soporte hemodinámico, endoscopia urgente con ligadura con bandas y/o escleroterapia, somatostatina y antibióticos. En caso de fracaso de estas medidas, se recurrió a la implantación de una derivación portosistémcica percutánea intrahepática (DPPI). Ambos grupos (IHA y no-IHA) fueron comparados.
ResultadosDurante un periodo de 5 años, se recogieron 68 pacientes con HDA por VEG. La incidencia de IHA fue del 16,2%. Tras el análisis univariante, los factores asociados con IHA fueron la diabetes mellitus (OR: 7,5; IC: 1,9-29), shock (OR: 8,5; IC: 2,06-34) y la persistencia de la hemorragia (OR: 9, IC: 1,6-49, p=0,03). En el análisis multivariante solo mostraron significación estadística la diabetes mellitus (OR: 8,61; IC: 1,4-52,5) y el shock (OR: 7,58; IC: 1,26-45,51). La mortalidad del grupo de IHA fue mayor (45%) que en el grupo no-IHA (10,5%) (p=0,012).
ConclusionesLa IHA tras una hemorragia digestiva por VEG en el paciente cirrótico ocurrió en el 16,2%, asociándose con un peor pronóstico y una mortalidad del 45%. Nuestros resultados sugieren que la diabetes mellitus y el shock hipovolémico son factores de riesgo para el desarrollo de IHA. La detección precoz de estos pacientes en riesgo podría por tanto ayudar a prevenir la IHA.
Acute hepatic ischaemia, also called ischaemic or hypoxic hepatitis, is the result of hypoxia of the liver tissue, causing acute centrilobular necrosis.1 Ischaemia-hypoperfusion of liver tissue is not the only mechanism involved, so many authors prefer the name acute hypoxic hepatitis (AHH) for this entity.2 The liver is much more resistant than other organs to hypoperfusion due to its dual blood supply system, with 75% from the portal vein and 25% from the hepatic artery. However, AHH can occur in different conditions such as cardiovascular disease, respiratory failure or circulatory shock (sepsis, hypovolaemia). A prospective study conducted in an intensive care unit (ICU) over a 10-year period found that incidence of AHH could be as high as 0.9%.3 The severity of this entity is determined by the underlying disease, and can reach a mortality rate of 72%.4
AHH is diagnosed when transient markedly elevated transaminases are observed within a compatible clinical picture such as those mentioned above, ruling out other causes of elevated enzymes.5 This cut-off point has been debated, and an increase in transaminases of up to 10 times the upper limit of normal is considered reasonable for the diagnosis of AHH.6
Cirrhotic patients have impaired liver function, so it seems logical that they might be more susceptible to the development of AHH than patients with no previous liver disease. As a result of portal hypertension, cirrhotic patients can develop gastric and/or oesophageal varices (GEV) during the course of their disease. Gastrointestinal (GI) bleeding in these cases has a mortality of 15% per se, which can increase to 80% in the event of development of AHH.7–9 According to Amitrano et al.,9 incidence of AHH in cirrhotic patients following an upper gastrointestinal bleed (UGIB) varies from 1.5% to 12%.9 Factors that have been related with higher mortality are high international normalised ratio (INR), sepsis-related organ failure assessment (SOFA) score, renal replacement therapy and septic shock.10,11
The aim of this study was to analyse the incidence and characteristics of AHH after a variceal UGIB, and to assess the associated factors.
Patients and methodsA total of 68 patients with liver cirrhosis who presented variceal UGIB between January 2007 and March 2012 were included retrospectively. Patients under 18 years of age, liver transplant recipients, patients in whom endoscopy was not performed within the first 24h of the UGIB, and patients whose progress during admission was insufficiently documented were excluded. The diagnosis of cirrhosis was made either by previous liver biopsy or by clinical-laboratory and ultrasound findings. The diagnosis of bleeding was made based on the clinical presentation and analytical findings, and active bleeding due to GEV was considered in accordance with the Baveno III criteria.12 The recommendations of the Baveno V consensus were used to define failure to control bleeding: presence of fresh haematemesis or nasogastric tube output greater than 100mL≥2h after the start of specific pharmacological or endoscopic treatment; development of hypovolaemic shock; or 3g drop in haemoglobin within a period of 24h if no transfusion is administered.13 Re-bleeding was considered when the above-mentioned events occurred ≥24h after the initially controlled UGIB.
The cause of the bleeding was identified by oral endoscopy (gastroduodenal endoscopy), which was performed within the first 12h of the episode in all cases. Diagnosis of AHH was based on the following criteria: transaminase levels of over 10 times the upper limit of normal (aspartate aminotransferase [AST]>500IU/mL and alanine aminotransferase [ALT]>400IU/mL) and absence of other causes of elevated transaminases.6 To that end, serologies were performed (hepatitis A, hepatitis B, hepatitis C and in some cases other more specific serologies such as hepatitis E, cytomegalovirus or Epstein Barr virus), autoimmune antibodies, ceruloplasmin, and blood and urinary copper were determined, and a detailed clinical history was taken to rule out toxic causes. Liver biopsy was not performed in any case of AHH.
All patients received the standard established treatment in the acute phase of the bleeding: fluid therapy, somatostatin (500μg bolus followed by 6mg/250cc of physiological saline in continuous infusion at 21mL/h) and infection prophylaxis with 3rd generation cephalosporin. The endoscopic treatment of choice was rubber band ligation, in some cases combined with injection of aetoxisclerol, with transjugular intrahepatic portosystemic shunt (TIPS) placement in those patients in whom primary haemostasis failed. Blood products were transfused according to the following general guidelines, although administration was individualised in each case according to clinical judgement: packed red cells if haemoglobin (Hb)<8g/dL, fresh frozen plasma if INR>2 and platelets if the platelet count was less than 50,000IU/mL.
Various clinical and analytical variables from the 68 patients with variceal UGIB were recorded and subsequently compared in patients who presented AHH and those who did not. The baseline characteristics analysed were: age, sex, comorbidity (heart disease, chronic lung disease, hypertension), medication with anti-platelet or anticoagulant drugs, aetiology of the liver disease, presence of hepatocarcinoma and/or portal vein thrombosis, Child–Pugh stage and model for end-stage liver disease (MELD) score. The following were taken into account in the acute phase of the bleeding: vital signs (heart rate and mean arterial pressure, presence of shock), endoscopic findings, need for transfusion of blood products, and laboratory test values (haemoglobin, creatinine, transaminases, lactate dehydrogenase, blood glucose). The evolution of the transaminases after admission was reviewed in all patients, identifying the time at which they peaked and their subsequent evolution. Patient progress was also assessed until hospital discharge or exitus, taking into account the development of complications and treatments received.14
The statistical package SPSS 20.00 was used for the statistical analysis. Quantitative variables were expressed as medians (interquartile range) or means (standard deviation) and qualitative variables as percentages and absolute values. The Student t test and Mann–Whitney U test were used for analysis of the former, as applicable, and Fisher's test for the latter. Statistical significance was considered when p<0.05. We later performed a multivariate logistic regression analysis of the statistically significant variables in the univariate analysis and those that we considered relevant, taking into account current scientific evidence and biological plausibility.
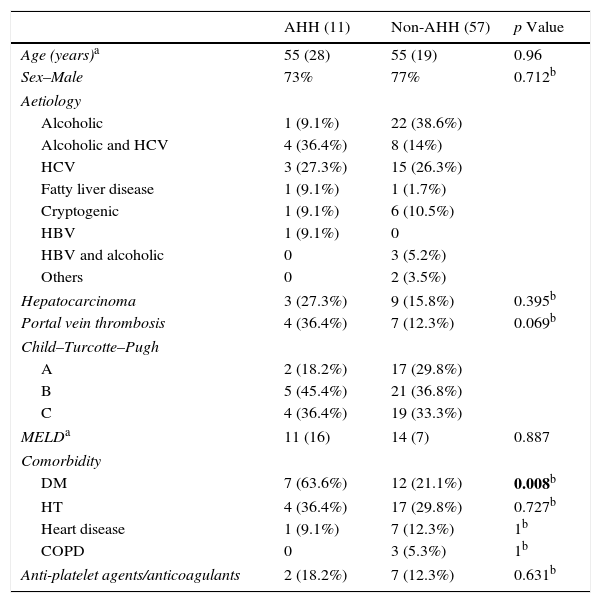
ResultsOf the 68 patients analysed, 11 (16.2%) presented AHH. Demographic characteristics of the patients with UGIB are shown in Table 1. As can be seen, both groups were made up mostly of men (73–77%), with the most common cause of liver disease being alcohol consumption and hepatitis C virus. Portal vein thrombosis (36.4% vs 12.3%; p=0.069) and hepatocarcinoma (27.3% vs 15.8%) was more prevalent in the group of patients with AHH compared with the non-AHH group, although this did not reach statistical significance in the univariate analysis. In contrast, the AHH group included a significantly greater number of patients with diabetes mellitus (DM) (odds ratio [OR]: 7.5; confidence interval [CI]: 1.9–29; p=0.008).
Demographic characteristics.
| AHH (11) | Non-AHH (57) | p Value | |
|---|---|---|---|
| Age (years)a | 55 (28) | 55 (19) | 0.96 |
| Sex–Male | 73% | 77% | 0.712b |
| Aetiology | |||
| Alcoholic | 1 (9.1%) | 22 (38.6%) | |
| Alcoholic and HCV | 4 (36.4%) | 8 (14%) | |
| HCV | 3 (27.3%) | 15 (26.3%) | |
| Fatty liver disease | 1 (9.1%) | 1 (1.7%) | |
| Cryptogenic | 1 (9.1%) | 6 (10.5%) | |
| HBV | 1 (9.1%) | 0 | |
| HBV and alcoholic | 0 | 3 (5.2%) | |
| Others | 0 | 2 (3.5%) | |
| Hepatocarcinoma | 3 (27.3%) | 9 (15.8%) | 0.395b |
| Portal vein thrombosis | 4 (36.4%) | 7 (12.3%) | 0.069b |
| Child–Turcotte–Pugh | |||
| A | 2 (18.2%) | 17 (29.8%) | |
| B | 5 (45.4%) | 21 (36.8%) | |
| C | 4 (36.4%) | 19 (33.3%) | |
| MELDa | 11 (16) | 14 (7) | 0.887 |
| Comorbidity | |||
| DM | 7 (63.6%) | 12 (21.1%) | 0.008b |
| HT | 4 (36.4%) | 17 (29.8%) | 0.727b |
| Heart disease | 1 (9.1%) | 7 (12.3%) | 1b |
| COPD | 0 | 3 (5.3%) | 1b |
| Anti-platelet agents/anticoagulants | 2 (18.2%) | 7 (12.3%) | 0.631b |
AHH: acute hypoxic hepatitis; COPD: chronic obstructive pulmonary disease; DM: diabetes mellitus; HBV: hepatitis B virus; HCV: hepatitis C virus; HT: hypertension.
Variables with significant p-values are shown in bold.
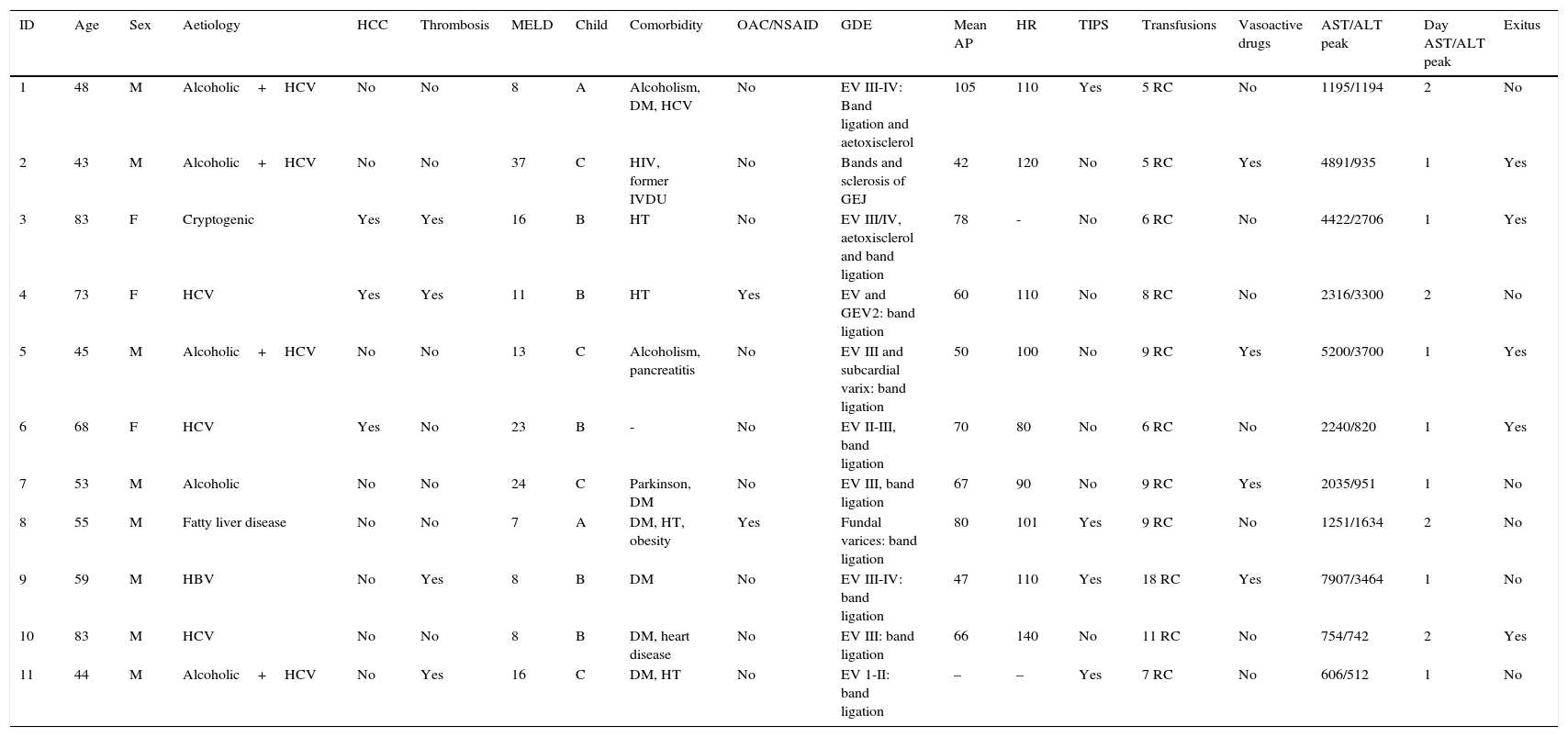
The group of patients who developed AHH was composed of 8 men and 3 women, with a median age of 55 (28) years. The Child–Pugh stage was distributed as follows: 5 stage B (45.4%), 4 stage C (36.4%) and 2 stage A (18.2%). The MELD score was 11 (16).
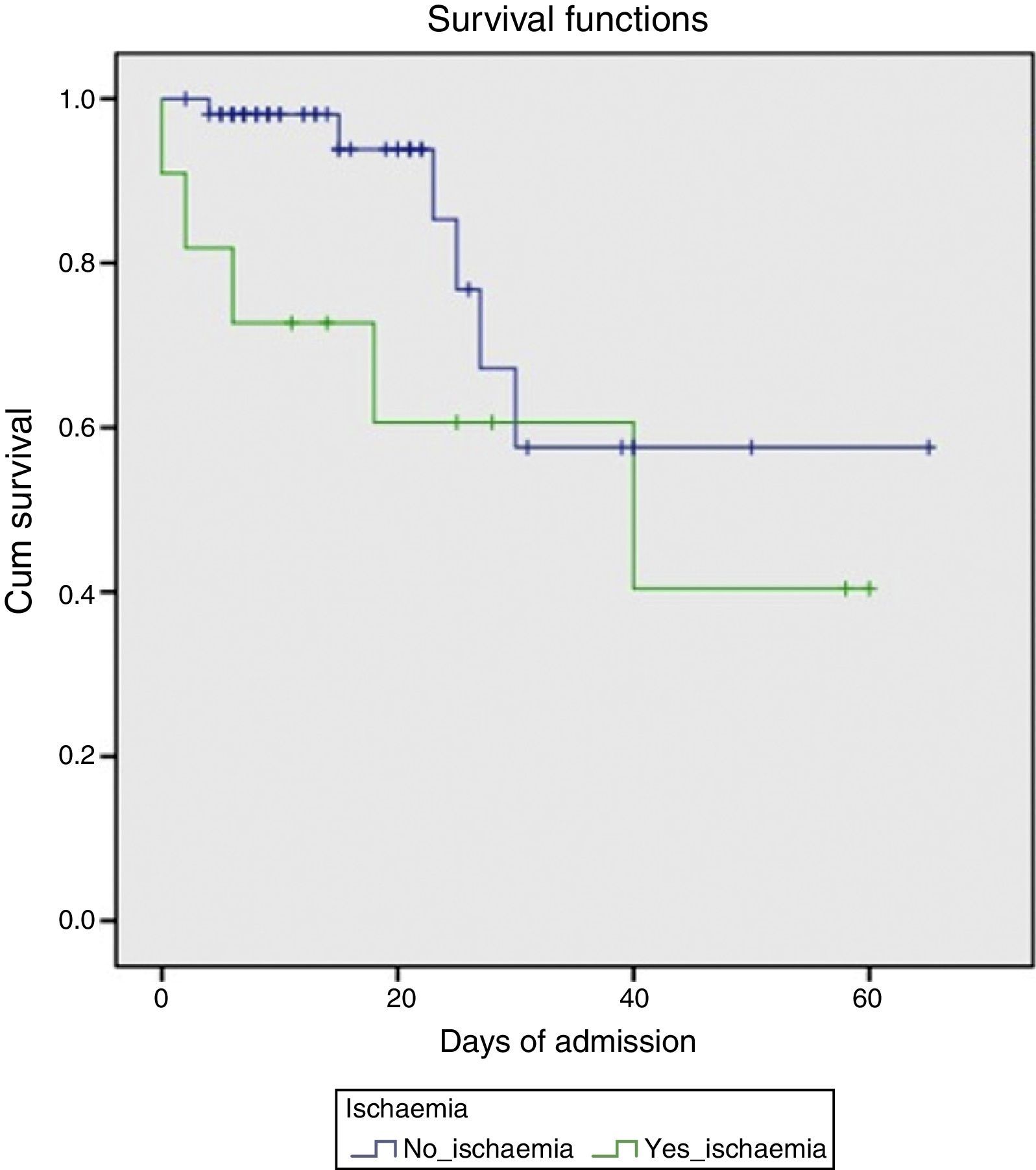
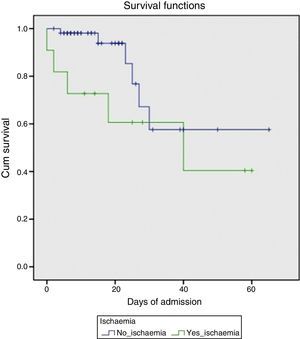
Endoscopic findings in the acute phase of the bleed are shown in Table 2. Active bleeding with blood spurting was observed in 8 of the patients. Rubber band ligation was performed in all cases; in 3 of these, the therapy was complemented with aetoxisclerol injection. Four patients later required TIPS implantation, either because of poor initial control of the bleeding (days 0 and +1 of the UGIB) or re-bleeding (day +4 and +10). All were survivors at 6 months. Transfusion of blood products was required in all cases, with a median of 7 (SD 3) units of packed red cells per patient. With respect to laboratory changes in transaminase levels, the maximum peak was reached at day +1 (7 patients) or day +2 (4 patients). A gradual decrease was observed, falling to below 400IU/mL on day +5 on average (SD 2) and returning to normal on day +8 (SD 2). Mortality in this patient group was markedly higher (45%) compared to the group that did not develop AHH (10.5%) (p=0.012). The survival probability in both groups is shown in Fig. 1.
Characteristics of patients who developed AHH.
| ID | Age | Sex | Aetiology | HCC | Thrombosis | MELD | Child | Comorbidity | OAC/NSAID | GDE | Mean AP | HR | TIPS | Transfusions | Vasoactive drugs | AST/ALT peak | Day AST/ALT peak | Exitus |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 48 | M | Alcoholic+HCV | No | No | 8 | A | Alcoholism, DM, HCV | No | EV III-IV: Band ligation and aetoxisclerol | 105 | 110 | Yes | 5 RC | No | 1195/1194 | 2 | No |
| 2 | 43 | M | Alcoholic+HCV | No | No | 37 | C | HIV, former IVDU | No | Bands and sclerosis of GEJ | 42 | 120 | No | 5 RC | Yes | 4891/935 | 1 | Yes |
| 3 | 83 | F | Cryptogenic | Yes | Yes | 16 | B | HT | No | EV III/IV, aetoxisclerol and band ligation | 78 | - | No | 6 RC | No | 4422/2706 | 1 | Yes |
| 4 | 73 | F | HCV | Yes | Yes | 11 | B | HT | Yes | EV and GEV2: band ligation | 60 | 110 | No | 8 RC | No | 2316/3300 | 2 | No |
| 5 | 45 | M | Alcoholic+HCV | No | No | 13 | C | Alcoholism, pancreatitis | No | EV III and subcardial varix: band ligation | 50 | 100 | No | 9 RC | Yes | 5200/3700 | 1 | Yes |
| 6 | 68 | F | HCV | Yes | No | 23 | B | - | No | EV II-III, band ligation | 70 | 80 | No | 6 RC | No | 2240/820 | 1 | Yes |
| 7 | 53 | M | Alcoholic | No | No | 24 | C | Parkinson, DM | No | EV III, band ligation | 67 | 90 | No | 9 RC | Yes | 2035/951 | 1 | No |
| 8 | 55 | M | Fatty liver disease | No | No | 7 | A | DM, HT, obesity | Yes | Fundal varices: band ligation | 80 | 101 | Yes | 9 RC | No | 1251/1634 | 2 | No |
| 9 | 59 | M | HBV | No | Yes | 8 | B | DM | No | EV III-IV: band ligation | 47 | 110 | Yes | 18 RC | Yes | 7907/3464 | 1 | No |
| 10 | 83 | M | HCV | No | No | 8 | B | DM, heart disease | No | EV III: band ligation | 66 | 140 | No | 11 RC | No | 754/742 | 2 | Yes |
| 11 | 44 | M | Alcoholic+HCV | No | Yes | 16 | C | DM, HT | No | EV 1-II: band ligation | – | – | Yes | 7 RC | No | 606/512 | 1 | No |
AP: arterial pressure; DM: diabetes mellitus; EV: oesophageal varices; F: female; GDE: gastroduodenal endoscopy; GEJ: gastro-oesophageal junction; GEV: gastro-oesophageal varices; HBV: hepatitis B virus; HCC: hepatocarcinoma; HCV: hepatitis C virus; HR: heart rate; HT: hypertension; ID: patient identification number; IVDU: intravenous drug user; M: male; NSAID: non-steroidal anti-inflammatory drugs; OAC: oral anticoagulants; RC: units of red cells; TIPS: transjugular intrahepatic portosystemic shunt.
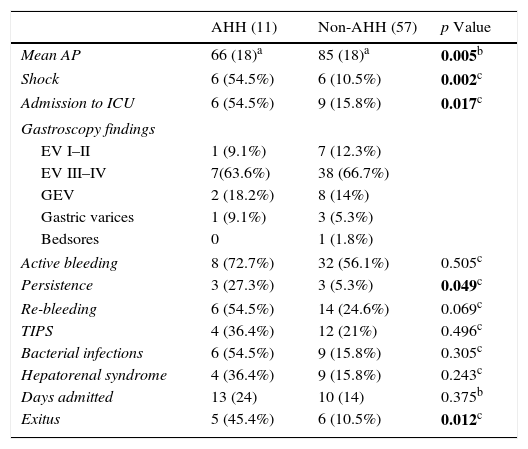
The characteristics of the acute phase of the bleeding as well as the laboratory values and subsequent changes are shown in Table 3.
Characteristics and evolution of bleed.
| AHH (11) | Non-AHH (57) | p Value | |
|---|---|---|---|
| Mean AP | 66 (18)a | 85 (18)a | 0.005b |
| Shock | 6 (54.5%) | 6 (10.5%) | 0.002c |
| Admission to ICU | 6 (54.5%) | 9 (15.8%) | 0.017c |
| Gastroscopy findings | |||
| EV I–II | 1 (9.1%) | 7 (12.3%) | |
| EV III–IV | 7(63.6%) | 38 (66.7%) | |
| GEV | 2 (18.2%) | 8 (14%) | |
| Gastric varices | 1 (9.1%) | 3 (5.3%) | |
| Bedsores | 0 | 1 (1.8%) | |
| Active bleeding | 8 (72.7%) | 32 (56.1%) | 0.505c |
| Persistence | 3 (27.3%) | 3 (5.3%) | 0.049c |
| Re-bleeding | 6 (54.5%) | 14 (24.6%) | 0.069c |
| TIPS | 4 (36.4%) | 12 (21%) | 0.496c |
| Bacterial infections | 6 (54.5%) | 9 (15.8%) | 0.305c |
| Hepatorenal syndrome | 4 (36.4%) | 9 (15.8%) | 0.243c |
| Days admitted | 13 (24) | 10 (14) | 0.375b |
| Exitus | 5 (45.4%) | 6 (10.5%) | 0.012c |
AP: arterial pressure; EV: oesophageal varices; GEV: gastro-oesophageal varices; ICU: intensive care unit; TIPS: transjugular intrahepatic portosystemic shunt.
Variables with significant p-values are highlighted in bold.
In the univariate analysis, haemodynamic shock was more frequent in patients who developed AHH (OR: 8.5; CI: 2.06–34; p=0.002) compared with the non-AHH group, with mean arterial pressure values of 85 and 68mmHg, respectively. Consequently, the rate of ICU admission was higher in the former (54.5% vs 15.8%; p=0.017). Similarly, baseline Hb values in the first group were lower (8 vs 10g/dL; p=0.025).
Bleeding was also more persistent in the AAH group (OR: 9, CI: 1.6–49; p=0.03), although no statistically significant difference was found in the re-bleeding rate (OR: 2; CI: 0.6–8; p=0.228).
During evolution of the UGIB, changes in blood glucose metabolism occurred more often in the AHH group (72.7% vs 35.1%; p=0.041), which is in line with the higher proportion of diabetic patients in this group.
Multivariate logistic regression was performed on the above data (Table 4). Given the equivalence of some variables, the analysis was simplified by entering the most representative variable: the variable “shock” was used, omitting the variables “mean arterial pressure” and “admission to ICU”, while the variable “DM” was included, without considering changes in blood glucose values. Furthermore, given its biological plausibility, portal vein thrombosis was included despite being non-significant in the univariate analysis. As a result, DM (OR: 8.61; CI: 1.4–52.5; p=0.02) and the presence of shock (OR: 7.58; CI: 1.26–45.51; p=0.03) reached statistical significance.
DiscussionGastrointestinal bleeding due to GEV involves major haemodynamic changes. Liver function is already impaired in cirrhotic patients, so it seems reasonable that patients with these characteristics will be more susceptible to ischaemic damage than those with preserved liver function. In accordance with this hypothesis, we observed an incidence of 16.2%, although evidence in the literature is controversial: while Fuhrmann et al.4 and Amitrano et al.9 describe an incidence of 15% and 6.8% in cirrhotic patients, respectively, Henrion et al.8 found this phenomenon to be very rare.
AHH is typically accompanied by elevated transaminases, with a sharp increase in the first few days and subsequent gradual decrease.15 In our series, we found that levels peaked on day +1 (7 patients) or day +2 (4 patients) following the UGIB, decreasing gradually until they returned to normal values on day +8 (SD) in all patients.
After univariate analysis of the data, we observed that the factors associated with the development of AHH were the presence of DM (OR: 7.5; CI: 1.9–29), shock (OR: 8.5; CI: 2.06–34) and persistence of bleeding (OR: 9; CI: 1.6–49). Neither the presence of hepatocarcinoma nor portal vein thrombosis showed a statistically significant difference. However, the latter was found to be more common in patients with AHH (36.4% vs 12.3%; p=0.069). This, together with the evidence reported in literature and biological plausibility, might suggest that this is a problem of insufficient study power to reach statistical significance. The Child–Pugh score on admission was not among the possible predictive factors, either.
Multivariate analysis only confirmed the statistical significance of the presence of DM (OR: 8.61; CI: 1.4–52.5) and shock (OR: 7.58; CI: 1.26–45.51).
These results partially coincide with the findings published by Amitrano et al.,6 in which DM, the persistence of bleeding and portal vein thrombosis were identified as statistically significant risk factors.
Considering the natural history of DM, these results are plausible. Diabetic patients suffer micro- and macrovascular impairment throughout the course their disease, later developing complications such as nephropathy, retinopathy, cardiopathy and diabetic arteriopathy. This vasculopathy can also remain silent until it manifests itself in situations that alter the haemodynamic balance, as would be the case of variceal UGIB.16 Therefore, since diabetic patients do not have the same compensatory mechanisms as those without this disease, it is logical to assume they may have a higher tendency to develop AHH in a situation of hypovolaemia.17
The presence of shock is also an indicator of a severe bleed and/or failure of the cardiovascular system to maintain haemodynamic stability. In both situations, impaired tissue perfusion leads to situations of hypoxia, and can trigger AHH.
AHH has high mortality, particularly in cirrhotic patients: rates of 45% were observed in the AHH group in our series. Although high, our findings are more optimistic than those of other studies, in which mortality reached 83%.9 This could be related with the implantation of TIPS in 4 of our patients (36%), with a 100% 6-month survival in this group. TIPS was performed early in 2 patients (day 0 and day 1) and later in the other 2 (day +4 and +10). Early TIPS implantation (less than 72h after GI bleed) has been shown to be effective in controlling GI bleeding and in improving re-bleeding and mortality rates.18 Nevertheless, this was not the aim of our study.
The relevance of this study lies in the detection of possible risk factors for AHH, thus allowing physicians to select high-risk patients and implement preventive measures. At present, the best prevention strategy is to avoid the cause, but we know that once the possible trigger has occurred, attempts should be made to maintain haemodynamic balance as far as possible in each patient. Based on these premises, initial control of bleeding in cirrhotic patients with variceal UGIB or immediate implementation of measures in cases of re-bleeding is essential to prevent the development of AHH and consequently reduce the risk of mortality.
Our study has a number of limitations that prevent us from drawing categorical conclusions. Firstly, the retrospective nature of this study makes it impossible to rule out selection bias. Furthermore, the small sample size diminishes the study power. Similarly, the small number of cases of AHH limits the possibilities of the multivariate analysis, and can give OR values that are probably oversized.
In conclusion, despite the limitations of our study, our results show that the incidence of AHH in the context of a GI bleed due to GEV in cirrhotic patients is 16.2%, and that development of AHH clearly worsens the prognosis, with mortality rates as high as 45%. Our study shows that some factors, such as DM and hypovolaemic shock, are associated with a higher incidence of AHH; therefore, identifying these at-risk patients could be a preventive measure in AHH. The small study sample size prevented us from drawing definitive conclusions. Multicentre studies with a larger number of patients are needed to verify our hypothesis.
Conflict of interestsThe authors declare that they have no conflict of interests.
Please cite this article as: Senosiain Lalastra C, Arribas Anta J, Moreira Vicente V, Martínez González J, Maroto Castellanos M, García Sánchez MC, et al. Isquemia hepática aguda tras hemorragia digestiva alta por varices esofagogástricas. Gastroenterol Hepatol. 2016;39:590–596.