Colorectal cancer (CRC) is a major health problem. An improvement in survival has been demonstrated by performing screening colonoscopies and removing their precursor lesions, the polyps. However, colonoscopy is not infallible and multiple strategies have been proposed aimed at improving the quality of it. This review describes the endoscopic systems available to improve the detection and characterization of polyps, the different classifications for histological prediction and the current indications of advanced endoscopic diagnostic techniques.
El cáncer colorrectal (CCR) constituye un problema de salud importante. Se ha demostrado una mejoría de la supervivencia mediante la realización de colonoscopias de cribado y la extirpación de sus lesiones precursoras, los pólipos. Sin embargo, la colonoscopia no es infalible y se han propuesto múltiples estrategias dirigidas a mejorar la calidad de la misma. En esta revisión se describen los sistemas endoscópicos de que disponemos para mejorar la detección y caracterización de los pólipos, las diferentes clasificaciones utilizadas para la predicción histológica y las indicaciones actuales de las técnicas de diagnóstico endoscópico avanzado.
Colorectal cancer (CRC) screening colonoscopies with the removal of polyps has led to an improvement in survival of this cancer.1 However, a direct consequence of implementing screening programmes has been the exponential increase in the number of colonoscopies and the saturation of Endoscopy and Pathology Units.
Due to the low risk of advanced or invasive histology of most of the polyps resected in routine practice, it has been suggested that many do not need pathology assessment (“resect and discard” strategy).2 Moreover, avoiding unnecessary polypectomies of hyperplastic polyps could represent significant cost savings (“leave in situ” strategy).3,4 These strategies are based on an in vivo prediction of histology.
However, although colonoscopy is the best technique available, it is not infallible, as up to 21 % of polyps can go unnoticed,5 especially if they are small and/or flat or hidden in the folds of the colon.
For these reasons, many strategies have been developed with the aim of improving the quality of colonoscopy to optimise the detection and characterisation of lesions. These include better tolerated bowel cleansing solutions, improvements to endoscopes, devices to enhance visualisation of the mucosa and the use of computer support systems.6 In this review we describe the endoscopic systems proposed for improving the detection of colorectal polyps and prediction of histology, the different classifications used for prediction of histology, and the current indications for advanced endoscopic diagnostic techniques.
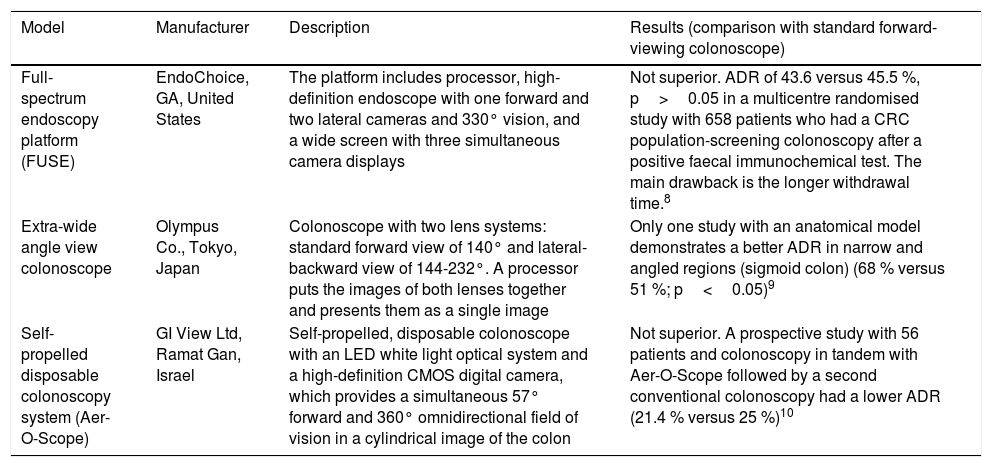
New endoscopes, devices and imaging systemsColonoscopes with wider viewing angleOne of the potential causes of polyp detection failure is the standard colonoscope's relatively narrow viewing angle (140-170°). There are currently 3 models with a wider viewing angle, although only one has demonstrated an adenoma detection rate (ADR) higher than conventional colonoscopy, with the disadvantage of a longer withdrawal time7–10 (Table 1).
Colonoscope models with wider viewing angle.
| Model | Manufacturer | Description | Results (comparison with standard forward-viewing colonoscope) |
|---|---|---|---|
| Full-spectrum endoscopy platform (FUSE) | EndoChoice, GA, United States | The platform includes processor, high-definition endoscope with one forward and two lateral cameras and 330° vision, and a wide screen with three simultaneous camera displays | Not superior. ADR of 43.6 versus 45.5 %, p>0.05 in a multicentre randomised study with 658 patients who had a CRC population-screening colonoscopy after a positive faecal immunochemical test. The main drawback is the longer withdrawal time.8 |
| Extra-wide angle view colonoscope | Olympus Co., Tokyo, Japan | Colonoscope with two lens systems: standard forward view of 140° and lateral-backward view of 144-232°. A processor puts the images of both lenses together and presents them as a single image | Only one study with an anatomical model demonstrates a better ADR in narrow and angled regions (sigmoid colon) (68 % versus 51 %; p<0.05)9 |
| Self-propelled disposable colonoscopy system (Aer-O-Scope) | GI View Ltd, Ramat Gan, Israel | Self-propelled, disposable colonoscope with an LED white light optical system and a high-definition CMOS digital camera, which provides a simultaneous 57° forward and 360° omnidirectional field of vision in a cylindrical image of the colon | Not superior. A prospective study with 56 patients and colonoscopy in tandem with Aer-O-Scope followed by a second conventional colonoscopy had a lower ADR (21.4 % versus 25 %)10 |
ADR: adenoma detection rate.
A number of different devices have been designed to be attached to the endoscope with the principal aim of increasing the lesion detection rate. However, with some of these devices the only studies carried out have been feasibility studies and the results we have are limited. Table 2 shows the main models available with a summary of the results.6,7,11–15 A meta-analysis was recently published on Endocuff including 12 clinical trials and 8376 patients. The ADR was significantly higher in the group that used Endocuff compared to standard colonoscopy (41.3 versus 34.2 %; relative risk [RR]=1.20, 95 % confidence interval [CI] 1.06–1.36; p=0.003), with the difference more marked in endoscopists with low-to-moderate ADR (<35 %): RR 1.51, 95 % CI 1.35–1.69; p<0.001).16 A recent randomised Spanish study with 711 patients comparing Endocuff with cap found no differences in ADR (50.4 % vs. 50.6 %).17
Complementary devices to the endoscope to optimise lesion detection.
| Technology/Reference | Manufacturer | Operation | Clinical application | Limitations | Impact on ADR |
|---|---|---|---|---|---|
| Third-Eye Retroscope (TER)a | Avantis Medical Systems, Inc, Sunnyvale, CA, United States | Auxiliary imaging device through the working channel to obtain retrograde image during withdrawal | Improves visualisation of the proximal side of the folds | Reduces absorption capacity by 50 %, poor image quality, has to be withdrawn to pass accessories through the working channel | Increase |
| Third-Eye Panoramica | Avantis Medical Systems, Inc, Sunnyvale, CA, United States | Cap with two LED and two lateral cameras connected by a catheter which runs through the endoscope to an external processor connected to the monitor | Wider field of vision (>300°) from three partially superimposed images | Difficulty with intubation of the ileocaecal valve | Unknown (single viability study) |
| Endocuff™, Endocuff-Vision™b | Arc Medical Design, Leeds, United Kingdom | 2 cm flexible sleeve with 1–2 rows of flexible spikes on the tip of the colonoscope | Improves visualisation of the proximal side of the folds | May cause laceration of the mucosa, exit during the procedure | Improvement, especially small lesions and endoscopists with low/moderate ADR |
| Capb | Various | Plastic adapter which is placed at the distal end of the endoscope for stability | Flattens the folds of the colon and improves exposure of the mucosa during withdrawal | It reduces the field of vision, the interposition of faecal material can hamper vision | Increases the detection of polyps but with no benefits in the ADR |
| EndoRingsb | EndoAid Ltd, Caesarea, Israel | Smooth, flexible, circular silicone rings at the tip of the colonoscope. | Improves visualisation of the proximal side of the folds and increases stability | Can hamper insertion | Modest improvement, limited data |
| G-EYEb | SMART Medical Systems Ltd, Ra’ anana, Israel | High-definition forward-viewing with a reusable and reprocessable ball at the distal end | Partially inflated balloon that straightens out the folds, reduces slippage and centres the visual optics during withdrawal | None reported | Increase |
Video endoscopy equipment uses white light which is reflected onto the tissues and recovered by a Charge-Coupled Device at the end of the endoscope. The white light covers virtually the entire spectrum of visible light, from 400nm to 700nm. High-definition endoscopy has more scan lines and horizontal pixels than conventional systems (>850,000 pixels instead of the conventional 367,000), allowing structures to be observed in more detail. Additionally, the magnification means the image can be enlarged up to 150 times.18
Study of the pit pattern using the Kudo classification is performed with high-definition endoscopy, chromoendoscopy (with methylene blue, indigo carmine or crystal violet) and magnification19 and has been validated in numerous studies. A meta-analysis, which included 20 studies with a total of 5111 colorectal lesions in 3418 patients, obtained a sensitivity of 89 % and a specificity of 85.7 % for the differentiation of neoplastic and non-neoplastic lesions with magnification. The results were even better in the chromoendoscopy with magnification subgroup (sensitivity 92.7 % and specificity 87.3 %).20 In addition, there is good inter-and intra-observer agreement in endoscopists who are experts in prediction of histology using the Kudo classification, with average kappa values of 0.776 and 0.862 respectively.21
In Europe, endoscopes with magnification are uncommon. A prospective study which randomly assigned 660 patients to have a colonoscopy with or without magnification obtained a diagnostic accuracy of 92 % (372/405) in the magnification group, significantly higher than in the non-magnification group (68 %, 278/407), for differentiation of neoplastic/non-neoplastic lesions ≤10mm.22
The degree of experience also has an effect on results and inexpert endoscopists tend to confuse small neoplastic (≤5mm) with non-neoplastic lesions and, conversely, wrongly label large non-neoplastic lesions (>5mm) as neoplastic.22,23
Regarding the detection of lesions, in a recent meta-analysis of 40 studies comparing different endoscopic modalities, chromoendoscopy obtained better results in the detection of adenomas than white light, whether standard (OR 1.53; 95 % CI 1.22–1.93) or high definition (OR 1.30; 95 % CI 1.06–1.60). However, the authors conclude that additional studies are required to confirm these findings.24
Narrow band imagingThe Olympus Narrow Band Imaging (NBI) system (Table 3)25 uses a filter system that narrows the wavelength of the emitted light. The equipment filters the bands corresponding to blue and green light (415nm and 540nm) and rejects the red (650nm). The short-wavelength blue light penetrates the wall of the organ very little and is strongly absorbed by the haemoglobin in the capillary network near the surface of the mucosa which has an absorption peak of 415nm. The green light penetrates further and reproduces images of the deeper vessels. These properties accentuate the vascularisation of polyps and they are therefore easier to see.
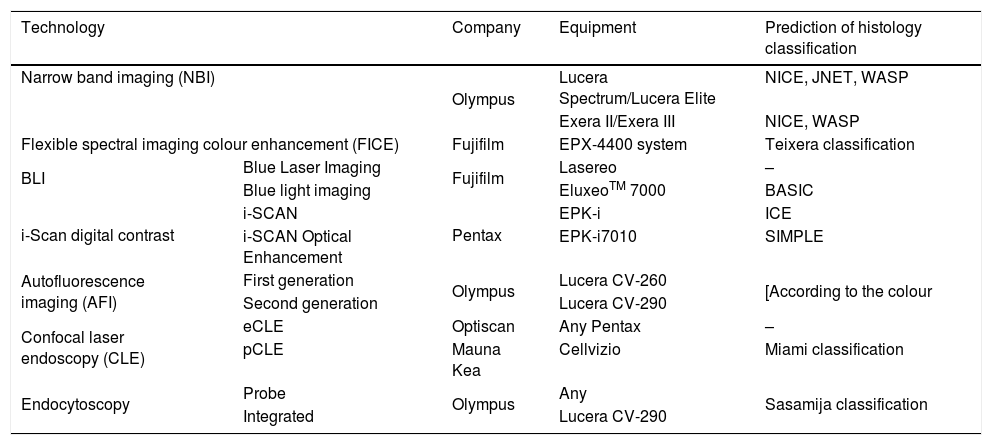
Advanced endoscopic imaging technologies: specific equipment and classification systems for prediction of histology diagnosis.
| Technology | Company | Equipment | Prediction of histology classification | |
|---|---|---|---|---|
| Narrow band imaging (NBI) | Olympus | Lucera Spectrum/Lucera Elite | NICE, JNET, WASP | |
| Exera II/Exera III | NICE, WASP | |||
| Flexible spectral imaging colour enhancement (FICE) | Fujifilm | EPX-4400 system | Teixera classification | |
| BLI | Blue Laser Imaging | Fujifilm | Lasereo | – |
| Blue light imaging | EluxeoTM 7000 | BASIC | ||
| i-Scan digital contrast | i-SCAN | Pentax | EPK-i | ICE |
| i-SCAN Optical Enhancement | EPK-i7010 | SIMPLE | ||
| Autofluorescence imaging (AFI) | First generation | Olympus | Lucera CV-260 | [According to the colour |
| Second generation | Lucera CV-290 | |||
| Confocal laser endoscopy (CLE) | eCLE | Optiscan | Any Pentax | – |
| pCLE | Mauna Kea | Cellvizio | Miami classification | |
| Endocytoscopy | Probe | Olympus | Any | Sasamija classification |
| Integrated | Lucera CV-290 | |||
Adapted from the review by East et al.25.
Atkinson et al. recently published a meta-analysis with 11 clinical trials, which included a total of 4491 individuals and 6636 polyps, and the ADR was superior with NBI than with high-definition white light (45.2 % vs 42.3 %; p=0.04); the improvement was more marked the better the bowel preparation.26
Narrow band imaging International colorectal endoscopic classificationBoth the colour of the lesion and the thickness of the vessel are subjective estimations. This has led to the development of the "NBI International Colorectal Endoscopic" (NICE) classification, based on colour, vascularisation and surface pattern.27 One key advantage of this classification system is that it can be applied in colonoscopies with or without optical magnification. Moreover, the NICE system has been validated, achieving 89 % diagnostic accuracy, 98 % sensitivity and 95 % negative predictive value to rule out the diagnosis of adenoma with high confidence for 75 % of small polyps.28
A multicentre clinical trial with 421 small colorectal polyps which compared NBI using the NICE classification and high-definition white light in optical diagnosis (neoplastic versus non-neoplastic) found no significant differences in diagnostic accuracy, sensitivity or negative predictive value (NPV) (73.7 %, 82.4 % and 75.5 % in the NBI group and 79.2 %, 79.8 % and 73.4 % in the white-light group).29 However, the diagnostic accuracy of NBI in this study was lower than reported in the literature, so the results should be interpreted with caution.
Japan narrow band imaging classificationAnother classification system developed after the NICE system is the "Japan NBI Expert Team" (JNET) classification,30 which subdivides NICE 2 lesions into type 2 A (low-grade adenomas), and type 2 B (high-grade adenomas including cancer superficially infiltrating the submucosa). However, the JNET classification is more complex owing to the need for magnification, which means not all endoscopists are able to use it.
Workgroup serrated polyps and polyposis classificationSessile serrated polyps are not considered in the NICE classification. The Workgroup serrAted polypS and Polyposis (WASP) classification, however, combines the NICE system and four features of sessile serrated polyps: clouded surface, poorly defined borders, irregular shape and dark spots inside the crypts. The presence of at least two features is sufficient to diagnose a sessile serrated lesion. During the validation phase, the optical diagnosis made with a high degree of confidence showed a diagnostic accuracy of 84 % and an NPV of 91 % for tiny neoplastic lesions.31
Flexible spectral imaging colour enhancementFlexible spectral imaging colour enhancement or Fujinon Intelligent Chromoendoscopy (FICE) is a post-processing technology for improving vascular and surface images using spectroscopy. Spectroscopy consists of the interaction between electromagnetic radiation (light) and matter (tissue surface), with absorption or emission of radiant energy. Unlike NBI, which uses physical optical light filters, FICE selects certain wavelengths from digitised data. The system is flexible as it has ten preset digital filter settings with the capacity to program more. For example, filter 2 highlights lesions with superficial vessels while filter 4 is useful for visualising the pattern of glandular crypts of the mucous surface.
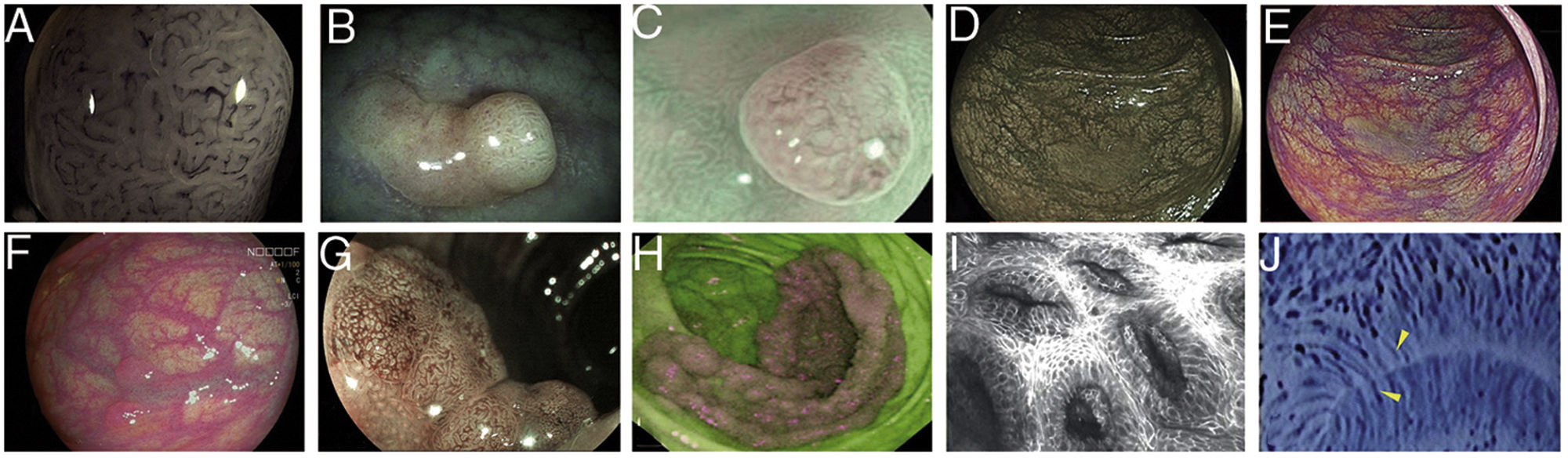
In 2009, Teixeira et al. described a classification system with a diagnostic accuracy of 98 % for differentiating non-neoplastic from neoplastic colon polyps, based on magnification and microvascularisation patterns which took into account the thickness and arrangement in relation to the glands of the vessels (Fig. 1A).32 Inter-observer agreement (0.80) and intra-observer agreement (0.73-0.88) were also very good.33
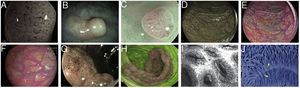
Images of polyps with different endoscopic technologies. A) Adenoma with high-grade dysplasia corresponding to category IV of the Teixeira classification for Flexible spectral imaging colour enhancement (FICE). Many long, spiriform vessels can be seen, some thicker and with disperse dilations. B) i-SCAN image (mode 3, demarcation) of a non-adenomatous colon polyp, pale in colour, similar to the surrounding mucosa. C) Mode 3 of i-SCAN Optical enhancement (OE) of a diminutive colon adenoma, which provides optical improvement of the vascular characterisation. Representative case of sessile serrated polyp in Blue Laser Imaging-Bright (BLI-Bright) mode D) and Linked Colour Imaging (LCI) mode E). F) Also with LCI, two adenomas with flat-elevated diminutive low-grade dysplasia (Paris IIa) of sigmoid colon. G) Image in Blue Light Imaging (BLI) mode of 10mm flat-elevated colon adenoma with low-grade dysplasia (courtesy of H. Uchima). H) Image of an adenomatous polyp of granular laterally spreading tumour (G-LST) of 5cm in caecum; with autofluorescence (AFI) the lesion appears purple, which contrasts with the normal surrounding mucosa, which is green. I) Hyperplastic polyp visualised by laser confocal endomicroscopy (iCLE) in which the crypts have regular openings with a homogeneous epithelial cell layer. J) Example of colon polyp with high-grade dysplasia and viewed by endocytoscopy with 450x magnification; the glands are irregularly shaped with large and distorted nuclei (category 2).
One study which applied the NICE classification (developed for NBI) to videos of polyps recorded using FICE to differentiate adenomas from hyperplastic polyps showed a diagnostic accuracy of only 77 %, with modest inter-observer and intra-observer agreement (0.51 and 0.40 respectively). This suggests that classification systems may not be interchangeable between advanced imaging modalities.34
Contrast digital i-SCANi-SCAN is another digital contrast technology based on the post-processing of the captured image. This system consists of three improvement characteristics: surface improvement; contrast enhancement, where darker (depressed) areas appear more blue; and improved tone, a type of digital image with some similarities to FICE, as the white light image is divided into its red, green and blue components. Each component can be modified independently, and the three colours then recombined to build a new digital image35 (Fig. 1B).
The i-SCAN classification systems for polyps have also been developed using pit patterns and microvascularisation characteristics. Bouwens et al.36 developed a simple system called ICE (i-SCAN Classification for Endoscopic diagnosis) based on the Kudo and NICE systems, where the colour, the epithelial surface pattern and the vascular pattern were assessed independently. The sensitivity, specificity and diagnostic accuracy for the diagnosis of adenomas were 79 %, 86 % and 81 %, respectively. Of the diagnoses, 81 % were made with a high degree of confidence and these were associated with significantly higher diagnostic accuracy compared to the remaining diagnoses.
A meta-analysis that included 925 patients and 2312 polyps obtained a sensitivity and specificity of approximately 90 % for differentiating neoplastic from non-neoplastic polyps after assessment with i-SCAN.37
i-SCAN optical enhancementi-SCAN Optical enhancement (OE) is a later version of i-SCAN which, while maintaining the post-processing, has added pre-processing using optical filters that limit the light spectrum; i.e. a combination of optical and digital chromoendoscopy. The OE technology aims to achieve a higher global transmission by connecting the peaks of the haemoglobin absorption spectrum (415, 540 and 570nm), thereby creating a continuous wavelength spectrum. It is similar to NBI as red light is reduced by increasing blue and green to highlight the morphology of the vascular pattern. However, the image appears brighter than NBI. The use of optical filters also makes it easier to see microsurface patterns on the surface of the mucosa and the vascularisation38 (Fig. 1C).
The Simplified Identification Method for Polyp Labelling during Endoscopy (SIMPLE) has recently been published for classification of tiny and small colorectal polyps using i-SCAN OE. Experts achieved diagnostic accuracy with this system of 83 % before training and 94 % after training (p=0.002). The sensitivity, specificity, positive predictive value (PPV) and NPV after training were 97 %, 88 %, 95 % and 91 % respectively. Inter-observer agreement on polyp diagnosis improved from 0.46 before training to 0.66 after. It was also demonstrated that this classification system is applicable with NBI without magnification, with comparable diagnostic accuracy post-training (0.69 versus 0.71 in NICE and SIMPLE systems, respectively; p=0.40).38
Blue laser imagingThe utility of most reduced-spectrum technologies can be limited by a dark field of vision. Blue Laser Imaging (BLI) may overcome this limitation by combining two laser light sources with 410nm and 450nm wavelengths. The 450nm laser acts on phosphorus, inducing fluorescent light which is equivalent to a xenon light source. The other laser provides better information on the mucosal surface and vascularisation by applying a limited wavelength spectrum of 410nm of blue light, similar to NBI.
The BLI system uses four modes (BLI, BLI-bright, white light or WLI and linked colour imaging or LCI) which can be selected by the endoscopist using a button on the control handle of the colonoscope. Each mode has a different effect on the contrast and it is more suitable for inspecting near images, to detect or better recognise the mucosal or microvascular pattern.39,40 (Figs. 1D and E).
One study that combined the LCI methodology with laser and the NICE classification obtained good results for the prediction of type 2 and 3 neoplastic lesions: sensitivity 96.5 %, specificity 83.8 %, PPV 90.2 % and NPV 93.9 %.41
Blue light imagingThe Blue Light Imaging (BLI) and Linked Colour Imaging (LCI) system with 4 LED technology (4-LED Multi Light technology) has recently become available in Europe42 (Figs. 1F and 1G).
The BLI Adenoma Serrated International Classification (BASIC) system is specific for the characterisation of colorectal polyps with BLI and includes surface, pit-pattern and vascularisation features . Inter-observer agreement improves if magnification is used.43
AutofluorescenceSome molecules, such as collagen, flavin and nicotinamide adenine dinucleotide phosphate, are fluorophores, which means they emit fluorescence after being excited with short-wave light. Autofluorescence imaging (AFI) is obtained in real time and its signal is altered by changes in mucosal thickness, mucosal blood flow and endogenous tissue fluorophores. Thick tissue with increased blood flow, as in adenomas, attenuates both excitement and autofluorescence signals.25
Differences in fluorescence emission between neoplastic and non-neoplastic tissues are detected by an additional CCD image sensor equipped with a filter which cuts out the blue excitation light. The video processor combines the autofluorescence signal with the mucosa reflectance of the green light used for illumination to produce a false-colour image, which can be purple, violet or green. A dysplastic lesion would stand out as a purple lesion on a green background corresponding to normal mucosa44 (Fig. 1H).
The drawbacks of this technique are that the image resolution is even lower than with standard definition white light, there may be ambiguous lesions and inflammatory or ischaemic lesions may also be violet (false positives).25
A classification system has been developed for optical diagnosis in the colon: if the lesion of interest is purple, it indicates neoplastic tissue; if green, it is not neoplastic; and if violet (intermediate), NBI should be used for discrimination.45
In a retrospective study with the first-generation AFI system, the sensitivity, specificity, PPV and NPV for differentiation of neoplastic lesions were 98.8 %, 86.4 %, 96.4 % and 95.0 %, respectively.46 In a prospective study they were 94.7 %, 88.9 %, 95.9 % and 85.2 %, respectively.47 Lastly, in a retrospective study with the second generation AFI system, the results were 94.2 %, 91.8 %, 92.5 % and 93.8 %, respectively.48
Confocal laser endomicroscopyConfocal laser endomicroscopy (CLE) was developed to visualise cell and subcellular images up to 250μm below the surface of the mucosa. It is termed "confocal" because the lighting and detection systems are in the same focal plane. Successive points in a region are scanned to build a digitised raster image. The image created is an optical section that represents one focal plane within the examined area and appears in shades of grey. Unlike the other technologies described above, CLE requires the use of contrast. The most commonly used are fluorescein, administered intravenously, and acriflavine and crystal violet, which are applied topically.
There are two CLE-based systems. The endoscope-CLE (eCLE) system is integrated into the distal tip of the endoscope and the probe-CLE (pCLE) system consists of a flexible mini-probe which can be introduced through the working channel of a standard endoscope. The eCLE allows a higher resolution, wider field of vision and deeper imaging depth, at the expense of dynamic imaging planes, unlike pCLE, which uses fixed planes and variable imaging depth.
The Mainz classification was the first classification system for colon polyps with the integrated-CLE (iCLE) method (first system integrated into the endoscope, no longer commercially available) which differentiated normal, regenerative and dysplastic epithelium25,49 (Fig. 1I). In a study with three observers, it showed a high degree of diagnostic accuracy and inter- and intra-observer agreement (0.68-0.84).50
The Miami classification was proposed for the pCLE system in 2009.51 A validation study of these criteria for histological diagnosis of colon polyps (hyperplastic versus tubular adenomas) obtained diagnostic accuracy of 84.9 %, which rose to 91 % if the prediction was made with high degree of confidence. Inter-observer agreement was k = 0.73.52
EndocytoscopyEndocytoscopy is based on the principle of contact light microscopy and allows real-time visualisation of the cellular structure of the superficial epithelial layer in a plane parallel to the surface of the mucosa. The system uses a fixed-focus, high-power objective lens that projects highly magnified images from areas less than 0.5mm in diameter. There are two prototypes: endoscope-integrated or a device which is inserted through the working channel of a therapeutic endoscope (channel ≥3.7mm). It requires contact with the mucosa, prior mucolytic application, often the use of a cap to stabilise the image, and the use of an absorptive contrast such as 0.5–1 % methylene blue or crystal violet. Endocytoscopy diagnosis is based on the assessment of various cytological and architectural features such as density, size, cell arrangement, nucleolus size and shape, nucleus-cytoplasm ratio and staining pattern53,54 (Fig. 1J).
Sasamija et al. proposed a classification system for colorectal lesions by endocytoscopy which was subsequently validated by Rotondano et al., with a PPV of 100 %, 93.1 %, 90.1 % and 100 % for non-neoplastic lesions, low-grade dysplasia, high-grade dysplasia and invasive cancer, respectively.54,55
Current indications for advanced diagnostic endoscopic techniquesThe aim of all the technologies described in the preceding section is to improve the visualisation of the mucosa with enhancement of structures and microvascular detail. In expert hands they can improve diagnostic accuracy; however, in routine practice their superiority over high-definition white light has not been systematically demonstrated. For that reason, current guidelines do not systematically recommend their use.
In terms of the detection of sporadic polyps in individuals with medium risk, in a summary of 6 meta-analyses (range 5–14 studies, 1,199-5,074 patients) examining NBI, FICE, i-SCAN and AFI, no significant benefit was found with any modality for the detection of adenomas or polyps.56 The European Society of Gastrointestinal Endoscopy (ESGE) guidelines do not support the clinical use of NBI, FICE or i-SCAN to improve polyp detection. They only recommend performing pan-colonic conventional chromoendoscopy with dyes and the use of high-definition endoscopy systems in patients with Lynch syndrome or serrated polyposis syndrome and for detection of dysplasia in long-standing ulcerative colitis.57
A meta-analysis that included 91 studies analysing the ability to characterise polyps as adenomatous or hyperplastic using NBI, FICE, i-SCAN, AFI or CLE concluded that all these techniques, except AFI (sensitivity 87 %, specificity 66 %), could be used by endoscopists appropriately trained to make optical diagnoses.58 Consequently, the ESGE supports the clinical use of NBI, FICE and i-SCAN for the optical diagnosis of diminutive polyps (≤5mm) by experts,57 while the American Society of Gastrointestinal Endoscopy (ASGE) only supports the use of NBI.59
Table 4 shows the utility of the different techniques in the detection and characterisation of polyps.25
Utility of detection techniques and optical diagnosis in clinical practice.
| High-definition white light | Magni-fication | Conventional chromoendoscopy | NBI | i-SCAN | i-SCAN OE | FICE | AFI | CLE | BLI | EC | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| "Optical biopsy" assessment of polyps | ++ | ++ | ++ | ++ | ++ | NA | ++ | +/− | ++ | +a | + |
| Sporadic detection of polyps | ++ | NA | ++ | – | +/− | + | – | +/− | NA | NA | NA |
Adapted from the review by East et al.25 The clinical utility represents the evidence and the clinical impact: ++ very useful; + useful; +/- undetermined; - no additional benefit; NA, not available.
In recent years, advances in technology have led to the development of advanced endoscopic imaging and new endoscopes and devices to improve the detection and characterisation of colorectal polyps. However, these techniques are not universally available, are dependent on the endoscope manufacturer, and require specific training, so current guidelines do not consider the use of many of them systematically. The incorporation of artificial intelligence could be a good option in the near future.
At present, based on the published results, the use of conventional chromoendoscopy is recommended in clinical practice in patients with serrated polyposis syndrome and Lynch syndrome to improve the detection of colorectal polyps. NBI technology is, however, useful to improve the characterisation of polyps, and also probably FICE and i-SCAN (both recommended by the ESGE, but not by the ASGE). Nevertheless, in view of the wide range of new systems, the best tool available in each centre should be used. There are also other techniques available to all endoscopists which can increase the detection of lesions with standard endoscopes, such as retroflexion in the right colon, double inspection in the right colon, water-assisted colonoscopy and chromoendoscopy with dyes.
Conflict of interestMaría Pellisé was a consultant for Norgine Iberia from 2012 to 2017. She has received fees for holding conferences for Norgine Iberia, Casen Recordati and Olympus Spain in the last 5 years. Gloria Fernández-Esparrach has received fees for organising courses for Norgine Iberia and Olympus Spain in the last two years and has been a consultant for CDx Diagnostics.
This work was supported by the Government of Spain through the funded projects iVENDIS (DPI2015-65286-R) and HISINVIA (PI17/00894), by the Fundación de la Sociedad Española de Endoscopia Digestiva (FSEED) [Foundation of the Spanish Society of Gastrointestinal Endoscopy], by the Secretaria d'Universitats i Recerca de la Generalitat de Catalunya [Catalan Autonomous Government Secretary of Universities and Research] (2014-SGR-1470, 2014-SGR-135, SGR-2017-1669 and SGR-2017-653), and by the Generalitat de Catalunya CERCA programme.
Please cite this article as: Sánchez-Montes C, García-Rodríguez A, Córdova H, Pellisé M, Fernández-Esparrach G. Tecnologías de endoscopia avanzada para mejorar la detección y caracterización de los pólipos colorrectales. Gastroenterol Hepatol. 2020;43:46–56.