Ustekinumab is an effective treatment for inflammatory bowel diseases. However, some patients do not respond to conventional doses. The aim of the study was to evaluate the effectiveness of intravenous maintenance ustekinumab in patients with secondary failure.
MethodsSingle-center, retrospective study in adult patients with intravenous maintenance ustekinumab. The reduction of biochemical activity markers, ustekinumab trough levels and clinical indices of activity were evaluated. Biological remission was defined as the percentage decrease fecal calprotectin ≥80% and/or final fecal calprotectin ≤250 and C reactive protein <5mg/L.
ResultsThirty-one patients were included: Crohn's disease 77.4%. All included patients were bio-exposed and 61.3% had carried ≥2 biologics.
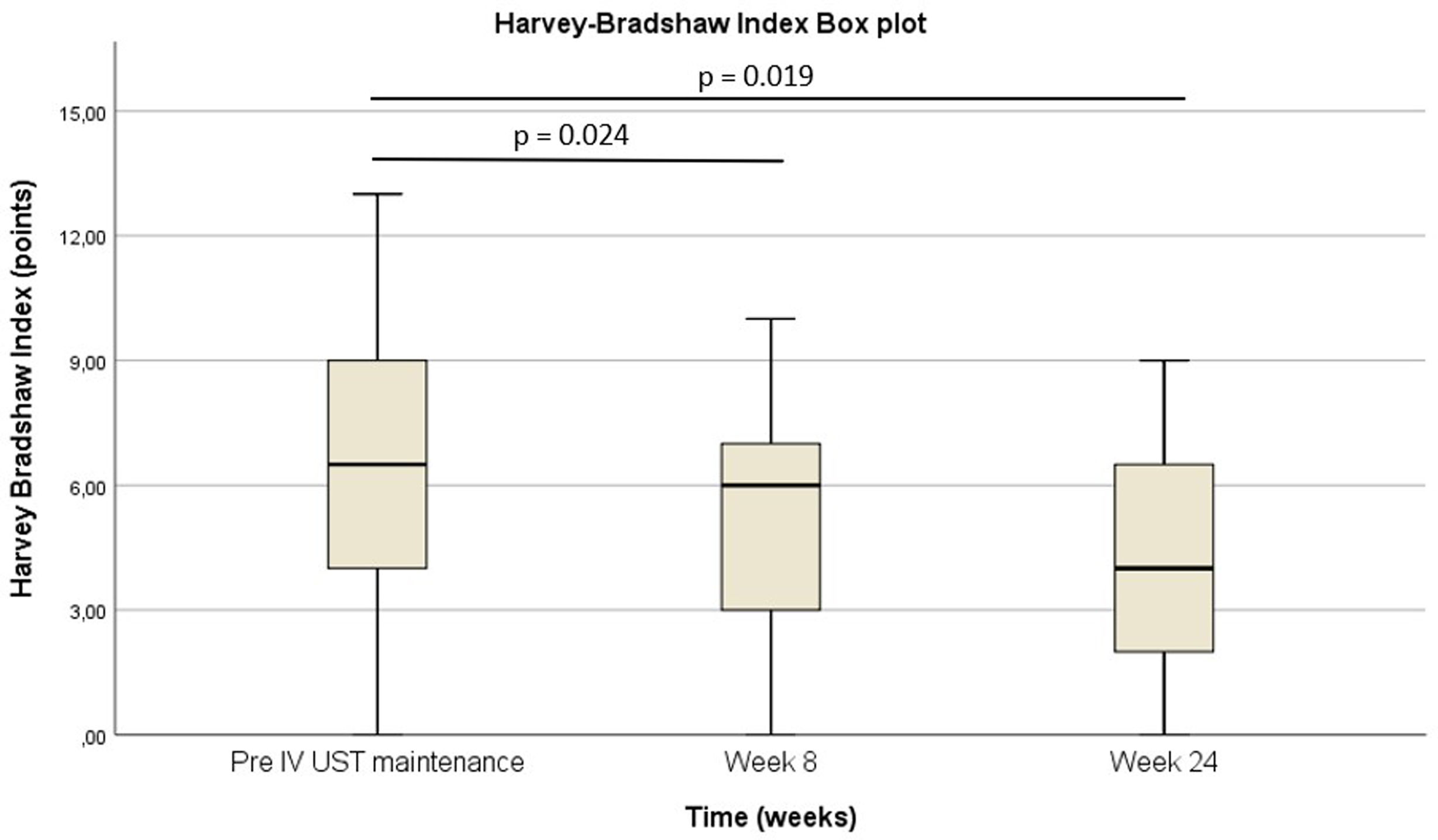
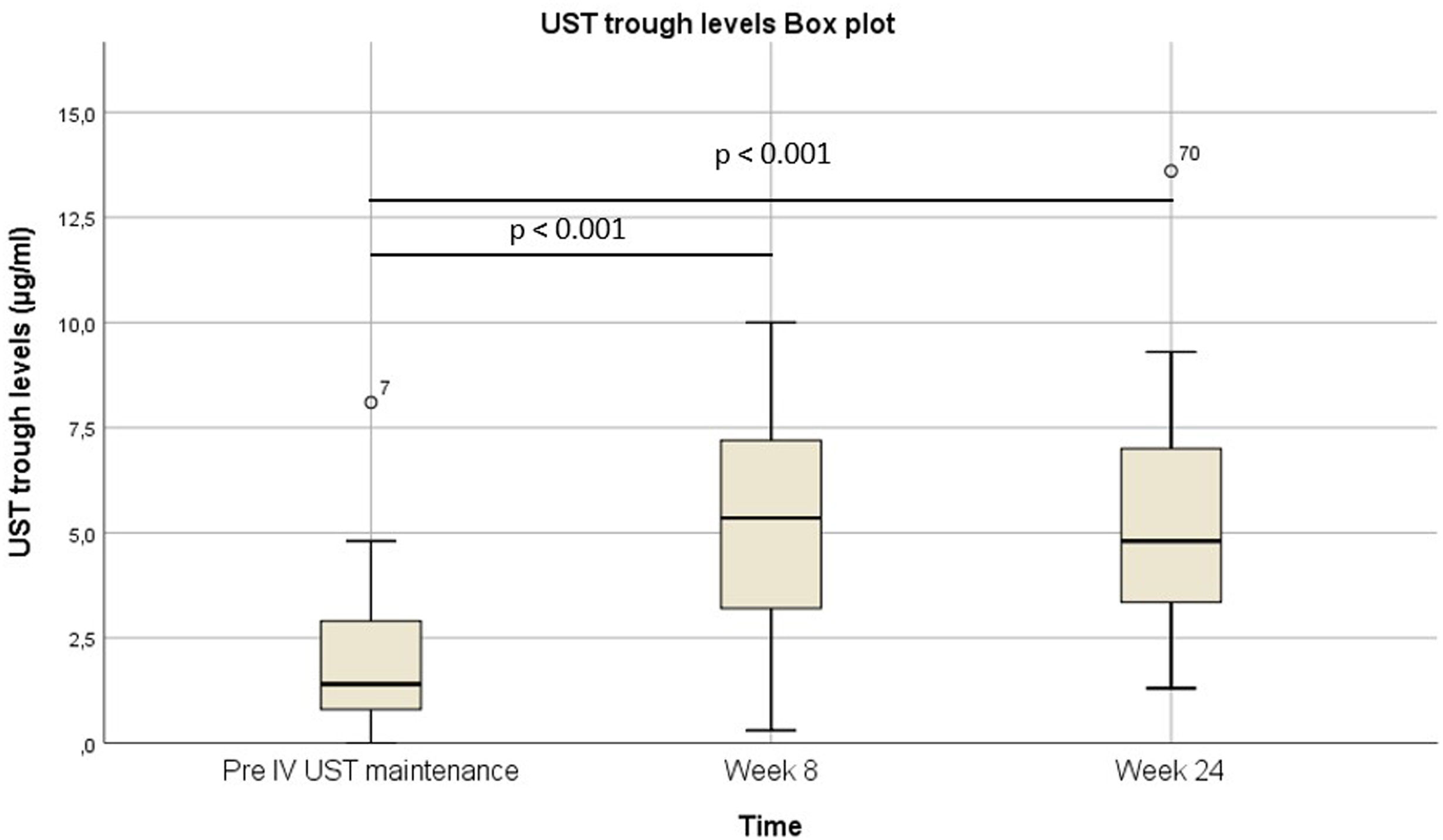
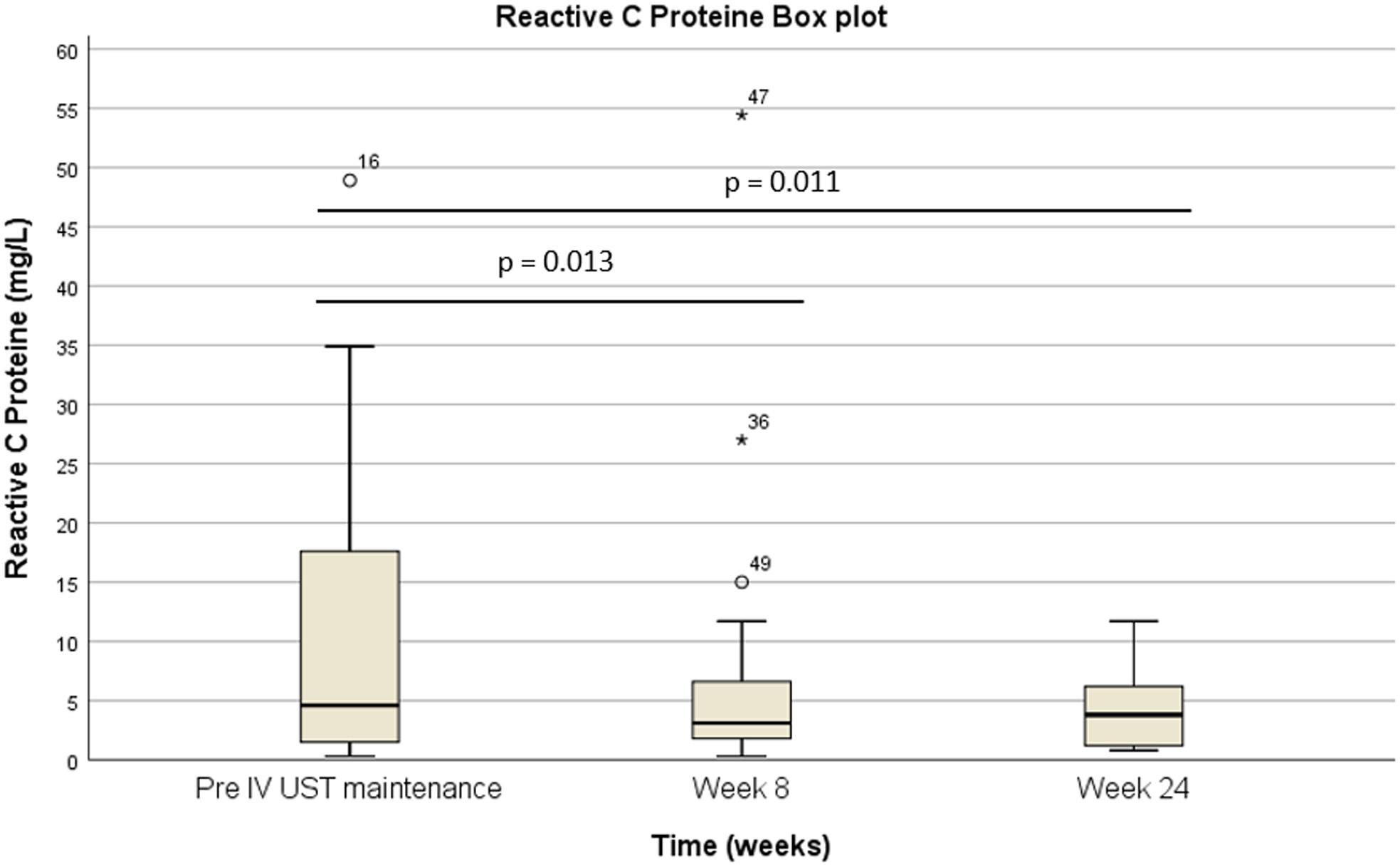
Pre-intravenous maintenance mean Harvey–Bradshaw Index was 6.5±4.38 vs 5±3.1 at week 8 (p=0.024) vs 4.1±3.1 at week 24 (p=0.019). The median ustekinumab trough level pre-intravenous maintenance was 1.40μg/ml [IQR 2.3] vs 5.35μg/ml [IQR 4.1] at week 8 (p<0.001) vs 4.8μg/ml [IQR 3.9] at week 24 (p<0.001). The pre-intravenous maintenance median fecal calprotectin was 809μg/g [IQR: 2256] vs 423μg/g [IQR: 999] at week 8 (p=0.025) vs 333μg/g [508] (p=0.001) at week 24.
At the end of follow-up 48% went into biological remission. The presence of perianal disease was associated with lower biological remission (70.6% vs 27.3%, p=0.025). Median intravenous ustekinumab maintenance time was 8.55 [IQR 23.9] months. In 83.9% of patients no serious infections or malignancy were documented.
ConclusionsThe use of maintenance intravenous ustekinumab appears to be an effective and safe strategy that can be evaluated as a salvage treatment especially in highly bio-exposed patients.
Ustekinumab es un tratamiento eficaz para la enfermedad inflamatoria intestinal. Sin embargo, algunos pacientes no responden a las dosis convencionales. El objetivo del estudio fue evaluar la eficacia de ustekinumab intravenoso de mantenimiento.
MétodosEstudio retrospectivo unicéntrico en pacientes adultos con ustekinumab intravenoso de mantenimiento. Se evaluó el descenso de los marcadores bioquímicos de actividad, los niveles valle de ustekinumab y los índices clínicos de actividad. La remisión biológica se definió como la disminución porcentual de calprotectina fecal ≥80% y/o calprotectina fecal final ≤250 y proteína C reactiva <5mg/l.
ResultadosSe incluyeron 31 pacientes: enfermedad de Crohn 77,4%. Todos los pacientes incluidos estaban bioexpuestos, y el 61,3% habían llevado ≥2 biológicos.
La mediana del índice Harvey-Bradshaw antes del mantenimiento intravenoso fue de 6,5±4,38 vs. 5±3,1 en la semana 8 (p=0,024) vs. 4,1±3,1 en la semana 24 (p=0,019). La mediana de los niveles valle de ustekinumab antes del mantenimiento intravenoso fue de 1,40μg/ml (IQR: 2,3) vs. 5,35μg/ml (IQR: 4,1) en la semana 8 (p<0,001) vs. 4,8μg/ml (IQR: 3,9) en la semana 24 (p<0,001). La mediana de calprotectina fecal antes del mantenimiento intravenoso fue de 809μg/g (IQR: 2.256) vs. 423μg/g (IQR: 999) en la semana 8 (p=0,025) vs. 333μg/g (508) (p=0,001) en la semana 24.
Al final del seguimiento, el 48% entró en remisión biológica. La presencia de enfermedad perianal se asoció a una menor remisión biológica (70,6 vs. 27,3%; p=0,025). La mediana del tiempo de mantenimiento con ustekinumab intravenoso fue de 8,55 (IQR: 23,9) meses. En el 83,9% de los pacientes no se documentaron infecciones graves ni neoplasias.
ConclusionesEl uso de ustekinumab intravenoso de mantenimiento parece ser una estrategia eficaz y segura que podría servir como tratamiento de rescate especialmente en los pacientes altamente bioexpuestos.