Chronic diarrhoea is a common presenting symptom in both primary care medicine and in specialised gastroenterology clinics. It is estimated that >5% of the population has chronic diarrhoea and nearly 40% of these patients are older than 60 years. Clinicians often need to select the best diagnostic approach to these patients and choose between the multiple diagnostic tests available. In 2014 the Catalan Society of Gastroenterology formed a working group with the main objective of creating diagnostic algorithms based on clinical practice and to evaluate diagnostic tests and the scientific evidence available for their use. The GRADE system was used to classify scientific evidence and strength of recommendations. The consensus document contains 28 recommendations and 6 diagnostic algorithms. The document also describes criteria for referral from primary to specialised care.
La diarrea crónica es un síntoma de presentación frecuente, tanto en las consultas de medicina de familia como en las de digestivo. Se estima que >5% de la población sufre diarrea crónica y que cerca del 40% de estos sujetos son mayores de 60 años. El clínico se enfrenta con frecuencia a la necesidad de decidir cuál es el mejor enfoque diagnóstico de estos pacientes y elegir entre las múltiples pruebas diagnósticas existentes. En 2014 la Societat Catalana de Digestologia creó un grupo de trabajo con el objetivo principal de crear algoritmos diagnósticos en base a la práctica clínica y evaluar las pruebas diagnósticas disponibles y la evidencia científica para su utilización. Para clasificar la evidencia científica y la fuerza de las recomendaciones se utilizó el sistema GRADE. Se han establecido 28 recomendaciones y 6 algoritmos diagnósticos. Se describen los criterios de derivación desde medicina primaria a digestivo de un paciente con diarrea crónica.
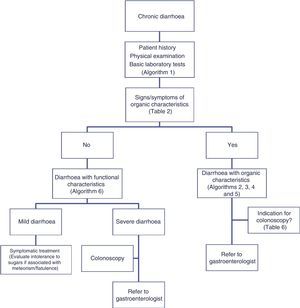
Chronic diarrhoea is a common complaint seen by both primary care doctors and gastroenterologists. According to estimates, chronic diarrhoea has a prevalence of over 5%, with over 40% of cases occurring in the over-60 age group.1 The list of possible causes is long (Table 1), and various diagnostic tests are usually needed before reaching a definitive diagnosis.1–5 Clinicians are often faced with the challenge of deciding the best diagnostic approach in these patients, and must choose between the broad array of diagnostic tests currently available. A definitive diagnosis all too often proves elusive, and many patients are diagnosed with functional or idiopathic diarrhoea.
Classification of chronic diarrhoea.
| Chronic watery diarrhoea |
| 1. Osmotic |
| Osmotic laxatives (Mg+2, PO−, SO4−2) |
| Carbohydrate malabsorption |
| Excessive consumption of poorly absorbed carbohydrates |
| Lactulose |
| Sorbitol and mannitol (“sugar-free” chewing gum) |
| Fructose (fruit, soft drinks) |
| 2. Secretory |
| Congenital chloride diarrhoea |
| Bacterial enterotoxins |
| Bile acid malabsorption |
| Inflammatory bowel disease |
| Ulcerative colitis |
| Crohn disease |
| Microscopic colitis |
| Vasculitis |
| Laxative abuse |
| Drugs |
| Food allergies |
| Heavy metal poisoning |
| Dysmotility |
| Post-vagotomy diarrhoea |
| Post-sympathectomy diarrhoea |
| Diabetic autonomic neuropathy |
| Irritable bowel syndrome |
| Faecal impaction |
| Faecal incontinence |
| Endocrine disorders |
| Addison disease |
| Hyperthyroidism |
| Gastrinoma |
| VIPoma |
| Somatostatinoma |
| Carcinoid syndrome |
| Mastocytosis |
| Other neoplasms |
| Colorectal cancer |
| Small bowel lymphoma |
| Secretory villous adenoma of the rectum |
| Idiopathic secretory diarrhoea |
| Others: amyloidosis |
| Chronic inflammatory diarrhoea |
| Inflammatory bowel disease |
| Ulcerative colitis |
| Crohn disease |
| Diverticulitis |
| Ulcerative jejunoileitis |
| Infectious diseases |
| Bacteria: Shigella, Salmonella, Campylobacter, Yersinia, Clostridium difficile |
| Viruses: herpes simplex, CMV |
| Parasites: amoebiasis, strongyloidesstercoralis |
| Ischaemic colitis |
| Radiation colitis |
| Neoplasms |
| Colorectal cancer |
| Lymphoma |
| Chronic diarrhoea with steatorrhoea |
| Enteric causes |
| Mucosal diseases: coeliac, Whipple, giardiasis, lymphoma, Crohn, radiation enteritis, gastrointestinal lymphangiectasia, amyloidosis, eosinophilic gastroenteritis, tropical sprue, sprue, collagenous colitis. |
| Short bowel syndrome |
| Bacterial overgrowth |
| Chronic mesenteric ischaemia |
| Maldigestion syndromes |
| Exocrine pancreatic insufficiency |
| Low bile acid levels in the intestinal lumen |
Several clinical practice guidelines have been published for the purpose of establishing the best investigation protocol in patients with chronic diarrhoea.2,4,6 The aim of these recommendations is to maximise positive diagnoses while minimising examinations. These guidelines need to be updated and adapted to current clinical practice, as in recent years a series of diseases with an underlying organic cause presenting with the characteristics of a “functional” disease have been identified. In fact, some authors have challenged the very existence of functional diarrhoea per se.7,8
In 2014, with the aim of helping clinicians diagnose chronic diarrhoea, the SocietatCatalana de Digestologia proposed creating a working group to draw up a consensus document on the issue. The document was presented at the association's annual conference in January 2015, and an abbreviated version is available online (http://www.scdigestologia.org/index.php?link=docs_posicio). The primary aim of the group was to create diagnostic algorithms based on clinical practice, and to evaluate existing diagnostic tests and the clinical evidence supporting their use.
Scientific evidence and strength of recommendation were classified according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system (http://www.gradeworkinggroup.org/). Table 2 shows the different categories used to grade certainty (or quality) of the evidence (CE) and strength of recommendation (SR).9
GRADE system.
| Quality of the evidence (CE) | |
| High | Further research is very unlikely to change our confidence in the estimate of effect. |
| Moderate | Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. |
| Low | Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. |
| Very low | Any estimate of effect is very uncertain. |
| Grade of recommendation (SR) | |
| Strong | Benefits clearly outweigh risk and burdens, or vice versa. |
| Weak | Benefits closely balanced with risks and burdens, or some uncertainty in the estimates of benefits and risks. |
Chronic diarrhoea is defined as the passage of loose or liquid stools, urgent need to evacuate or feelings of abdominal discomfort, or increased frequency of these, lasting more than 4 weeks.2,5 Stool consistency is determined by the relationship between faecal water and the water-holding capacity of insoluble faecal solids. As stools consist predominantly of water (60–85%), consistency is difficult to quantify, and for this reason stool weight is used as a reasonable indirect estimation of consistency. Diarrhoea, therefore, can be defined by the weight or volume of stools measured over 24–72h (on average, 2–3 days). The normal weight of stool output over a 24-h period in children and adults is less that 200g; thus, stool weight >200g/24h is an objective definition of diarrhoea. However, it is important to note that up to 20% of patients with liquid diarrhoea, and thus a lower stool weight, are excluded from this definition.
A pragmatic definition incorporates the following elements: passage of loose or liquid stools more than 3 times daily and/or an output of 200g/day of loose or liquid stools.
Patient history and classificationA detailed medical history and physical examination are essential in the assessment of patients with chronic diarrhoea.3–5 When taking the medical history, clinicians should first evaluate the patient's family history of diseases such as coeliac disease or inflammatory bowel disease, both of which have a familial component, their history of travel to regions where diarrhoea is endemic, engagement in risky sexual practices, history of systemic diseases (for example, diabetes mellitus, systemic or neurological diseases, amyloidosis, etc.) and gastrointestinal surgery (for example, cholecystectomy, intestinal resection), use of medicinal products that could cause diarrhoea, or use of chewing gum or sweets with a high sorbitol content.
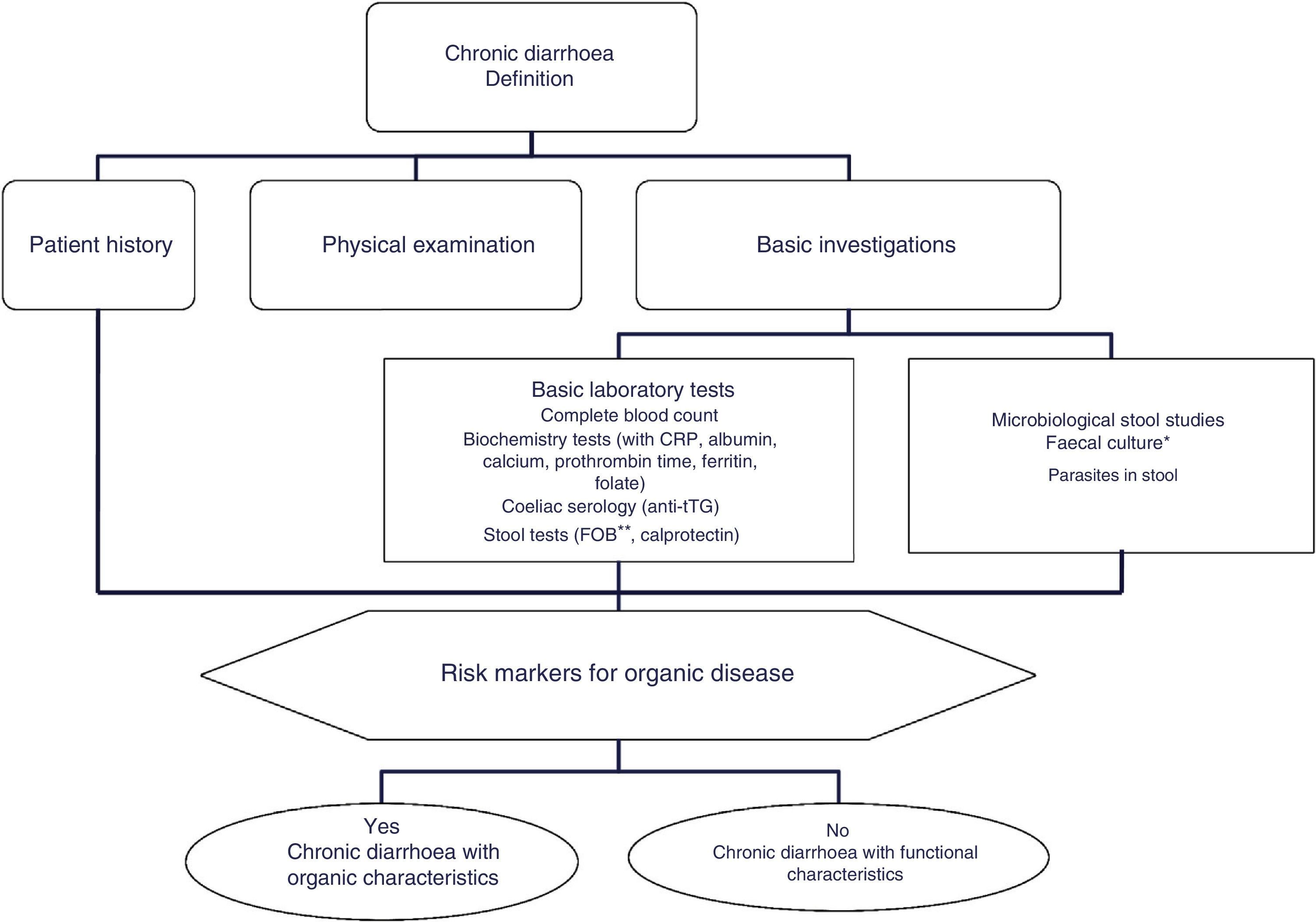
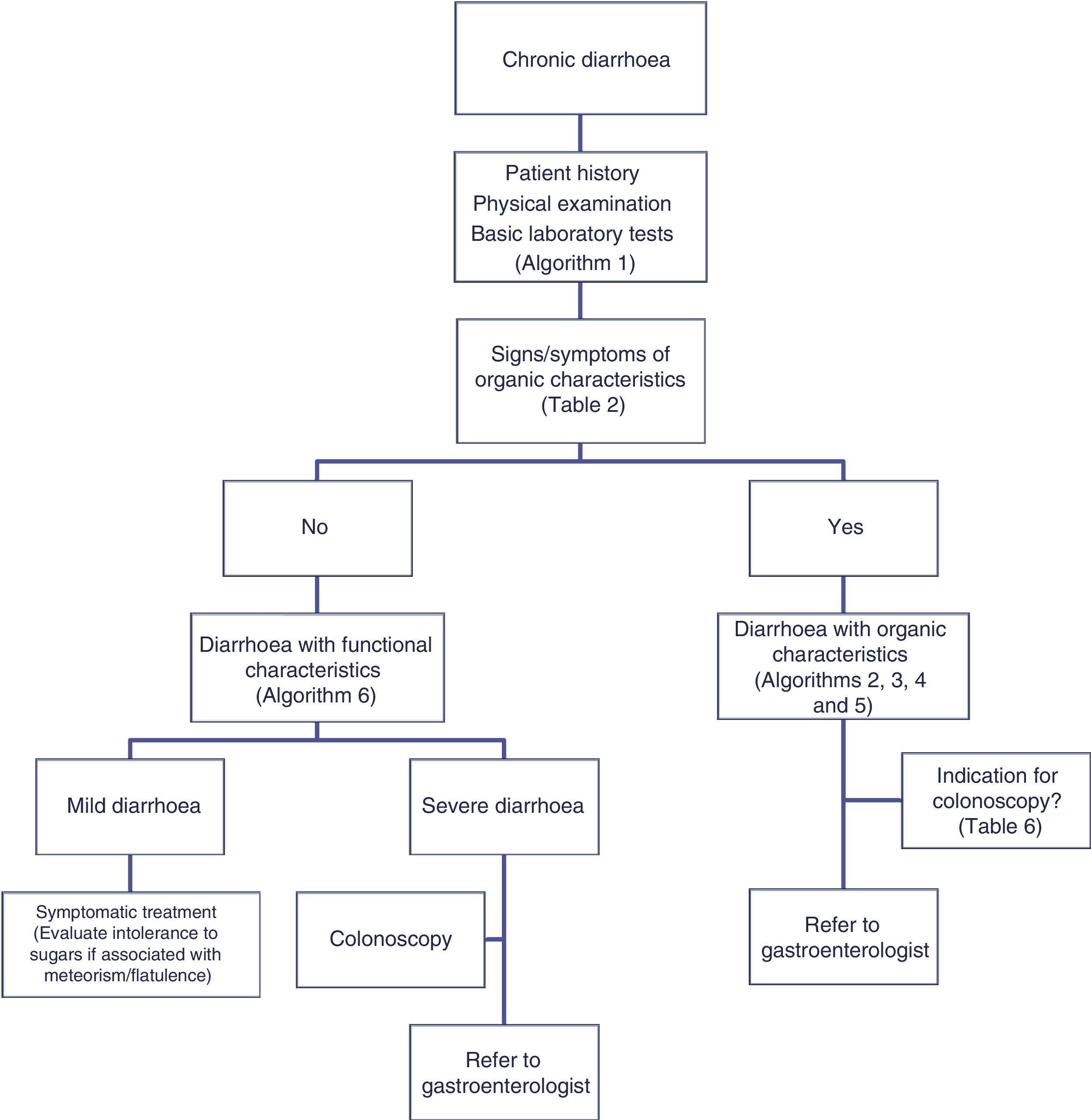
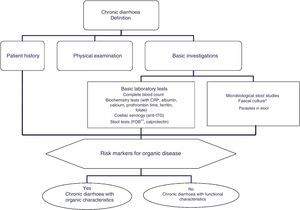
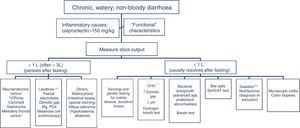
The differential diagnosis of diarrhoea has traditionally been based on its causative mechanisms. Diarrhoea is associated with 4 pathophysiological mechanisms: osmotic, secretory, exudative and altered motility. This classification, however useful from an academic perspective, is impractical in routine practice, because, in addition to other considerations, more than 1 mechanism is often present. From a practical standpoint, it is more useful to classify patients presenting with symptoms of diarrhoea according to “functional” or “organic” characteristics (Algorithm 1, Fig. 1).
Algorithm 1. Initial approach in patients with chronic diarrhoea.
*Faecal culture is only indicated in immunocompromisedpatients or patients receiving immunosuppressants (in many cases these tests will have been performed during the acute phase of the disease, before the duration of symptoms suggests a chronic process).
**The faecal occult blood test has high sensitivity for intestinal inflammation.
CRP: C-reactive protein; FOB: faecal occult blood; tTG: tissue transglutaminase IgA test.
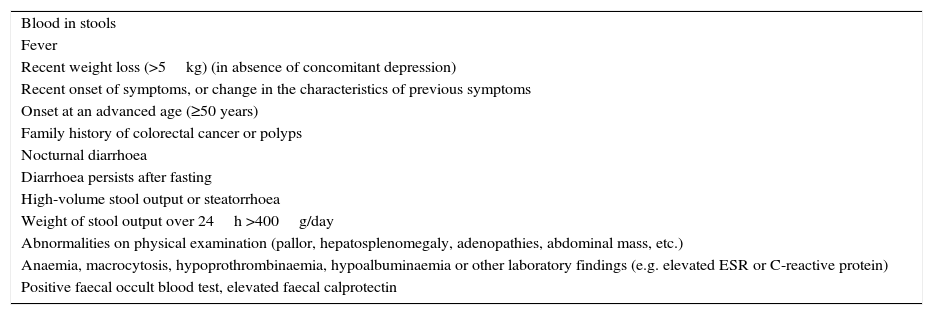
Accordingly, the first step in the diagnostic process involves evaluating signs, symptoms and analytical tests suggestive of organic disease (Table 3). Alarm symptoms or abnormal findings in blood tests point to an organic cause. In these cases, diarrhoea can be characterised as inflammatory, malabsorption (steatorrhoea), or watery diarrhoea. The medical history will often help locate the intestinal segment causing the diarrhoea. The presence of large quantities of liquid or pasty stools with a shiny appearance, accompanied by cramp-like pain in the umbilical area (suggestive of malabsorption diarrhoea), would point to an origin in the proximal small intestine or pancreas. Small quantities of loose or liquid stools, however, mixed with blood, mucous or pus, associated with urgent evacuation or tenesmus and pain in the hypogastrium or sacrum, are more suggestive of an origin in the left colon and/or rectum (inflammatory diarrhoea).
Chronic diarrhoea: signs, symptoms and analytical findings suggestive of an organic cause.
| Blood in stools |
| Fever |
| Recent weight loss (>5kg) (in absence of concomitant depression) |
| Recent onset of symptoms, or change in the characteristics of previous symptoms |
| Onset at an advanced age (≥50 years) |
| Family history of colorectal cancer or polyps |
| Nocturnal diarrhoea |
| Diarrhoea persists after fasting |
| High-volume stool output or steatorrhoea |
| Weight of stool output over 24h >400g/day |
| Abnormalities on physical examination (pallor, hepatosplenomegaly, adenopathies, abdominal mass, etc.) |
| Anaemia, macrocytosis, hypoprothrombinaemia, hypoalbuminaemia or other laboratory findings (e.g. elevated ESR or C-reactive protein) |
| Positive faecal occult blood test, elevated faecal calprotectin |
The “functional” diarrhoea group, meanwhile, includes some entities with an organic origin and functional diarrhoea per se; these will be differentiated in the section on functional diarrhoea. These patients frequently present chronic non-bloody diarrhoea in either recurrent form, with frequent watery stools interspersed with episodes of normal bowel function, or in a persistent form, with passage of loose or liquid stools. The frequency of bowel movement will vary in these cases, but some patients may report up to 10–15 movements daily. Other common symptoms are urgency and faecal incontinence, which can prevent the patient from engaging in their normal activity and diminish their quality of life. Patients can also present moderate weight loss secondary to reduced food intake associated with an astringent or restrictive diet designed to prevent diarrhoea.
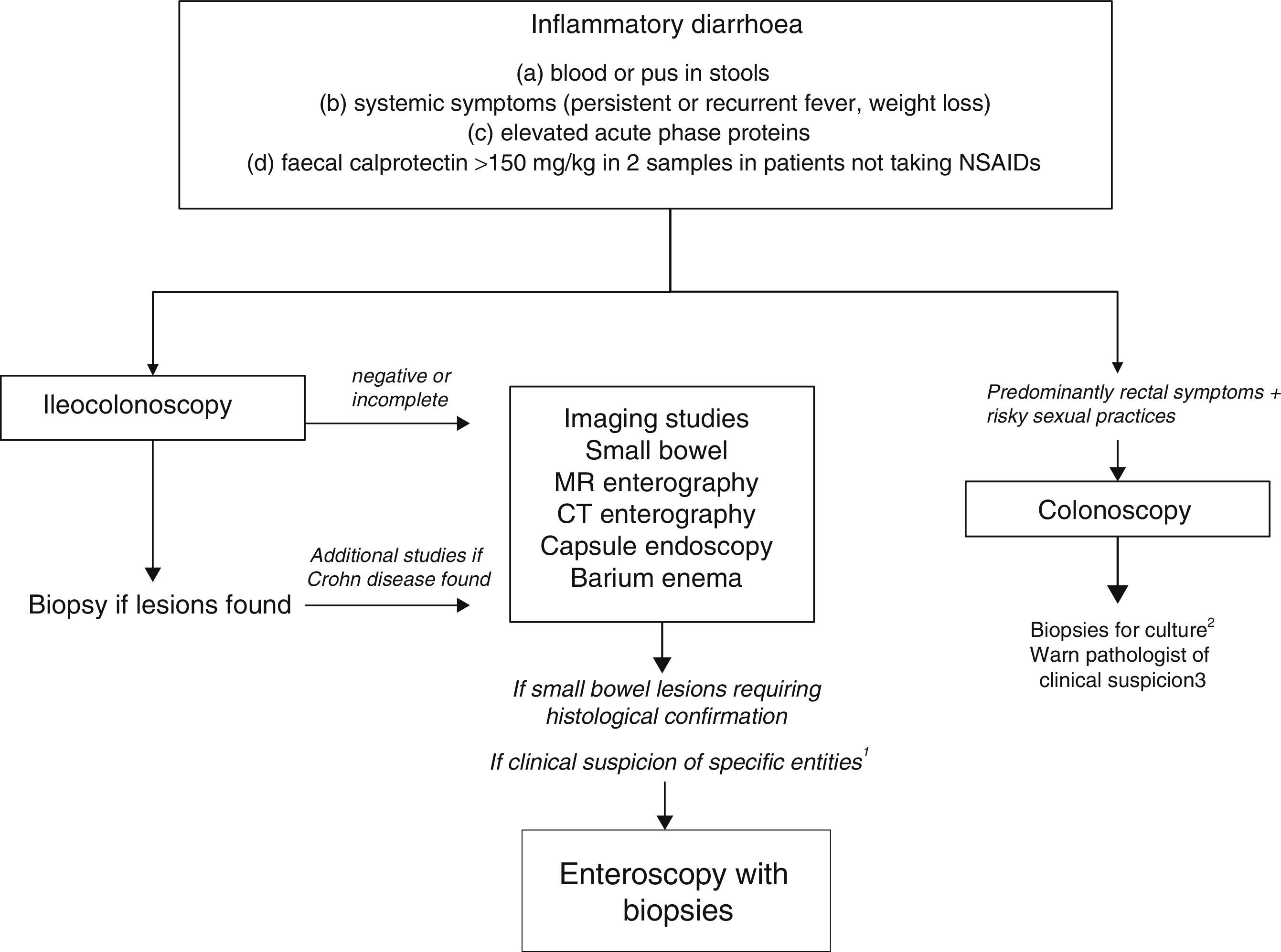
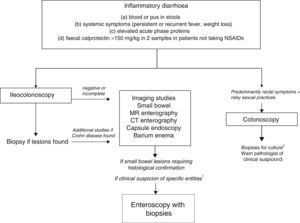
Chronic diarrhoea with organic characteristicsInflammatory diarrhoeaChronic inflammatory diarrhoea is traditionally defined as the presence of white blood cells in stools. As these tests are not performed in most centres, a more practical definition is the following: (a) blood or pus in faeces; (b) accompanied by systemic symptoms (persistent or recurring fever, weight loss) or extra intestinal inflammatory manifestations (mainly affecting the joints, skin or eyes); and (c) elevated acute phase reactants (C-reactive protein, erythrocyte sedimentation rate, platelet count) or faecal calprotectin >150mg/kg in 2 samples taken at different times in patients not taking NSAIDs.10,11
As evaluation of the intestinal mucosa and histological confirmation are required for the diagnosis of most entities causing chronic inflammatory diarrhoea (Table 1), colonoscopy should initially be performed (with or without ileoscopy, depending on symptoms and colonoscopic findings as far as the caecum) (Algorithm 2, Fig. 2). If colonoscopy (with or without ileoscopy) is incomplete, or if findings are unremarkable, other small bowel imaging techniques should be used. Of these, magnetic resonance (MR) enterography, which is similar to CT enterography as regards diagnostic accuracy but with no harmful radiation, is the technique of choice. Alternatively, abdominal ultrasound with or without IV contrast medium can be performed. This technique is similar in terms of diagnostic accuracy to both MR and CT, but is less costly, available in most centres, and does not irradiate the patient.12 Because of this, abdominal ultrasound is an attractive choice for a preliminary study, particularly in paediatric patients. Ultrasound, however, can only accurately explore the terminal ileum, and is operator-dependent, two drawbacks that considerably limit its usefulness in our setting. If ultrasound, MR or CT are unavailable, a barium enema can be performed, although this technique has several drawbacks: lower diagnostic accuracy than MR, because it cannot detect extra intestinal complications; considerable radiation; and lack of training in this technique among the younger generation of radiologists. For all these reasons, barium enemas should be avoided. Abnormal findings from any of the foregoing will indicate the need for further studies, such as gastroduodenoscopy, enteroscopy, capsule endoscopy or specific imaging scans.
Algorithm 2. Diagnosis of chronic inflammatory diarrhoea
1Certain entities may be hard to diagnose without initial clinical suspicion (for example, Whipple disease). In these cases, enteroscopy is indicated to obtain biopsy samples from the small bowel.
2Biopsy for gonorrhoea culture.
3Tissue PCR for lymphogranulomavenereum and herpes simplex.
Chronic inflammatory diarrhoea secondary to infection is rarely found except in the immuno compromised patient population; the principle causes are summarised in Table 4. Faecal cultures, therefore, are only indicated in immuno compromised patients or those receiving immuno suppressants, and are typically tested for Salmonella, Shigella, Yersinia and Campylobacter. Serial sampling is not necessary, and samples can be refrigerated. Several methods can be used to test for Clostridium difficile (stool toxin assay, faecal culture, cytotoxin assay, and glutamate dehydrogenase tests), although none has been shown to be clearly superior13; no single test will give maximum sensitivity and specificity while minimising costs and analysis time, and diagnostic algorithms recommend basing diagnosis on 2 or 3 consecutive tests.
Causative agents of chronic infectious diarrhoea.a
| Bacteraemia (rare) | Protozoa | Viruses (Immunocompromised) |
|---|---|---|
| Salmonella | Giardia | Norovirus |
| Shigella | Cryptosporidium | Citomegalovirus |
| Yersinia | Blastocystishominis | |
| Campylobacter | Entamoebahistolytica | |
| Aeromonas | Diantomoeba | |
| Escherichia coli (enteroinvasive) | Helmintos (Strongyloides) | |
| Clostridium difficile |
Testing for parasites must be performed on 2 fresh samples taken on alternate days. If fresh samples cannot be obtained, patients can be given an appropriate container and medium for providing “fixed” refrigerated faeces samples. Parasites (trophozoite, cysts and eggs) are identified under direct microscopy, and the sensitivity of the test will depend on the intensity of colonisation, the freshness of the sample, and the expertise of laboratory personnel.
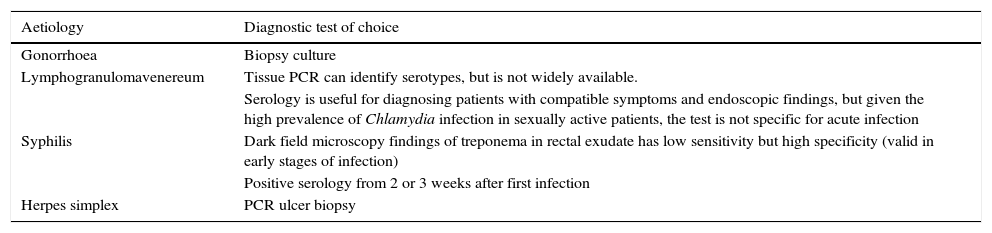
Special mention should be made of sexually transmitted gastrointestinal infections,14 which are usually characterised by rectal symptoms (rectal discharge, tenesmus, or faecal incontinence). These infections are associated with specific aetiological agents, they can mimic inflammatory lesions or tumours on endoscopy, and require specific diagnostic methods (Table 5).
Diagnosis of sexually transmitted proctitis.
| Aetiology | Diagnostic test of choice |
|---|---|
| Gonorrhoea | Biopsy culture |
| Lymphogranulomavenereum | Tissue PCR can identify serotypes, but is not widely available. |
| Serology is useful for diagnosing patients with compatible symptoms and endoscopic findings, but given the high prevalence of Chlamydia infection in sexually active patients, the test is not specific for acute infection | |
| Syphilis | Dark field microscopy findings of treponema in rectal exudate has low sensitivity but high specificity (valid in early stages of infection) |
| Positive serology from 2 or 3 weeks after first infection | |
| Herpes simplex | PCR ulcer biopsy |
- 1
Determination of faecal calprotectin is recommended as a useful biomarker for chronic diarrhoea caused by inflammation (CE, high; SR, strong).
- 2
Initial investigation in a patient with suspected chronic inflammatory diarrhoea is colonoscopy (CE, high; SR, strong).
- 3
If colonoscopy is negative, incomplete, or ileoscopy could not be performed, other studies, preferably MR enterography, should be performed to evaluate the small intestine (CE, high; SR, strong).
Maldigestion is typically defined as decreased intraluminal hydrolysis of foodstuffs, and malabsorption as the reduced mucosal absorption of nutrients. Although this distinction is useful in terms of pathophysiology, maldigestion and malabsorption have similar clinical presentations and complications. For this reason, only the term malabsorption will be used in these guidelines.
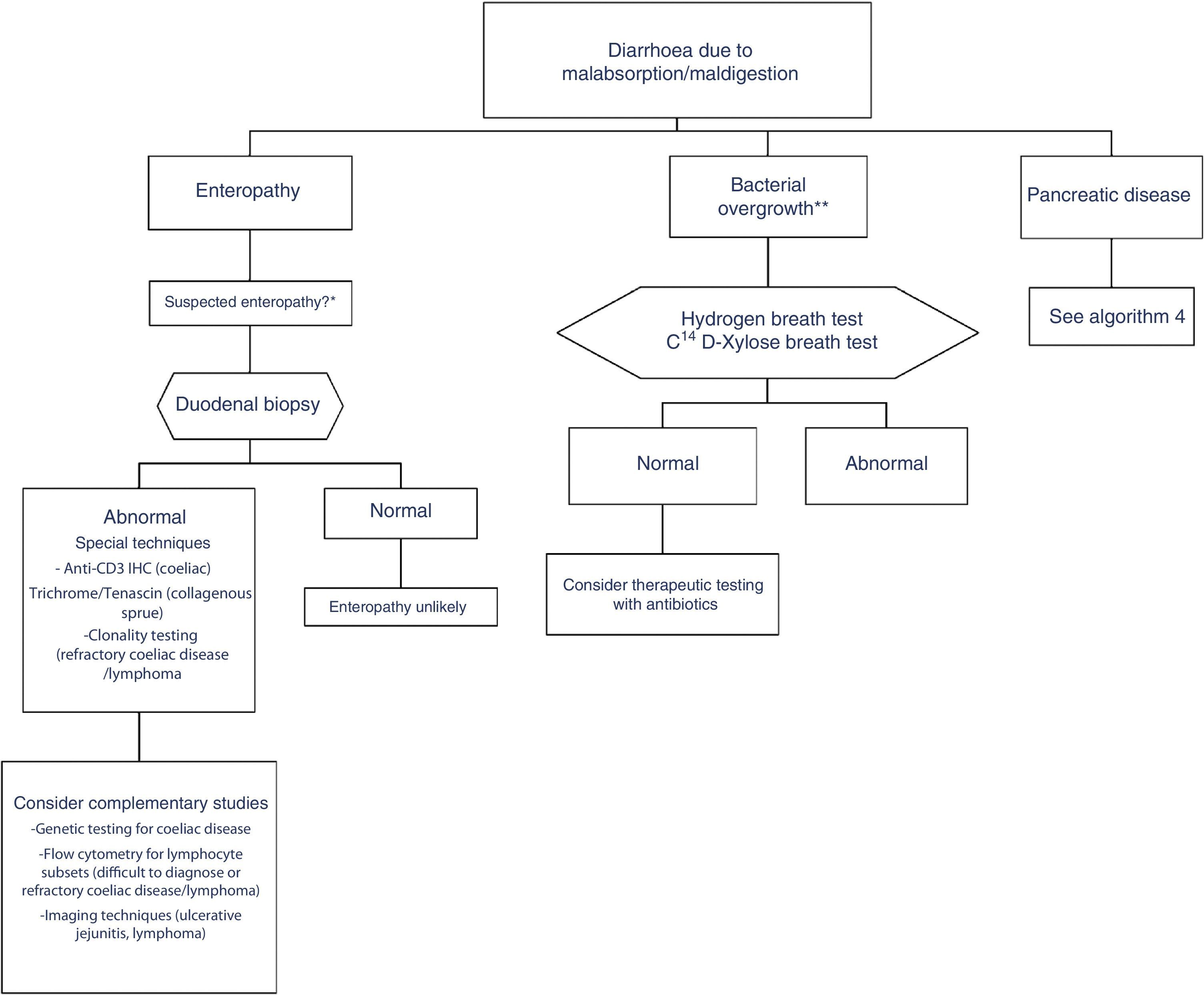
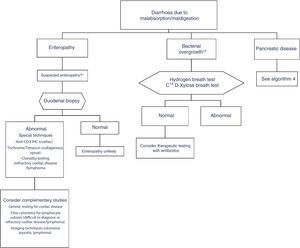
In clinical practice, it is important to differentiate between diarrhoea due to enteropathy, diarrhoea due to bacterial overgrowth, and diarrhoea due to pancreatic disease. Diagnosis of each of these entities is described in a specific algorithm (Algorithms 3 and 4, Figs. 3 and 4).
Algorithm 3. Diagnosis of chronic diarrhoea due to enteropathy and bacterial overgrowth
*Suspectedenteropathy: risk group for coeliac disease (family members, Down syndrome, organ-specific systemic autoimmune diseases, compatible symptoms, etc.), visits to tropical countries, poorly controlled coeliac disease, olmesartan therapy, etc.
**Suspected bacterial overgrowth: structural changes in the small bowel (e.g. by-pass, stenosis) or motility disorder.
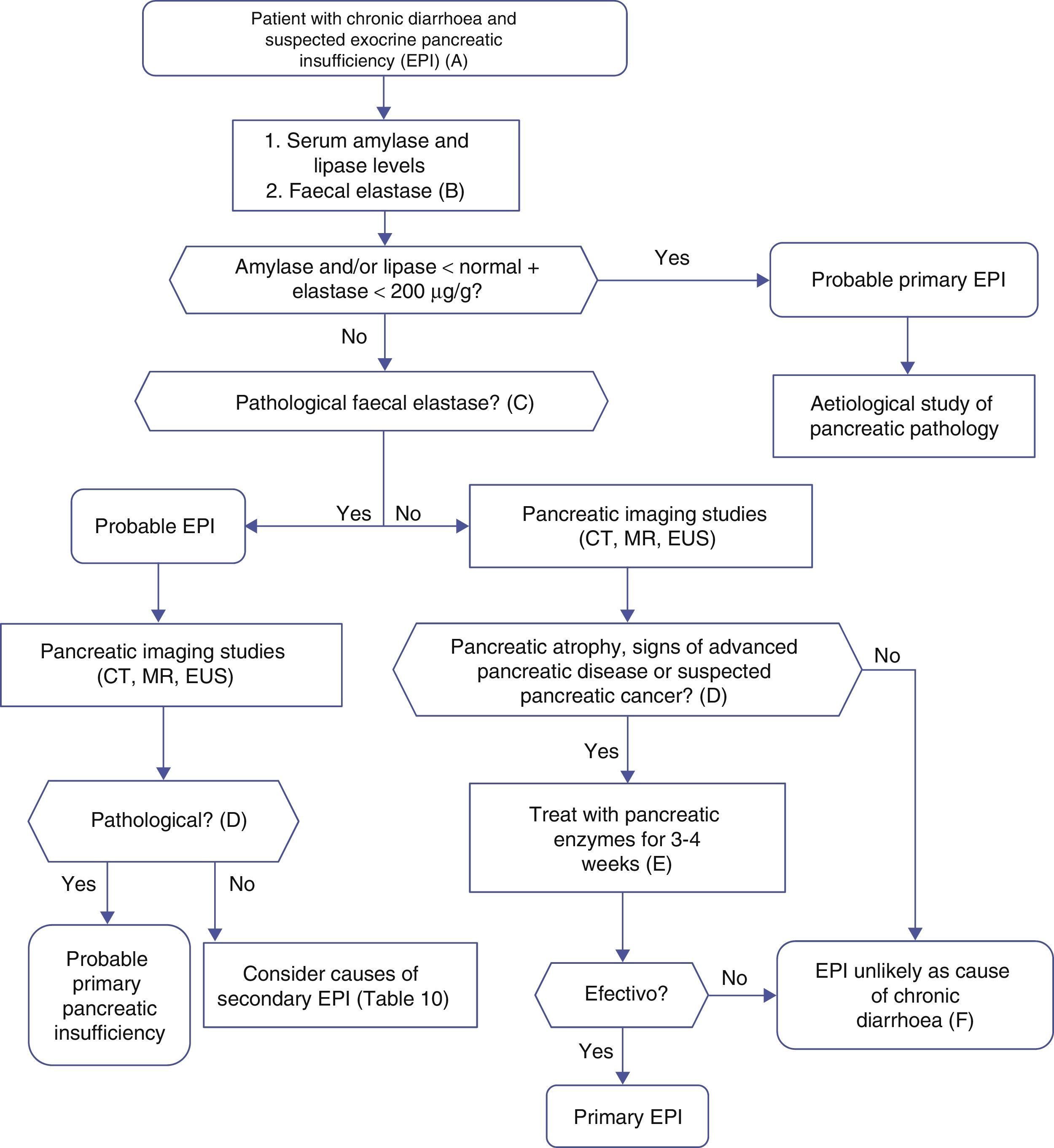
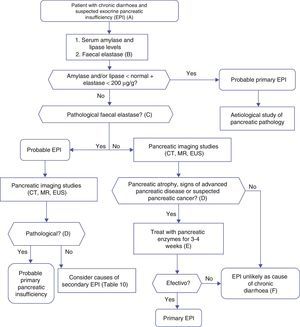
Algorithm 4. Diagnosis of chronic diarrhoea due to exocrine pancreatic insufficiency.
(A) Strong suspicion. Patients with heavy alcohol consumption and smoking habit, history of acute or recurrent pancreatitis, chronic pancreatic-type abdominal pain, suspicion of mutation in the cystic fibrosis gene (bronchiectasis, male sterility, family history of cystic fibrosis), suspicion or diagnosis of pancreatic cancer, previous pancreatectomy.
Suspicion. Occasional smoker, diabetes mellitus, gastrectomy, advanced age, diarrhoea improves with fasting, no anorexia.
(B) Faecal elastase is the most widely used pancreatic function test, and is available in most hospitals. It is performed on a solid stool sample. Liquid stool samples can give falsely low elastase levels.
(C) Concentrations below 100μg/g are definitely pathological. Concentrations between 100 and 200μg/g are also pathological, but with lower sensitivity and specificity; they should be viewed with caution. In patients with pancreatectomy, concentrations <200μg/g can indicate exocrine insufficiency.
(D) Although it is a cause of primary pancreatic insufficiency, pancreatic atrophy can in itself be primary (due to pancreatic disease) or secondary to other factors, such as prolonged malnutrition or advanced age.
(E) Response to enzyme therapy should be evaluated following administration of an appropriate dose, and in the absence of bacterial overgrowth or other causes of enzyme inactivation or that contribute to the diarrhoea. Recommended initial lipsasedose: 25000IU with small meals, and 50000IU with large meals (±proton pump inhibitor).
(F) Therapeutic testing can be used when no other cause for diarrhoeais found, and suspicion of exocrine insufficiency persists.
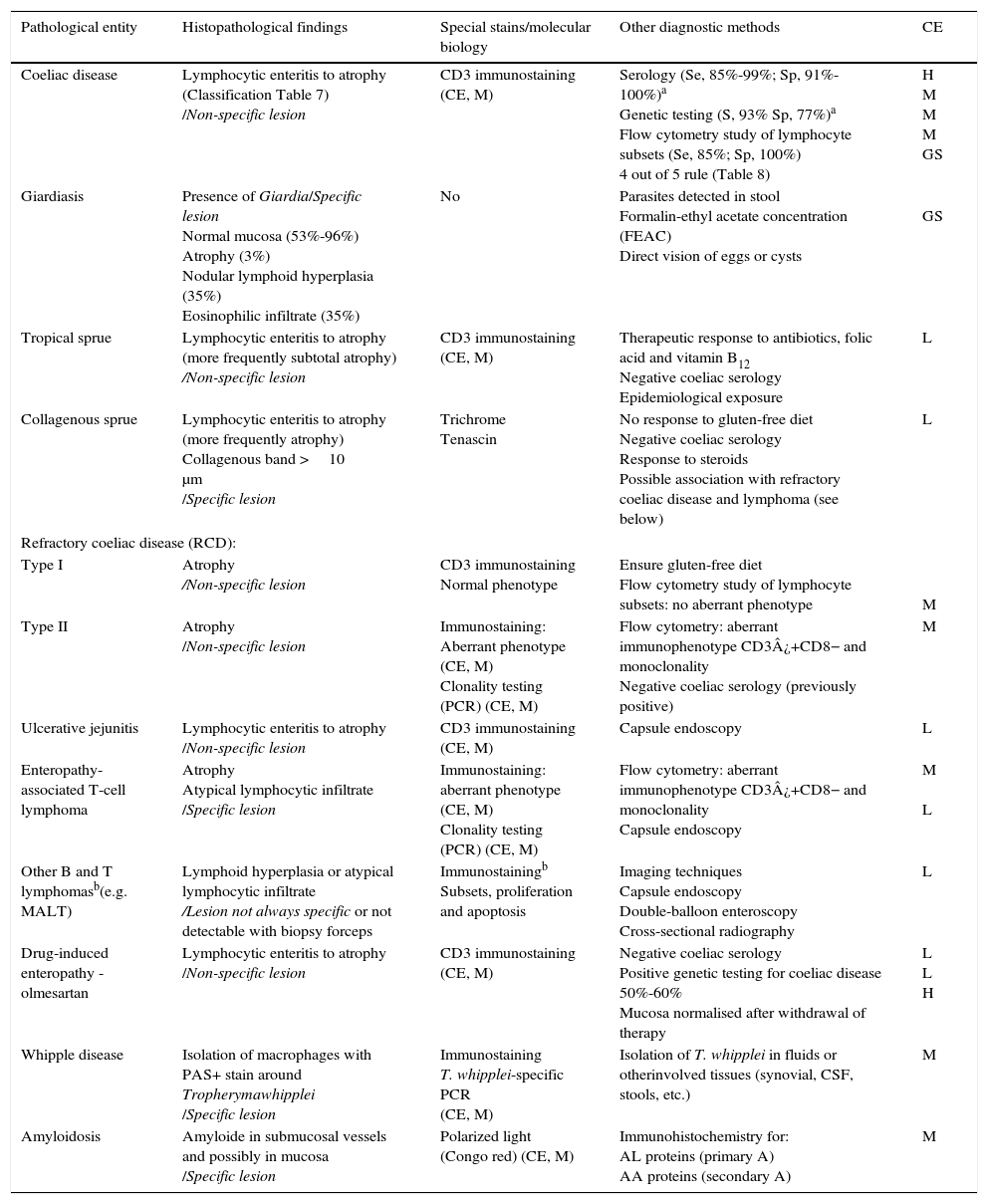
This is caused by malabsorption of nutrients secondary to enterocyte dysfunction. Coeliac disease is the paradigm of chronic diarrhoea due to enteropathy, and is by far the most frequent cause of villous atrophy, although it can also be caused by other entities (Table 6).15–20 Duodenal biopsy (6 samples: 2 from the duodenal bulb and 4 from the distal duodenum) will usually yield a diagnosis, or at least confirm the existence of enteropathy, irrespective of its aetiology. In some cases, such as amyloidosis or Whipple disease, a duodenal biopsy is diagnostic due to the characteristic histopathological appearance of the tissue.21 Intestinal lesions caused by coeliac disease, however, are non-specific (for any grade of lesion, from lymphocytic enteritis to atrophy) (Tables 6 and 7).22–25 Diagnosis should be complemented or confirmed with other analytical tests (serology, genetic studies, lymphocyte subset tests) and clinical evaluations (good response to gluten-free diet).26 As none of the foregoing criteria are in themselves sufficient for a diagnosis of coeliac disease, the use of score-based tests, such as the “4 out of 5” rule, has been suggested (Table 8).27 Nonetheless, when diarrhoea due to enteropathy is suspected, an intestinal biopsy must be performed to either confirm suspicion or determine the extent of the lesion. The clinical context (epidemiological and/or family history, radiation enteritis caused by radiation therapy, bone marrow transplant in the case of graft-versus-host disease, pharmaceutical therapy, etc.) will guide diagnosis. Table 6 shows the main causes of enteropathy-related diarrhoea, together with diagnostic methods and the quality of the supporting evidence. It also describes the diagnostic process for some specific entities, such as tropical sprue,28 refractory coeliac disease and gastrointestinal lympho proliferative disorders.20,29,30
Principle causes of enteropathy that can cause diarrhoea, and diagnosis.
| Pathological entity | Histopathological findings | Special stains/molecular biology | Other diagnostic methods | CE |
|---|---|---|---|---|
| Coeliac disease | Lymphocytic enteritis to atrophy (Classification Table 7) /Non-specific lesion | CD3 immunostaining (CE, M) | Serology (Se, 85%-99%; Sp, 91%-100%)a Genetic testing (S, 93% Sp, 77%)a Flow cytometry study of lymphocyte subsets (Se, 85%; Sp, 100%) 4 out of 5 rule (Table 8) | H M M M GS |
| Giardiasis | Presence of Giardia/Specific lesion Normal mucosa (53%-96%) Atrophy (3%) Nodular lymphoid hyperplasia (35%) Eosinophilic infiltrate (35%) | No | Parasites detected in stool Formalin-ethyl acetate concentration (FEAC) Direct vision of eggs or cysts | GS |
| Tropical sprue | Lymphocytic enteritis to atrophy (more frequently subtotal atrophy) /Non-specific lesion | CD3 immunostaining (CE, M) | Therapeutic response to antibiotics, folic acid and vitamin B12 Negative coeliac serology Epidemiological exposure | L |
| Collagenous sprue | Lymphocytic enteritis to atrophy (more frequently atrophy) Collagenous band >10 μm /Specific lesion | Trichrome Tenascin | No response to gluten-free diet Negative coeliac serology Response to steroids Possible association with refractory coeliac disease and lymphoma (see below) | L |
| Refractory coeliac disease (RCD): | ||||
| Type I | Atrophy /Non-specific lesion | CD3 immunostaining Normal phenotype | Ensure gluten-free diet Flow cytometry study of lymphocyte subsets: no aberrant phenotype | M |
| Type II | Atrophy /Non-specific lesion | Immunostaining: Aberrant phenotype (CE, M) Clonality testing (PCR) (CE, M) | Flow cytometry: aberrant immunophenotype CD3¿+CD8− and monoclonality Negative coeliac serology (previously positive) | M |
| Ulcerative jejunitis | Lymphocytic enteritis to atrophy /Non-specific lesion | CD3 immunostaining (CE, M) | Capsule endoscopy | L |
| Enteropathy-associated T-cell lymphoma | Atrophy Atypical lymphocytic infiltrate /Specific lesion | Immunostaining: aberrant phenotype (CE, M) Clonality testing (PCR) (CE, M) | Flow cytometry: aberrant immunophenotype CD3¿+CD8− and monoclonality Capsule endoscopy | M L |
| Other B and T lymphomasb(e.g. MALT) | Lymphoid hyperplasia or atypical lymphocytic infiltrate /Lesion not always specific or not detectable with biopsy forceps | Immunostainingb Subsets, proliferation and apoptosis | Imaging techniques Capsule endoscopy Double-balloon enteroscopy Cross-sectional radiography | L |
| Drug-induced enteropathy - olmesartan | Lymphocytic enteritis to atrophy /Non-specific lesion | CD3 immunostaining (CE, M) | Negative coeliac serology Positive genetic testing for coeliac disease 50%-60% Mucosa normalised after withdrawal of therapy | L L H |
| Whipple disease | Isolation of macrophages with PAS+ stain around Tropherymawhipplei /Specific lesion | Immunostaining T. whipplei-specific PCR (CE, M) | Isolation of T. whipplei in fluids or otherinvolved tissues (synovial, CSF, stools, etc.) | M |
| Amyloidosis | Amyloide in submucosal vessels and possibly in mucosa /Specific lesion | Polarized light (Congo red) (CE, M) | Immunohistochemistry for: AL proteins (primary A) AA proteins (secondary A) | M |
CE: quality of the evidence (H, high; M, moderate; L, low); GS: gold standard.
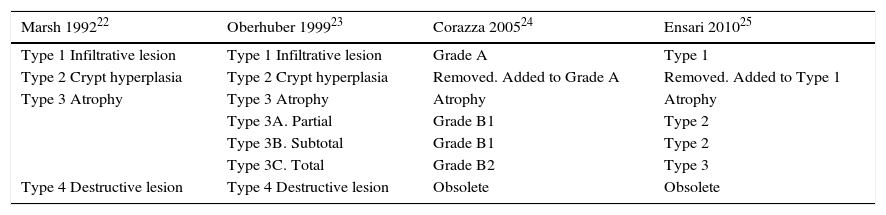
Histopathological classification systems in coeliac disease.
| Marsh 199222 | Oberhuber 199923 | Corazza 200524 | Ensari 201025 |
|---|---|---|---|
| Type 1 Infiltrative lesion | Type 1 Infiltrative lesion | Grade A | Type 1 |
| Type 2 Crypt hyperplasia | Type 2 Crypt hyperplasia | Removed. Added to Grade A | Removed. Added to Type 1 |
| Type 3 Atrophy | Type 3 Atrophy | Atrophy | Atrophy |
| Type 3A. Partial | Grade B1 | Type 2 | |
| Type 3B. Subtotal | Grade B1 | Type 2 | |
| Type 3C. Total | Grade B2 | Type 3 | |
| Type 4 Destructive lesion | Type 4 Destructive lesion | Obsolete | Obsolete |
The gold standard 4 out of 5 rule for the diagnosis of coeliac disease.
| 1. Typical symptoms (e.g., diarrhoea, growth delay, anaemia, etc.) |
| 2. Positive for high titres of class A antibodies (IgG accepted in case of IgA deficiency, IgG anti-deamidatedgliadinpeptideantibodies strengthen diagnosis) |
| 3. Positive for HLA-DQ2 and/or HLA-DQ8 genotypes |
| 4. Enteropathy on intestinal biopsy (includes Marsh 1 –positive serology or associated with subepithelial deposits – to Marsh 3) |
| 5. Clinical and serological improvement with gluten-free diet (histological improvement in sero-negative patients) |
Source: adapted from Catassi and Fasano.27
In addition to the foregoing, other less common diseases, such as abetalipoproteinemia, hypobetalipoproteinemia or hypogammaglobulinemia, can also cause diarrhoea. Diagnosis is generally made in childhood, and suspicion is rarely guided by the enteropathy caused by these conditions, but rather by the clinical context and laboratory results (low levels of apoprotein B, triglycerides and cholesterol, in the former, and hypogammaglobulinemia with no B lymphocytes in the latter).
Recommendations(See Table 6 for the quality of evidence of each diagnostic study.)
- 1
Determination of IgA anti-tissue transglutaminase antibody levels is the test of choice to screen for coeliac disease in patients with chronic diarrhoea (CE, high; SR, strong).
- 2
If blood tests are negative for coeliac disease, the presence of HLA-DQ2.5 should be determined. If positive, intestinal biopsies should be taken to rule out coeliac disease (CE, moderate; SR, strong).
- 3
In the case of suspected malabsorption syndrome, distal duodenal biopsy is indicated to diagnose coeliac disease or other types of enteropathy (CE, moderate; SR, strong).
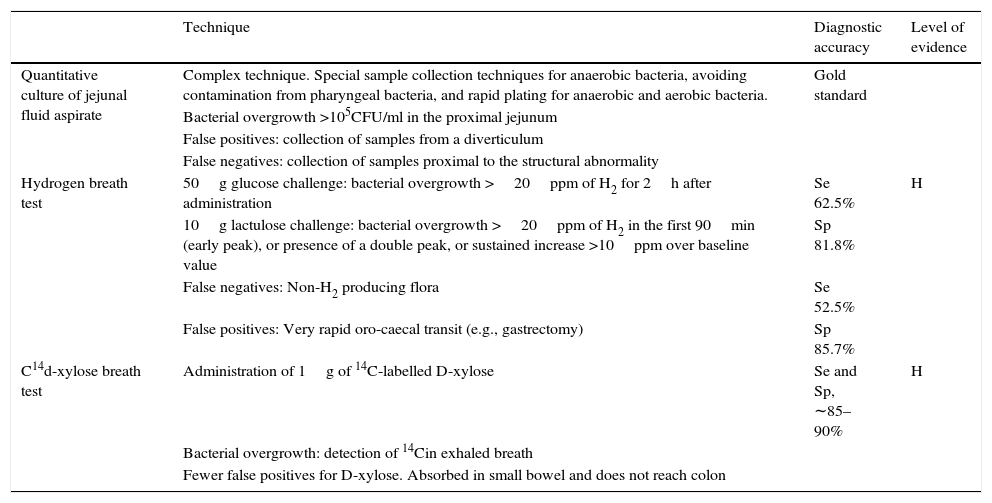
Diarrhoea due to bacterial overgrowth (BO) is caused by malabsorption of foodstuffs, including fats and carbohydrates.31,32 Certain situations involving high pH values, stasis or delayed gastric emptying can give rise to qualitative and quantitative changes in the bacteria colonising the proximal intestine, with a significant impact on the gut microbiota. The usual gut flora, consisting of lactobacilli, enterococci, and gram-positive facultative anaerobes, may be partially replaced with colic bacteria (coliforms and anaerobes: Bacteroides and Clostridium), causing an increase in certain enzymes, such as colilamidase and protease. Colilamidasesde conjugate bile salts, which are then more easily absorbed by passive transport in the proximal jejunum. This hampers micelle production, leading to malabsorption of fats. The increased proteases reduce villous surface enzymes, such as the disaccharidases, thus causing carbohydrate malabsorption. Small bowel bacterial overgrowth (SBBO) can be caused by a number of diseases, and should be suspected in any situation associated with impaired motility due to either structural (stenosis, by-pass, etc.,) or functional (pseudo-obstruction, diabetic enteropathy, etc.) disorders. Increased pH in the duodenum or proximal jejunum (caused by proton pump inhibitor [PPI] therapy, gastrectomy or atrophic gastritis) increases the risk of SBBO due to absence of the bacteriostatic effect of the acid environment. The role of SBBO in the pathophysiology of irritable bowel syndrome (particularly associated with diarrhoea and/or distension) is still unclear, although it could be a causative factor in some patient subgroups. There is insufficient conclusive evidence to recommend the routine study of SBBO in these patients. Table 9 summarises the studies recommended for detecting bacterial overgrowth. Small-bowel aspiration and quantitative culture is considered the gold standard, but the complexity of the test rules out its use in routine clinical practice. As an alternative, the 14C/13C d-xylose breath test has been shown to be the most accurate, but has less supporting evidence than hydrogen breath tests.33,34
Recommended tests for bacterial overgrowth.
| Technique | Diagnostic accuracy | Level of evidence | |
|---|---|---|---|
| Quantitative culture of jejunal fluid aspirate | Complex technique. Special sample collection techniques for anaerobic bacteria, avoiding contamination from pharyngeal bacteria, and rapid plating for anaerobic and aerobic bacteria. | Gold standard | |
| Bacterial overgrowth >105CFU/ml in the proximal jejunum | |||
| False positives: collection of samples from a diverticulum | |||
| False negatives: collection of samples proximal to the structural abnormality | |||
| Hydrogen breath test | 50g glucose challenge: bacterial overgrowth >20ppm of H2 for 2h after administration | Se 62.5% | H |
| 10g lactulose challenge: bacterial overgrowth >20ppm of H2 in the first 90min (early peak), or presence of a double peak, or sustained increase >10ppm over baseline value | Sp 81.8% | ||
| False negatives: Non-H2 producing flora | Se 52.5% | ||
| False positives: Very rapid oro-caecal transit (e.g., gastrectomy) | Sp 85.7% | ||
| C14d-xylose breath test | Administration of 1g of 14C-labelled D-xylose | Se and Sp, ∼85–90% | H |
| Bacterial overgrowth: detection of 14Cin exhaled breath | |||
| Fewer false positives for D-xylose. Absorbed in small bowel and does not reach colon |
CFU: colony forming units; Se: sensitivity; Sp: specificity.
Some authors have proposed therapeutic testing with antibiotics to detect SBBO when pre-test probability is high (clear predisposing cause and compatible clinical presentation).33 Nevertheless, it is important to bear in mind that SBBO should usually be treated cyclically, and thus calls for an objective diagnostic test.
Recommendations- 1
The hydrogen breath test has low sensitivity but adequate specificity for diagnosing SBBO, and is useful when results are positive. Hydrogen breath testing with glucose is recommended (CE, high; SR, strong).
- 2
A therapeutic test with antibiotics can be used to diagnose SBBO when pre-test probability is high (clear predisposing cause and compatible clinical presentation), and the hydrogen breath test is negative or not available (CE, low; SR, weak).
Pancreatic enzyme deficiency results in malabsorption of foodstuffs (particularly fats), which results in loose or liquid stools and increased daily stool output. Steatorrhoea is defined as the elimination of >7g/day fat with a dietary intake of 100g fat/day (fat absorption coefficient <93%). Pancreatic steatorrhoea only occurs when the functional reserve capacity of the pancreas is severely depleted.35 Moderate steatorrhoea might not be associated with diarrhoea. Constipation is a common symptom in patients with chronic pancreatitis or cystic fibrosis and severe pancreatic insufficiency. Diarrhoea secondary to primary pancreatic disease is not usually particularly voluminous, hardly ever watery (can be oily), and improves with fasting. Patients may suffer weight loss without anorexia, unless insufficiency is due to pancreatic cancer.
Pancreatic insufficiency can be primary (due to pancreatic disease) or secondary. In the latter, suboptimal enzyme levels are only partially responsible for the diarrhoea. However, administration of enzymes can improve symptoms in some cases, such as gastrectomy, diabetes mellitus and pancreatic cancer. Table 10 summarises the causes of pancreatic insufficiency. The most common primary cause is chronic pancreatitis, followed by cystic fibrosis and pancreatic cancer.
Causes of exocrine pancreatic insufficiency.
| Primary causes |
| 1. Chronic pancreatitis |
| 2. Cystic fibrosis |
| 3. Pancreatic cancer |
| 4. Acute pancreatitis (generally transient) |
| 5. Duct obstruction (including ampulloma, cysts and MPTs) |
| 6. Pancreatectomy |
| 7. Senile pancreatic atrophy, acquired (persistent severe malnutrition) or congenital (BDS, Johanson-Blizzard, pancreatic lipomatosis, dorsal pancreatic agenesis, MODY) atrophy |
| Secondary causes |
| 1. Coeliac disease and other enteropathies (Crohn disease, common variable immune deficiency with intestinal involvement, eosinophilic enteritis, etc.) |
| 2. Gastrectomy – pancreatic stent (postprandial asynchrony) |
| 3. Zollinger-Ellison syndrome |
| 4. Drugs (long-term octreotide therapy) |
| Of uncertain origin |
| 1. Diabetes mellitus |
| 2. Irritable bowel syndrome |
| 3. Chronic renal failure |
We will address the diagnosis of pancreatic insufficiency as a cause of chronic diarrhoea, and not as a factor in the diagnosis of chronic pancreatitis. More than 20 tests for pancreatic insufficiency have been described, but none give optimal results in clinical practice. Some are non-diagnostic (cerulein-secretin stimulation test), or are no longer in use (faecal chymotrypsin). As far as common clinical studies are concerned, the sensitivity, specificity and predictive value of the tests will depend to a large extent on the chosen reference value (steatorrhoea vs chronic pancreatitis) and the underlying disease (cystic fibrosis, pancreatectomy, chronic pancreatitis, etc.).
Tests that yield relevant clinical information- 1
Serum amylase, lipase and trypsin levels. Due to their low sensitivity, these tests are rarely used. However, low levels of these enzymes are indicative and specific (>90%) for primary exocrine insufficiency, particularly in patients with cystic fibrosis, advanced chronic pancreatitis, pancreatic cancer and Shwachman–Diamond syndrome. As diarrhoea only occurs with severely impaired pancreatic function, pancreatic enzymes could have adequate sensitivity in cases of chronic diarrhoea; however, this hypothesis has not been tested.
- 2
Evidence. In paediatric patients with cystic fibrosis, low lipase and trypsin levels correlate with steatorrhoea,36 with a sensitivity of 95% and 93%, respectively, and a specificity of 86% and 92%.37 In patients with chronic pancreatitis, low trypsin or amylase levels have a sensitivity of 70–85% for steatorrhea38, but less than 50% for mild exocrine insufficiency measured by the secretin stimulation test.39 Specificity for pancreatic steatorrhoea is between 90% and 100%.40 Low or undetectable serum lipase levels are typical of congenital lipase deficiency, a disease that presents with steatorrhoea and normal faecal elastase.
- 3
Faecal pancreatic elastase determination. This is the gold standard for detecting pancreatic insufficiency, and is recommended in British guidelines for chronic diarrhoea.5 The test determines levels of human pancreatic elastase (but not enzymes) in stool. Results are not affected by pancreatic enzyme therapy. It is a simple, inexpensive and reproducible test (15% individual variability),41 but can yield false positives when used with liquid stool samples. Concentrations of >200μg/gelastase per g of stool are normal, while concentrations of <100 indicate severe pancreatic insufficiency. Concentrations of between 100 and 200 are suggestive of pancreatic disease, but should be considered in the general context of the case. Evidence for sensitivity and specificity levels is conflicting, but it is generally considered a useful screening test for severe pancreatic insufficiency, which is the clinical situation associated with chronic diarrhoea, in the absence of other factors (for example, bacterial overgrowth). In a preliminary analysis, a cut off value of <200μg/g was found to have a sensitivity of 63% for mild and 100% for moderate to severe insufficiency (compared with the cerulein-secretin test), with a specificity of 93% and significant correlation with enzyme and bicarbonate secretion.41 Values under 100μg/g have a sensitivity and specificity for steatorrhea of 93% and 81%, respectively.42 In patients with pancreaticoduodenectomy, faecal elastase concentrations of <200μg/g are accompanied by steatorrhea.42 Sensitivity for detecting steatorrhoea in these cases is 91%, but specificity is only 35%.43 The difference in these cases arises because exocrine insufficiency is accompanied by anatomical abnormalities that prevent optimal mixing of nutrients with bile salts and with the enzymes and bicarbonate secreted by the pancreatic remnant (postprandial asynchrony).
- 4
Faecal chymotrypsin. Enzyme levels <3U/g have a specificity of between 49% and 100% and a sensitivity of between 50% and 90% for chronic pancreatitis. The specificity for pancreatic disease is extremely high (90–100%) for determining both the origin of steatorrhoea44 and for detecting pancreatic insufficiency in patients with cystic fibrosis.45 The test has a sensitivity of 69% and a specificity of 89% for detecting pancreatic insufficiency in patients with chronic pancreatitis.41 When used with watery stools, it can yield false positives. Test kits are not widely available.
- 5
13Cmixedtriglyceride breath test. In this test, concentrations of 13C in exhaled breath are measured following intake of triglycerides labelled with 13C. It has a sensitivity of 89% and a specificity of 81% for the diagnosis of pancreatic steatorrhoea.46 Compared with direct secretion tests (secretin-cerulein) and with faecal elastase and chymotrypsin, it has a sensitivity of 85% and a specificity of 100% for diagnosing severe pancreatic insufficiency, but only 69% and 46%, respectively, for moderate insufficiency. The test is not superior to the sensitivity and specificity of the faecal elastase test.47 Presence of bacterial overgrowth can give false negatives, particularly in patients with pancreatectomy.48 Other factors that can distort results are a diet rich in foodstuffs containing 13C, abnormal gastric emptying, physical exercise, and basal CO2 production. Drawbacks include the duration of the test (between 6 and 8h), the requirement for patients to fast and abstain from exercise, and the need for specially trained personnel.
- 6
Faecal fat and coefficient of fat absorption. Quantification of faecal fat is a good marker of exocrine insufficiency in patients with a known pancreatic parenchymal lesion. The total fat content in stool samples taken over 72h is measured. Patients are required to eat a diet containing 100g fat/day for 2 days prior to the start of the test. Faecal fat >7g/day indicates steatorrhoea (coefficient of fat absorption <93%). Although this is the gold standard for steatorrhoea screening,43 it is not specific for pancreatic disease.
- 7
Endoscopic pancreatic function test. This test is only performed in some hospitals Pancreatic secretions in response to secretin or cerulein stimulation are aspirated through the endoscope at certain time intervals. The test has a sensitivity of 83% and a specificity of 87% for diagnosis of chronic pancreatitis.49 A reduced version of the test, in which secretions are aspirated over 15min, also gives good results.50
- 8
Secretin-enhanced magnetic resonancecholangiopancreatography. The test quantifies duodenal secretions in response to secretin infusion. Imaging findings can discriminate patients (chronic pancreatitis) from healthy volunteers,51 and correlate with faecal elastase test findings.52 The test only measures volume of secretions, and is not standardised.
- 9
Morphological investigations. The greater the morphological abnormalities in imaging studies, the greater the pancreatic dysfunction. Pancreatic insufficiency rarely occurs in patients with normal pancreatic morphology. Correlation between imaging and direct function test results is good, but not perfect.53,54 In patients with partial pancreatectomy (in whom pancreatic function testing is difficult or inaccurate), the main duct diameter/parenchymal thickness ratio correlates well with exocrine insufficiency.55
- 10
Therapeutic testing with pancreatic enzymes. Therapeutic testing is used when there is a strong suspicion of pancreatic insufficiency and the results of other tests have proved inconclusive, and is a useful confirmatory test. For example, in a group of patients with diarrhoea-predominant irritable bowel syndrome, 6% had faecal elastase levels <100mg/g, and symptoms improved after enzyme therapy.56
- 1
In chronic diarrhoea investigations, determination of faecal elastase in non-watery stool samples is the gold standard for evaluating exocrine pancreatic insufficiency (CE, high; SR, strong). The labelled triglyceride test can be a good alternative in hospitals where this test has been validated (CE, moderate; SR, weak).
- 2
If the findings of the former test are inconclusive, morphological abnormalities on imaging studies can indicate exocrine pancreatic insufficiency (CE, low; SR, strong).
- 3
If diagnostic tests are inconclusive, or when pancreatic insufficiency is merely a contributing factor to chronic diarrhoea, therapeutic testing with pancreatic enzymes may be indicated (CE, low; SR, weak).
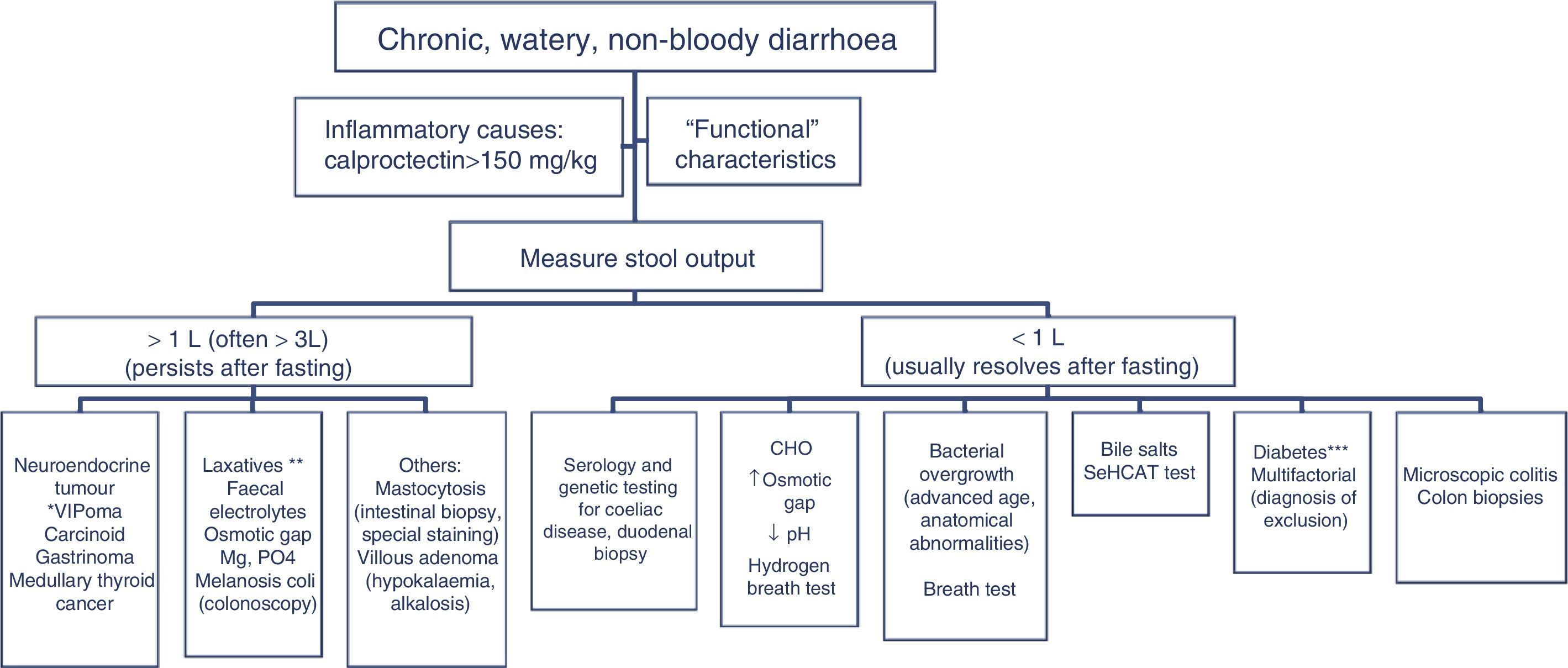
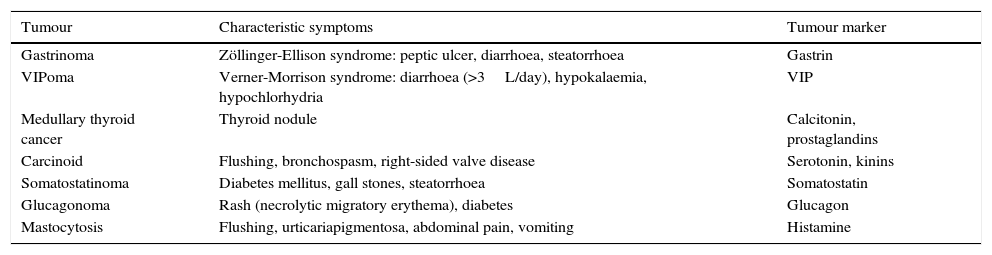
In patients with chronic watery, non-bloody diarrhoea with organic characteristics (Table 3), diagnosis is based on the strategy outlined in algorithm 5 (Fig. 5). These usually involve secretory diarrhoea characterised by large-volume, watery stools, often more than 1L per day; persistence of diarrhoea after fasting; and stool-water tests that show measured osmolality to be identical to that calculated from its electrolyte concentration (see below).57 Agents causing secretions that can lead to chronic diarrhoea include various hormones and substances produced by neuroendocrine tumours (Table 11).57–59 However, watery, secretory diarrhoea is only caused by such tumours in 1 in 5000 to 1 in 500,000 patients with chronic diarrhoea, depending on the type of tumour.
Algorithm 5. Diagnosis of chronic watery non-bloody diarrhoea.
Other causes of <1L/day: pharmacological therapy, hyperthyroidism, alcohol.
*Serum peptide panels have a false positive rate of 45% (positive predictive value <1%); only request these when there are signs and symptoms of neoplasm (Table 11) or when CT/MR or octreoscan findings indicate a tumour.
**Can be high- or low-volume, depending on the dose administered; can respond to fasting. A finding of melanosiscoli on colonoscopy suggests regular use of anthraquinone laxatives, such as senna and cascara sagrada.
***In diabetic patients, diarrhoea can have multifactorial causes: excess fructose consumption, bacterial overgrowth, increased risk of coeliac disease, and microscopic colitis in type 1 DM, some pharmacological therapies (metformin, acarbose), autonomic neuropathy.
CHO: carbohydrates (lactose, fructose and/or sorbitol).
Neuroendocrine tumours that cause diarrhoea, and their markers.
| Tumour | Characteristic symptoms | Tumour marker |
|---|---|---|
| Gastrinoma | Zöllinger-Ellison syndrome: peptic ulcer, diarrhoea, steatorrhoea | Gastrin |
| VIPoma | Verner-Morrison syndrome: diarrhoea (>3L/day), hypokalaemia, hypochlorhydria | VIP |
| Medullary thyroid cancer | Thyroid nodule | Calcitonin, prostaglandins |
| Carcinoid | Flushing, bronchospasm, right-sided valve disease | Serotonin, kinins |
| Somatostatinoma | Diabetes mellitus, gall stones, steatorrhoea | Somatostatin |
| Glucagonoma | Rash (necrolytic migratory erythema), diabetes | Glucagon |
| Mastocytosis | Flushing, urticariapigmentosa, abdominal pain, vomiting | Histamine |
Some patients present chronic secretory diarrhoea due to abuse of laxatives. The presence or absence of large-volume watery stools will depend on the laxative dose taken, and symptoms will improve with fasting. This aetiology should be suspected in the following circumstances60,61: (a) bulimic patients (usually young or adolescent women concerned about their body weight, or with confirmed eating disorders); (b) patients with an ulterior motive (economic, manipulation of family members); (c) Munchhausen syndrome (patients with a psychological need to be a diagnostic challenge); and (d) Munchhausen's syndrome by proxy (children or dependent adults given laxatives by their guardian or carer in order to seek personal benefit).
In patients with chronic secretory diarrhoea associated with hypokalaemia and metabolic alkalosis, large villous adenomas should be suspected
DiagnosisRoutine testing for gastrointestinal peptides in blood or urine has a false positive rate of 45%, and is not recommended in the diagnosis of patients with chronic diarrhoea.62 Considering, moreover, that the pre-test probability of diagnosing a neuroendocrine tumour as the cause of chronic secretory diarrhoea is extremely low, the positive predictive value of these tests is less than 1%. Serum determination of these peptides or their urinary metabolites should therefore only be performed in patients with chronic diarrhoea and signs and symptoms consistent with neoplasia (Table 11) or with evidence of a neuroendocrine tumour on imaging studies (CT, MR, EUS). Most neuroendocrine tumours secrete chromogranin A; however, routine testing for this protein contributes little to diagnosis, as high levels have been described in other types of cancer (pancreatic, prostate, small cell lung cancer), renal failure, diarrhoea-predominant IBS, inflammatory bowel disease, and collagenous colitis, as well as in atrophic gastritis and PPI therapy, probably due to enterochromaffin cell hyperplasia.63,64 The specificity of this marker for diagnosis of neuroendocrine tumours is only 10–35%, with a sensitivity of around 60%.63 An octreotide scan, or octreoscan, can be useful for identifying peptide-producing neuroendocrine tumours.65
A finding of melanosiscoli on colonoscopy suggests regular use of anthraquinone laxatives, such as senna and cascara sagrada. However, a histological finding of pseudomelanosiscoli in biopsy samples from a macroscopically normal colon is non-specific, and has been associated with increased epithelial apoptosis secondary to use of these laxatives and other drugs.66 Abuse of laxatives derived from magnesium sulphate, phosphates and other sulphates can be detected with electrolyte and stool osmotic gap panels67. Normal stool osmotic gap values are between 50 and 125 (the osmotic gap is the results of subtracting stool osmolality [290mOsm] from 2* Na+++K+ in stool water). In secretory diarrhoea, the osmotic gap is less than 50, while in osmotic diarrhoea it increases to >125. Patients with diarrhoea secondary to Mg2++ present high osmotic gap values and stool Mg2+ >50mmol/L. Diarrhoea caused by intake of sodium sulphate (Na2SO4) and disodium sulphate (Na2PO4) mimic secretory diarrhoea and can be diagnosed by findings of low Cl− concentration in faecal water (usually <20mmol/L).
In factitious diarrhoea, attempts to tamper with findings can involve the addition of water or urine to stool samples. This can be detected with stool osmolality testing, and is shown as reduced values (<25mOsm) in the case of contamination with hypotonic urine or water, or increased values (>375mOsm) in the case of contamination with concentrated urine. If suspicion is high, spectrofluorimetry or chromatography studies may be needed to determine the presence of laxatives in urine or stool samples.
Recommendations- 1
Serum peptide panels to screen for a neuroendocrine tumour in patients with chronic diarrhoea have a positive predictive value of less than 1%, and routine use is discouraged (CE, moderate; SR, strong).
- 2
CT and MR scans are useful for diagnosing and staging neuroendocrine tumours. An octreotide scan, or octreoscan, can be useful for identifying functioning neuroendocrine tumours (CE, high; SR, strong).
- 3
Routine determination of chromogranin A in patients with chronic watery diarrhoea has low specificity and is of little diagnostic value (CE, high; SR strong).
- 4
A histological finding of pseudomelanosiscoli in biopsy samples from a macroscopically normal colon is non-specific and should not be taken as a marker of laxative abuse, as it has also been associated with administration of other drugs (CE, moderate; SR, strong).
- 5
Stool osmotic gap measurements can be useful in the diagnosis of chronic large-volume watery diarrhoea (CE, moderate; SR, strong).
Chronic functional diarrhoea is defined as the persistent or recurrent passage of loose or liquid stools for more than 4 weeks with no obvious organic cause. If symptoms have lasted for at least 6 months, and occur in more than 75% of bowel movements in the previous 3 months, a diagnosis of functional diarrhoea can be established according to Rome III diagnostic criteria for functional gastrointestinal disorders. According these criteria, diarrhoea accompanied by abdominal pain that improves or is associated with loose stools indicates a diagnosis of diarrhoea-predominant irritable bowel syndrome.68
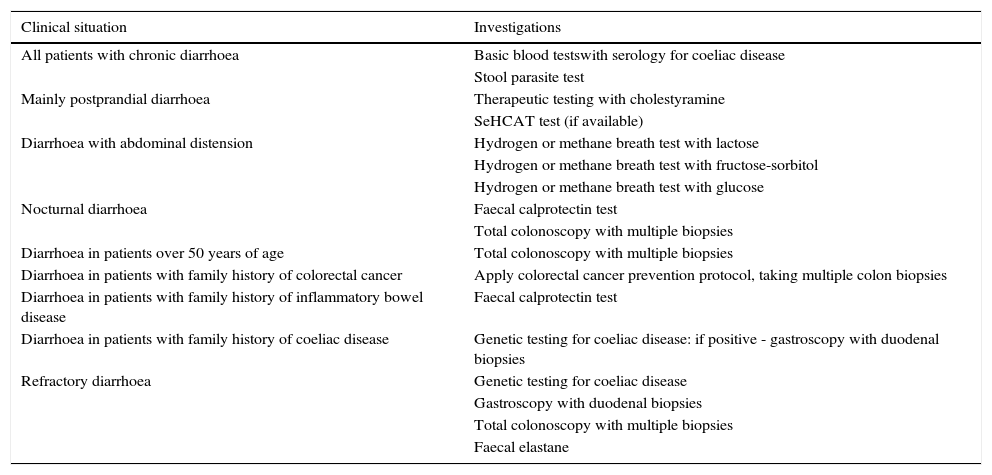
Functional diarrhoea may affect around 5% of the general population,68 and differential diagnosis must consider a wide range of disorders that can cause chronic diarrhoea (Table 12) (Algorithm 6, Fig. 6). In young patients with no alarm signs or symptoms, an unremarkable physical examination, and mild diarrhoea with no night symptoms and little impact on the patient's daily activity, general laboratory tests including serology for coeliac disease and a stool parasite test should be performed. If symptoms persist, are incapacitating, or significantly impact the patient's quality of life, other investigations will be needed to rule out an organic aetiology (Table 13).7,69–72 Some organic diseases, such as microscopic colitis, choleretic diarrhoea or sugar malabsorption diarrhoea, can mimic functional diarrhoea, and are discussed below.
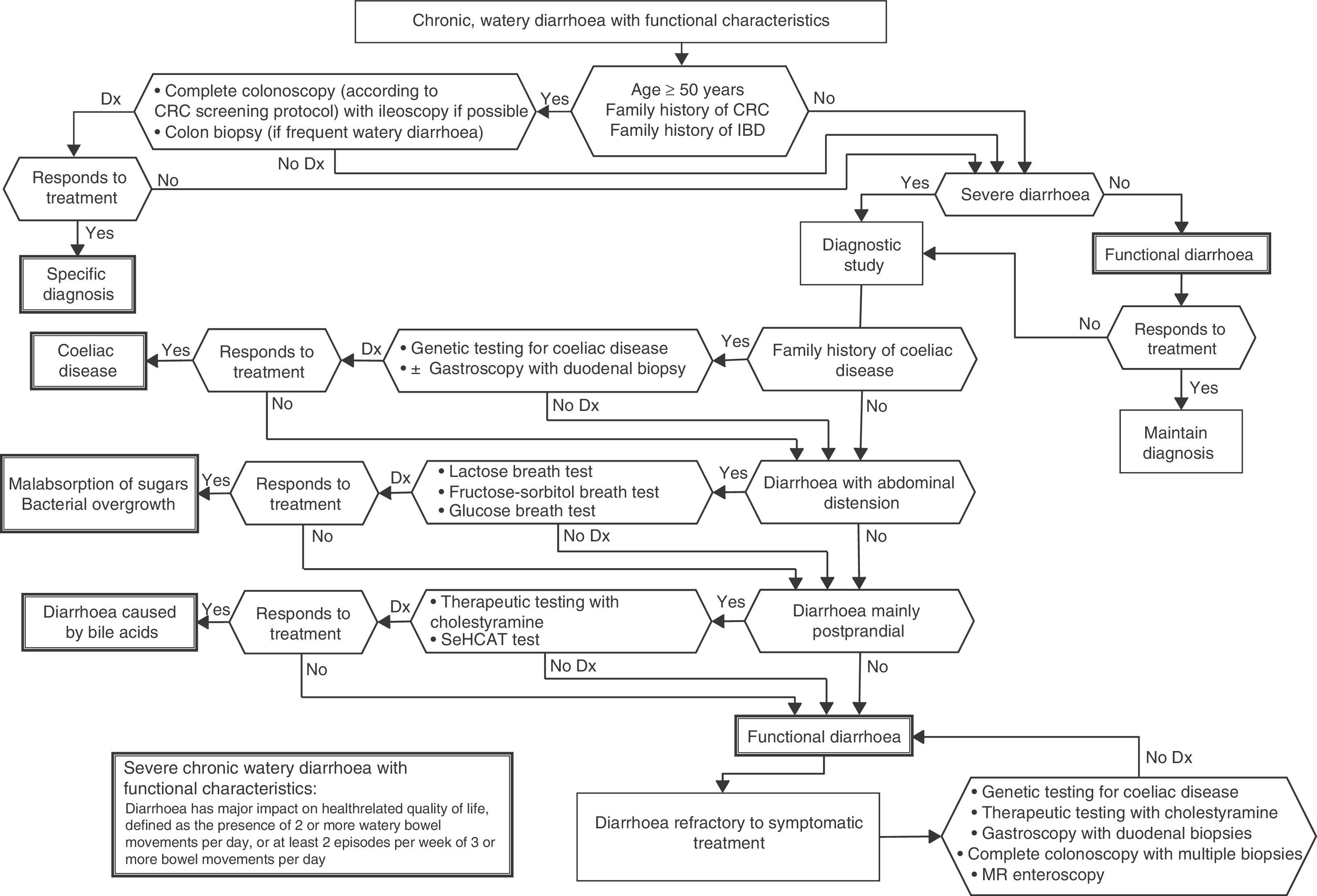
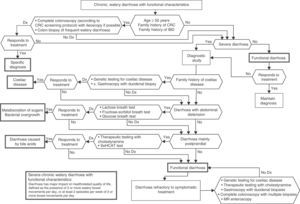
Algorithm 6. Diagnosis of watery diarrhoea with functional characteristics
All patients with chronic diarrhoea with no signs or symptoms of alarm, no abnormal findings on laboratory tests (including serology for coeliac disease) and no parasites in stool samples, are a priori diagnosed with functional diarrhoea. If such patients are aged 50 years or over, or have a family history of colorectal cancer (CRC), a complete colonoscopy should be performed as part of the screening protocol for individuals at moderate risk of CRC. During colonoscopy, biopsies should be taken from various sites along the colon to rule out microscopic colitis in patients with frequent passage of watery stools. A family history of inflammatory bowel disease is also an indication for total colonoscopy with ileoscopy. If no pathology is found, the protocol for patients aged under 50 years with no family history should be applied; in other words, if the diarrhoea is not serious, a diagnosis of functional diarrhoea should be established and the patient treated accordingly. In patients with severe diarrhoea, it is advisable to perform diagnostic tests. Therefore, in patients with a family history of coeliac disease, it is advisable to perform genetic testing for coeliac disease (HLA-DQ2/8). If positive, duodenal biopsy should be performed. In patients presenting diarrhoea with abdominal distension, it is important to bear in mind that, although distension can be caused by various functional gastrointestinal disorders, it is advisable to perform a hydrogen breath test to rule out intolerance to sugars (lactose or fructose and sorbital) or bacterial overgrowth. In patients with mainly postprandial diarrhoea, it should be borne in mind that although postprandial onset is common in functional gastrointestinal disorders, it is essential to rule out bile acid malabsorption, ideally using the SeHCAT test. If this is not available, then therapeutic testing with cholestyramine should be performed. If these tests are not diagnostic, or if they are diagnostic but with no therapeutic response, the diagnosis of functional diarrhoea should be maintained. All patients with undiagnosed severe functional diarrhoea that does not respond to symptomatic treatment should undergo a comprehensive study to rule out bile acid malabsorption, coeliac disease, microscopic colitis or inflammatory bowel disease.
Recommended investigations in patients with suspected functional diarrhoea.
| Clinical situation | Investigations |
|---|---|
| All patients with chronic diarrhoea | Basic blood testswith serology for coeliac disease |
| Stool parasite test | |
| Mainly postprandial diarrhoea | Therapeutic testing with cholestyramine |
| SeHCAT test (if available) | |
| Diarrhoea with abdominal distension | Hydrogen or methane breath test with lactose |
| Hydrogen or methane breath test with fructose-sorbitol | |
| Hydrogen or methane breath test with glucose | |
| Nocturnal diarrhoea | Faecal calprotectin test |
| Total colonoscopy with multiple biopsies | |
| Diarrhoea in patients over 50 years of age | Total colonoscopy with multiple biopsies |
| Diarrhoea in patients with family history of colorectal cancer | Apply colorectal cancer prevention protocol, taking multiple colon biopsies |
| Diarrhoea in patients with family history of inflammatory bowel disease | Faecal calprotectin test |
| Diarrhoea in patients with family history of coeliac disease | Genetic testing for coeliac disease: if positive - gastroscopy with duodenal biopsies |
| Refractory diarrhoea | Genetic testing for coeliac disease |
| Gastroscopy with duodenal biopsies | |
| Total colonoscopy with multiple biopsies | |
| Faecal elastane |
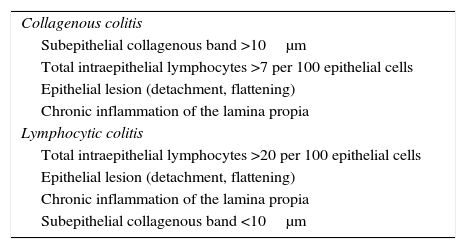
Microscopic colitis (MC) is a generic term used mainly to describe 2 entities: collagenous colitis (CC) and lymphocytic colitis (LC). MC describes a form of chronic, recurrent inflammatory bowel disease characterised by (a) chronic or intermittent passage of non-bloody watery stools; (b) macroscopically normal or nearly normal colonic mucosa on colonoscopy; and (c) characteristic histopathological findings (Table 14).73
Histopathological diagnostic criteria for microscopic colitis.
| Collagenous colitis |
| Subepithelial collagenous band >10μm |
| Total intraepithelial lymphocytes >7 per 100 epithelial cells |
| Epithelial lesion (detachment, flattening) |
| Chronic inflammation of the lamina propia |
| Lymphocytic colitis |
| Total intraepithelial lymphocytes >20 per 100 epithelial cells |
| Epithelial lesion (detachment, flattening) |
| Chronic inflammation of the lamina propia |
| Subepithelial collagenous band <10μm |
In both these entities, symptoms are usually similar to functional diarrhoea or irritable bowel syndrome, and general laboratory tests are usually negative. Both CC and LC are rare entities, and many clinicians and pathologists are unfamiliar with their diagnosis. A recent epidemiological study carried out in Spain reported a mean annual incidence of MC of 4.8/105 inhabitants/year.74 Although it can present in young patients, incidence peaks in women aged 60 years or more, and is higher than Crohn disease and ulcerative colitis in this population. MC is a common diagnosis in this age group, and should be one of the first options in the differential diagnosis of chronic non-bloody watery diarrhoea.
Diagnostic processBiopsies from different sites along a macroscopically normal colon is the gold standard for diagnosis of CC and LC in patients with chronic non-bloody watery diarrhoea.73 This will confirm a diagnosis of MC in between 8% and 16% of patients with chronic non-bloody watery diarrhoea referred for colonoscopy,74–78 in up to 17% of women aged 50 years or over, and in 23% of men aged 70 years or over.78 Histopathological diagnostic criteria are consistent and reproducible, with excellent inter-individual and intra-individual agreement to discriminate between MC, normal histology and others (inter-observer agreement >90%),79,80 with slightly less agreement to discriminate between CC, LC, and incomplete MC (70–80% agreement).80
A recent study suggests that at least 2 diagnostic biopsies from 2 different segments of the colon are needed to diagnose CC and LC, with at least mild chronic inflammation of the lamina propiain the other biopsies and segments.81 The study in question showed that a diagnostic collagenous band (>10μm) was found in 5 segments in only 47% of patients. It also showed that the diagnostic yield of biopsies taken from the ascending, transverse and descending colon was 96.2% higher than other sampling strategies. Therefore, it is recommended to take 2 biopsies each from, at least, the right, transverse and left colon, and store them in separate containers.
Recommendations- 1
Biopsies from different sites along a macroscopically normal colon is the gold standard for diagnosis of CC and LC in patients with chronic non-bloody watery diarrhoea (CE, moderate; SR, strong).
- 2
It is recommended to take 2 biopsies each from, at least, the right, transverse and left colon, and store them in separate containers (CE, moderate; SR, strong).
Bile acids are synthesised in the liver, secreted by the gall bladder and released in the duodenum, where they facilitate absorption of liposoluble vitamins and aid in fat digestion. They are then reabsorbed in the terminal ileum, from where enterohepatic circulation returns them to the liver. Less than 5% reach the colon.82 Elevated bile acid levels in the colon increase colonic motility and the transport of water and electrolytes in the intestinal lumen, causing diarrhoea.83 Symptoms are secretory, watery diarrhoea that can be associated with abdominal pain, distension, faecal urgency, incontinence, nocturnal defecation and, rarely, steatorrhoea.82,83
Bile acid malabsorption (BAM) is a common though little-known cause of chronic diarrhoea.82 Between 20% and 50% of patients with chronic functional-type diarrhoea (functional diarrhoea, diarrhoea-predominant or alternating irritable bowel syndrome) present BAM. BAM associated with a structurally normal ileum is usually called type 2, primary, or idiopathic BAM. In contrast, BAM associated with disease or ileectomy is known as type 1 or secondary BAM. It is very common in patients with Crohn disease (30%) or radiation enteritis (>50%), and nearly always presents after ileectomy (90%).84,85 BAM is also often associated with microscopic colitis (10–60%),86 and is the primary cause of postcholecystectomy chronic diarrhoea. Finally, BAM has also been associated with miscellaneous factors, such as peptic ulcer surgery, coeliac disease, chronic pancreatitis, diabetes mellitus, cystic fibrosis, and the use of some drugs (for example, NSAIDs, colchicine or olsalazine). When BAM presents with any of the foregoing, it is known as type 3 BAM or BAM secondary to gastrointestinal disorders. BAM has also been reported to be a common, and often under-diagnosed, cause of gastrointestinal symptoms associated with cancer therapies.87
DiagnosisThe most common diagnostic technique used in Europe (but not available in the USA) is the 75Se-homotaurocholic Acid (SeHCAT) test, which uses scintigraphy to evaluate abdominal retention of an orally-administered bile acid analogue labelled with selenium (75Se-homotaurocholic acid). The test is highly accurate, and abnormal retention values are predictive of good response to therapy with bile acid sequestrants. A retention value of less than 10% at 7 days is highly sensitive (80–90%) and specific (70–100%) for BAM.72,82,88 The drawbacks of the test are radiation exposure (equivalent to a chest X-ray) and the need for special equipment.
Although therapeutic testing with bile acid sequestrants (cholestyramine) is widely used, the diagnostic accuracy of the test has not been evaluated in the literature, and a definitive diagnosis must be established for a disorder such as BAM, which often requires long-term therapy.82,88,89 If therapeutic testing is performed, it is recommended to start treatment with low-dose bile acid sequestrants, and gradually uptitrate while monitoring patient response.
Recommendations- 1
BAM should be evaluated in patients with functional-type chronic diarrhoea (functional diarrhoea, diarrhoea-predominant irritable bowel syndrome), in intestinal diseases associated with the ileum (Crohn disease and radiation enteritis), and in microscopic colitis (CE, moderate; SR, strong).
- 2
BAM is a ubiquitous feature of chronic diarrhoea following cholecystectomy or ileectomy, and in these cases empirical treatment is advised (CE, low; SR, strong).
- 3
The recommended test for BAM is SeHCAT (CE, moderate; SR, strong).
- 4
If SeHCAT is unavailable, the best option is therapeutic testing with cholestyramine (CE, very low; SR, weak).
Malabsorption of carbohydrates can cause digestive intolerance symptoms, such as diarrhoea, abdominal pain, nausea and meteorism. The most common culprits are lactose, fructose and sorbitol. In a small percentage of cases, malabsorption is caused by rare congenital disorders that cause deficiency of disaccharidases (lactase, sucrase-isomaltase, trehalase, aldolase B) or affect intestinal transport (for example, congenital glucose-galactosemal absorption caused by a mutation in the SLC5A1 gene).
Any sugars that are not absorbed in the small intestine produce symptoms caused by osmotic imbalance and fermentation by the intestinal microbiota, producing short-chain fatty acids and gases (hydrogen, methane, carbon dioxide). Due to the rapid transit of carbohydrates through the gastrointestinal tract, symptoms will start shortly after sugar intake, and can persist for 6–9h. Typically, symptoms only occur when the patient ingests the offending carbohydrate. Symptoms are highly nonspecific, and differential diagnosis with other digestive pathologies is essential.
Lactose intoleranceLactose intolerance is the body's symptomatic response to lactose ingestion caused by hypolactasia or decreased β-galactosidase levels in intestinal villi90; this enzyme hydrolises lactose into monosaccharides (glucose and galactose) that can be absorbed by the jejunum. Hypolactasia is classed as primary (acquired or congenital) or secondary. The most common form is acquired hypolactasia, which is also called primary lactase deficiency or lactase non-persistence. Persistence or non-persistence of lactase is determined by autosomal-recessive inheritance of polymorphisms in the regulatory region of the lactase gene.91 In lactase non-persistent individuals, the activity of the enzyme gradually declines after weaning. It is a highly prevalent disorder that affects 70% of the world's population, although it is more predominant in certain regions. Most adults in Africa, Asia, Latin America and the Mediterranean region are lactase non-persistent, while most individuals in north and central Europe are lactase-persistent.
Lactose malabsorption is not always associated with intolerance and symptoms. Only 30–50% of individuals with lactose malabsorption are lactose intolerant. However, subjective perception of lactose intolerance does not always indicate lactose malabsorption,92–94 and therefore lactose malabsorption and lactose intolerance are not synonymous. For this reason, the patient history contributes little to a diagnosis of lactose intolerance, and diagnostic confirmation is needed before imposing life-long lactose restriction. The gold standard test is the determination of lactase levels in samples from intestinal biopsies,95 although it is rarely used because of its invasive nature, high cost, and limited results due to the patchy distribution of lactase in the gut.96 The most widely used, simplest, non-invasive, inexpensive and highly accurate diagnostic techniques are the H2 breath test with lactose challenge34 (sensitivity, 73%; specificity, 86%) and the gaxilose test97,98 (sensitivity, 93%; specificity, 92%). The breath test measures increased levels of H2 in exhaled breath (>20ppm) following intake of 50g of lactose. False positives can be caused by bacterial overgrowth and rapid intestinal transit. The test can trigger digestive symptoms but yield false negative results in individuals with non-H2-producing bacterial flora (15–20% of the population) or due to a nocebo effect (44% of individuals with a negative breath test).99 The gaxilose test measures the total amount of xylose in urine collected over 5h following oral administration of 4-galactosylxylose, with xylose levels of <37.87mg being diagnostic for hypolactasia.
Genetic testing evaluates the existence of polymorphisms (C/T-13910, G/A-22018) associated with lactase non-persistence. CC and GG genotypes are associated with non-persistence, while CT, TT, GA and AA genotypes are associated with lactase persistence, although some polymorphic variants can affect the accuracy of the test.100 The test has a high negative predictive value (98%), so the possibility of CT and TT-1390 genotypes presenting primary lactase deficiency is extremely remote.101 Agreement with a positive finding in a breath test is 100%.101–103 Genetic testing, however, is of no use in patients with suspected secondary hypolactasia, it is more costly than the gaxilose test or breath test, and is only available in a few hospitals.
Fructose and sorbitol intoleranceFructose is a monosaccharide found in fruit and some vegetables, and is widely used as a sweetening agent in the food industry. Fructose is absorbed by a number of pathways that facilitate transport across the intestinal epithelium. When fructose is present in excess of glucose (also called “free fructose”), the excess is absorbed by a low-capacity transporter (GLUT-5), so the greater the fructose overload, the greater the likelihood of malabsorption. Glucose facilitates fructose absorption along a more efficient pathway which uses the GLUT-2 transport system (facilitated glucose transporter).104,105
Sorbitol is a sugar alcohol found in some fruit that is widely used as a sugar substitute, and is found in cakes, jams, chewing gum and low-calorie products. It is absorbed in the small intestine by passive transport, and acts as an inhibitor of the GLUT-5 fructose transporter. Consumption of fructose and sorbitol together, therefore, worsens symptoms caused by fructose malabsorption.
Fructose intolerance is defined as the onset of symptoms following intake of <25–30g of fructose104. The H2 breath test with 25g fructose challenge is used to diagnose fructose malabsorption, while sorbitol malabsorption is confirmed by the H2 breath test 5g sorbitol challenge. Some authors, however, prefer to evaluate malabsorption of a combination of fructose and sorbitol, instead of fructose alone (20–25g fructose+3.5–5g sorbitol),104 as these sugars are frequently ingested together and, as already described, sorbitol obstructs absorption of fructose. Nevertheless, the main problem encountered in the diagnosis of both fructose and sorbitol malabsorption is uncertainty surrounding the normal absorption capacity of these sugars in healthy subjects.94,106 The correct dosage and concentration of fructose and/or sorbitol needed to differentiate between normal or impaired absorption remains unclear.
Other carbohydratesThe term “fermentable oligosaccharides, disaccharides, monosaccharides and polyols” (FODMAP) has recently been introduced to define a group of poorly absorbed short-chain carbohydrates that were previously thought to be unrelated, but have since been found to exhibit similar behaviour in the small and large intestine.104,107 The term includes malabsorbed fructose and lactose, different polyalcohol sugars (sorbitol, maltitol, xylitol, etc.) that are usually poorly absorbed, and fructans and galactans (galacto-oligosaccharides, such as raffinose and stachyose), which are always malabsorbed. All FODMAPs exert a similar osmotic effect in the colon, and are rapidly fermented by the gut microflora. The combination of fructose and fructans can aggravate symptoms in a similar way as the aforementioned dual action of fructose and sorbitol. This factor should be borne in mind when drawing up diets designed to restrict intake of malabsorbed sugars.
Recommendations- 1
Subjective manifestations of lactose intolerance do not always indicate lactose malabsorption. If diarrhoea secondary to primary lactase deficiency is suspected, diagnostic confirmation is needed before imposing life-long lactose restriction (CE, high; SR, strong).
- 2
The H2 breath test with lactose challenge, the gaxilose test, and genetic testing are recommended to confirm diagnosis of lactose malabsorption.
- 3
When fructose and/or sorbitol intolerance are suspected, a breath test using these sugars is advised (CE, low; SR, weak).
Motility abnormalities can be an aetiopathogenic factor shared by several different digestive disorders in which the primary symptom is chronic diarrhoea. In these entities, changes in motility are more a contributing factor than the underlying mechanism causing diarrhoea. This section will focus on chronic diarrhoea caused by gastrointestinal neuromuscular diseases in which the condition arises as a result of changes in motor function per se (for example, diabetes mellitus), or secondary to bacterial overgrowth (for example, scleroderma). Intestinal neuromuscular diseases (chronic intestinal pseudo-obstruction and intestinal dysmotility) are uncommon disorders that are rarely the cause of chronic diarrhoea. As such, they should only be investigated when other, more common, causes of chronic diarrhoea have been ruled out.108,109
ClassificationNeuromuscular diseases of the gastrointestinal tract are divided into:
- 1
Primary neuromuscular disease. This is characterised by:
- •
Chronic and recurrent subocclusive episodes or abdominal distension.
- •
Persistent or intermittent daytime or nocturnal diarrhoea interspersed with constipation, associated with weight loss and laboratory test findings of malnutrition/malabsorption. Bacterial overgrowth is a common feature (see corresponding section above), and the diarrhoea may improve or resolve after normalisation of bacterial colonisation.
- •
Endoscopic and radiological studies (CT, transit, MR enterography) used to rule out other structural diseases are usually unremarkable or show dilated bowel loops.
- •
There may also be extra intestinal symptoms due to involvement of other organs or systems (most often the urological, cardiovascular and autonomic nervous systems).
- •
- 2
Secondary to general disease with intestinal involvement:
- •
Connective tissue diseases: scleroderma, dermatomyositis, systemic lupus erythematosus.
- •
Endocrine and metabolic disorders: diabetes mellitus, hyperthyroidism.
- •
Neurological disorders: dysautonomia, MNGIE disease, Parkinson disease.
- •
Muscular diseases: muscular dystrophy, Steinert disease.
- •
Infiltrative diseases: amyloidosis.
- •
After gastric surgery with vagotomy.
- •
Diagnosis is based on: (a) compatible clinical history: suggestive symptoms, with other organic pathology ruled out by radiographic studies. Given the rarity of intestinal neuromuscular diseases, it is important to first rule out other, more common, causes of chronic diarrhoea. In milder cases, difficulties can be encountered in differentiating these diseases from other functional pathologies (IBS/functional diarrhoea); (b) test for bacterial overgrowth (see corresponding section above); and (c) test for motor dysfunction using small bowel manometry, which is the gold standard for studying small bowel motility.110–112
Small bowel manometry has high specificity but low sensitivity. Internationally accepted manometric diagnosis criteria are available.110 Manometry can be used to detect motor dysfunction and differentiate between myopathic and neuropathic disorders. It is a complex technique that requires skilled interpretation, and is only available in specialised centres.
Motor abnormalities correlate poorly with oro-caecal transit time, and the latter cannot be taken as an indication of motor dysfunction.
RecommendationsIntestinal manometry can be useful in the diagnosis of neuromuscular disorders in patients with recurrence of diarrhoea in an appropriate clinical context (CE, moderate; SR, weak).
Chronic diarrhoea secondary to food allergiesAdverse reactions to certain foods can be divided into 2 major subgroups: (a) food allergies, which are a subgroup of hypersensitivity reactions caused by immune mechanisms derived from IgE-dependent mast cell activation, or mediated by other immune mechanisms, often type 4 or delayed-type hypersensitivity reactions,113–115 and (b) food intolerance, which includes a number of reactions mediated by non-immune mechanisms and derived from enzyme or transport deficiencies, or the pharmacological properties of certain food components.116 Food allergies are more common in the paediatric population, and usually decrease with age; according to estimates based on diagnoses made using accepted techniques, prevalence ranges between 3.5% for cow's milk and 0.5–1.3% for other common food allergies (eggs, nuts, cereals, fruit, fish and seafood).114,117 The prevalence of food intolerance, in contrast, is estimated at between 15% and 20%, and is particularly associated with gastrointestinal functional diseases, such as irritable bowel syndrome and functional dyspepsia.118 The subgroup of food intolerance disorders includes both common and less frequent conditions that can cause diarrhoea and other gastrointestinal manifestations, such as flatulence, abdominal distension, nausea, vomiting and abdominal and/or epigastric pain. Sugar malabsorption, non-coeliac gluten sensitivity and FODMAP are among the most common disorders, and have been discussed elsewhere in this report. Less common disorders include intolerance to salicylates, biogenic amines, caffeine and glutamate, toxic reactions to microbial or fungal products, such as aflatoxins, and psychological reactions.116 It is important to differentiate between allergies and intolerance, as the gastrointestinal manifestations of food allergies are often comparable to symptoms of intolerance. Allergies, however, are commonly associated with other specific extra intestinal manifestations, such as rash or angiooedema, which usually appear immediately after exposure or intake of the offending allergen. In addition, unlike food intolerance, in which diarrhoea can persist while the individual continues to consume, albeit unwittingly, the offending foodstuff, fool allergies can cause acute diarrhoea, but are rarely the direct cause of chronic diarrhoea. A particular case in point is diarrhoea-predominant irritable bowel syndrome, in which it can be difficult to establish the role of food allergies and intolerance in the pathogenesis of the diarrhoea, as both entities often combine in these patients, and can even be associated with psychological adverse food reactions. In recent years, some authors have implicated IgG- or IgG4-mediated delayed food hypersensitivity reactions119–121 in the pathogenesis and severity of clinical manifestation, including diarrhoea. The association is still under debate, and no consensus has yet been reached.
DiagnosisThe first and most important step in diagnosing food allergies and differentiating them from food intolerance is to compile a comprehensive clinical and dietary history, focussing in particular on the food consumed by the patient in the 2h prior to the onset of symptoms. It is important to take into account levels of tolerance before and after ingestion of the offending foodstuff: good tolerance after ingestion will exclude the presence of an IgE-mediated mechanism, except when food allergies are facilitated or aggravated by co-factors (exercise, NSAIDs, stress or alcohol). Once the clinical suspicion has been confirmed, tests must be performed to establish whether the allergic symptoms are caused by an IgE-mediated hypersensitivity reaction. These include122: (a) skin prick tests using commercial extracts, or prick-prick tests using fresh foodstuffs; (b) determination of food-specific IgEin serum (UniCAP100, ImmunoCAP250, Immulite System, HYTEC-288 system); and to a lesser extent (c) the use of purified or recombinant molecules (component separation) to accurately identify the allergens causing reaction in each patient (microarray chip, ISAC; UNICAP, Thermofisher). These tests are mainly used to differentiate between true allergic reactions or cross reactivity; (d) screening tests for foods or pollens, particularly useful in primary care, that contain a mixture of the most common allergens (multiallergen screen IgE test, Phadiatop); and (e) other blood tests: tryptase, eosinophil cationic protein, carboxypeptidase, cathepsin G and mast cell chymase, IFN-γ, TNF-α, FGF, IL-4, IL-5, IL-6, IL-13, histamine, chondroitin sulphate, heparin, leukotriene C4, PGD2, total IgE and free IgE. Table 15 shows the specificity and sensitivity of the most widely used complementary tests for specific target foods.123 The double-blind, placebo-controlled oral food challenge is considered the gold standard diagnostic test. However, it is impractical, because it is time consuming and resource-intensive, and can cause anaphylaxis.
Approximate diagnostic accuracy of the main complementary food allergytests.
| Food | Technique | Diagnostic accuracy |
|---|---|---|
| Egg | s-IgE | Se, 93% (82–98); Sp, 49% (40–58) |
| SPT | Se, 92% (80–97); Sp, 58% (49–67) | |
| Cow's milk | s-IgE | Se, 87% (79–94); Sp, 48% (36–59) |
| SPT | Se, 88% (76–94); Sp, 68% (56–77) | |
| Wheat | s-IgE | Se, 83% (69–92); Sp, 43% (20–69) |
| SPT | Se, 73% (56–85); Sp, 73% (48–89) | |
| Soy | s-IgE | Se, 83% (64–93); Sp, 38% (24–54) |
| SPT | Se, 55% (33–75); Sp, 68% (52–80) | |
| Nuts | s-IgE | Se, 96% (92–98); Sp, 59% (45–72) |
| SPT | Se, 95% (88–98); Sp, 61% (47–74) | |
| Fish | s-IgE | Se, –% (67–94); Sp, –% (65–88) |
| SPT | Se, 91%-100% (–); Sp, 57% (–) | |
| Seafood | s-IgE | Se, 100% (80–100); Sp, 45% (23–68) |
| SPT | Se, 100% (–); Sp, --% (32–50) |
Se: sensitivity; Sp: specificity; SPT: Skin prick test.
Source: adapted from Soares-Weiser et al.123
Any methods that have not proved effective and safe in controlled trials should be classed as “unproven methods” or “non-validated methods”, and are therefore not recommended for the purpose of diagnosis.124 Such tests include: (a) controversial in vivo tests: subcutaneous, sublingual and intradermal provocation tests; the ricercaintolleranzealimentaria (DRIA) test, based on the sublingual administration of an allergenic extract and on measurement of muscle strength with an ergonometer; electroacupuncture and electrodermal testing (Voll method); applied kinesiology (manual measurement of muscle strength), and bioresonance; and (b) controversial in vivo tests: determination of IgG in blood samples using ELISA (A200 test, IADM test, and food intolerance screening [FIS]), and testing for IgG4 against foods (IgG4 Screen Nutritional); DNA analysis of hair samples to determine food intolerance, and measurement of white cells using the Coulter counter, either with or without incubation with food extracts (ALCAT test). There is currently insufficient evidence to recommend the use of detection of IgG or IgG4 against food components to guide the dietary management of chronic diarrhoea.
A recent recommendation indicates ruling out wheat allergy before diagnosing a patient with non-coeliac gluten sensitivity.125 Nevertheless, wheat allergy is rare and usually causes respiratory symptoms, above all rhinitis, particularly in association with co-factors such as exercise and stress. Diagnosis is based on the skin prick test (whole wheat, gluten and gliadin) and also by determination of IgE against ω5-recombinant gliadin.
Recommendations- 1
Taking a comprehensive medical history will help differentiate between diarrhoea associated with an immune reaction or with other mechanisms more typical of food intolerance.
- 2
If food allergy is suspected, the patient should be referred to an allergist for specific IgE determination and a skin prick test, both of which have good sensitivity but low specificity (CE, high; SR, strong).
- 3
The double-blind, placebo-controlled oral food challenge is considered the gold standard diagnostic test. However, it is impractical, because it is time consuming and resource-intensive, and can cause anaphylaxis (CE, high, SR, strong).
Primary care doctors make the initial evaluation of patients presenting with chronic diarrhoea (Algorithm 7, Fig. 7). It is important to determine whether the patient meets diagnostic criteria for chronic diarrhoea, to take a comprehensive medical history, and order basic blood and stool tests. It is essential to evaluate the presence of organic factors, to decide whether to refer the patient to a gastroenterologist, and whether to order a colonoscopy (Table 16).
Indications for colonoscopy in patients with chronic diarrhoea.
| Recent onset chronic diarrhoea in patients aged > 50 years |
| Chronic diarrhoea and history of colorectal cancer in first degree relatives |
| Chronic inflammatory diarrhoea (see definition in text) |
| Chronic non-bloody diarrhoea with positive faecal occult blood |
| Chronic non-bloody diarrhoea with calprotectin>150mg/kg |
| Chronic non-bloody diarrhoea with elevated C-reactive protein |
| Chronic watery diarrhoea with severe functional diarrhoea criteria or signs and symptoms and test findings suggestive of organic origin (see text) |
In many patients presenting with recurrent but mild (less than 2 episodes per week) watery diarrhoea, empirical therapy to treat symptoms (with loperamide or racecadotril) is often appropriate. In these cases, it is important to evaluate whether the complaint is associated mainly with symptoms of meteorism and abdominal distension. If so, the presence of sugar malabsorption (lactose and/or fructose or sorbitol) must be ruled out.
Conflict of interestsThe authors declare they have no conflicts of interest.
Please cite this article as: Fernández-Bañares F, Accarino A, Balboa A, Domènech E, Esteve M, Garcia-Planella E, et al. Diarrea crónica: definición, clasificación y diagnóstico. Gastroenterol Hepatol. 2016;39:535–559.