A severe shortage of suitable allografts is a long-standing and worldwide problem for patients who are waiting for organ transplantation. Hepatocyte transplantation has been proposed as an alternative therapeutic approach for liver disease patients to address this urgent and unmet medical need. The cell replacement approach does not replace orthotopic liver transplantation (OLT), but rather it complements OLT especially for patients who do not require whole liver replacement, such as those with congenital metabolic disorders. This review article summarizes the current knowledge and limitations of clinical hepatocyte transplantation and aims to advance our understanding toward the goal of developing novel cell replacement therapies for patients who are on the OLT waiting list.
Un antiguo problema en todo el mundo es la grave escasez de aloinjertos adecuados para pacientes que esperan un trasplante de órganos. El trasplante de hepatocitos se ha propuesto como un enfoque terapéutico alternativo para hacer frente a esta necesidad médica urgente y sin resolver en pacientes con hepatopatía. El enfoque del reemplazo celular no sustituye el trasplante ortotópico de hígado (OLT, por sus siglas en inglés) sino que lo complementa, sobre todo en pacientes que no requieren reemplazo hepático completo como en aquellos con trastornos metabólicos congénitos. Este artículo de revisión resume el conocimiento actual y las limitaciones del trasplante clínico de hepatocitos y tiene como objetivo mejorar nuestro conocimiento, con el objetivo de crear nuevas terapias de reemplazo celular para aquellos pacientes que están en lista de espera de un OLT.
Hepatocyte transplantation has been proposed as an alternative approach to orthotopic liver transplantation (OLT). A number of advantages of cell replacement therapy over OLT have been described, including being a less invasive and more cost-effective procedure.1 The concept of partially replacing damaged or malfunctioned hepatocytes is particularly suitable for patients who suffer from congenital metabolic disorders (CMDs) and for acute liver failure (ALF) patients who require transient support of their liver function. The keys to successful hepatocyte transplantation are the careful evaluation of eligible recipients, the appropriate preconditioning of the recipient's liver, and the quality of the donor cells. The cell transplantation procedure is technically simple compared to that of OLT. Unlike OLT, techniques in vascular and interventional radiology can be used to deliver the cells to the liver without major surgical interventions. Here, we briefly overview cell replacement therapy with regard to the reported clinical cases, suitable target diseases, clinical procedures, limitations of the procedures, and perspective on overcoming the limitations.
HistoryThe basic studies that led to clinical hepatocyte transplantation were initiated in the 1970s. Rodent models of metabolic disorders and models of acute liver failure induced by chemicals, partial hepatectomy, or ischemic–reperfusion liver injury were often used to demonstrate improvement of impaired liver function by injection of autologous liver cells. These pre-clinical studies indicated that allogeneic liver cell transplantation had a potential to correct various metabolic defects. The earliest published reports of pre-clinical hepatocyte transplantation were performed in 1976 by a group led by Najarian using UDP-glucuronyl-transferase-deficient rats, the Gunn rat.2 About a decade later, Mito et al. tested an idea to utilize the spleen as an ectopic liver by transplanting hepatocytes into the spleens of rodent and dog models.3
The first attempted human hepatocyte transplantation was performed in 1992 by the same group in Japan.4 The safety and therapeutic efficacy of hepatocyte splenic arterial infusion were confirmed by Strom et al. with chronic end-stage liver disease patients.5 They demonstrated that transplanted human hepatocytes were viable in splenic nidation and showed typical hepatic cord structures. Three of five treated patients fully recovered and successfully received OLT. Since then, more than 100 clinical hepatocyte transplantations have been reported.6 Over the last two decades, more than 15 institutions around the world have conducted clinical hepatocyte transplantation, and at least 7 groups are currently active. However, despite the successes of these clinical studies, hepatocyte transplantation has remained experimental due to the limited supply of donor liver tissue for hepatocyte isolation. Conducting a large-scale randomized clinical trial has been hindered by the limited and inconsistent supply of sufficient quality human hepatocytes.
IndicationsOrthotopic liver transplantation (OLT) is a remarkably efficient treatment to improve the prognosis in patients with fulminant hepatic failure, end-stage liver disease, and metabolic liver diseases. In theory, if we can replace 100% of a patient's damaged hepatocytes, hepatocyte transplantation can provide similar therapeutic efficacy to OLT in most of these diseases. Therefore, the indication for hepatocyte transplantation depends on the extent of cell replacement required to alleviate disease symptoms and the amount of donor cells that can functionally engraft in the patient's liver. Consequently, disease conditions that inhibit donor hepatocyte engraftments such as cirrhotic liver and acute hepatitis will not be suitable targets for hepatocyte transplantation. Contraindications for hepatocyte transplantation may include metastatic cancer outside of the liver, active drug or alcohol abuse, and active systemic infections. However, unlike OLT which is a major surgery with a high incidence of complications, the list of contraindications is shorter for hepatocyte transplantation.
Patients with congenital metabolic disorders may benefit from hepatocyte transplantation. Careful assessments of the impaired enzyme functions will be required to determine the indication for hepatocyte transplantation. In general, patients with metabolic diseases require only partial replacement of hepatocytes to compensate for the missing enzyme function. The required compensation level may vary by case and by disease. For example, an infusion of normal hepatocytes that is equivalent to 5% of the parenchymal mass achieved a medium-term reduction in serum bilirubin in a patient with Crigler-Najjar syndrome.7 An ornithine transcarbamylase deficient child who received 1.9×109 hepatocytes had normalization of plasma ammonia and glutamine levels on a normal diet without phenylbutyrate/phenylacetate therapy.8 These cases clearly demonstrate that CMDs can be effectively treated via cell replacement therapy. Patients with acute liver failure will also benefit from the cell replacement strategy. Although in these patients the liver is actively inflamed, injecting hepatocytes into the spleen may support the patients’ hepatic function temporarily until the own liver recovers or until OLT becomes available.
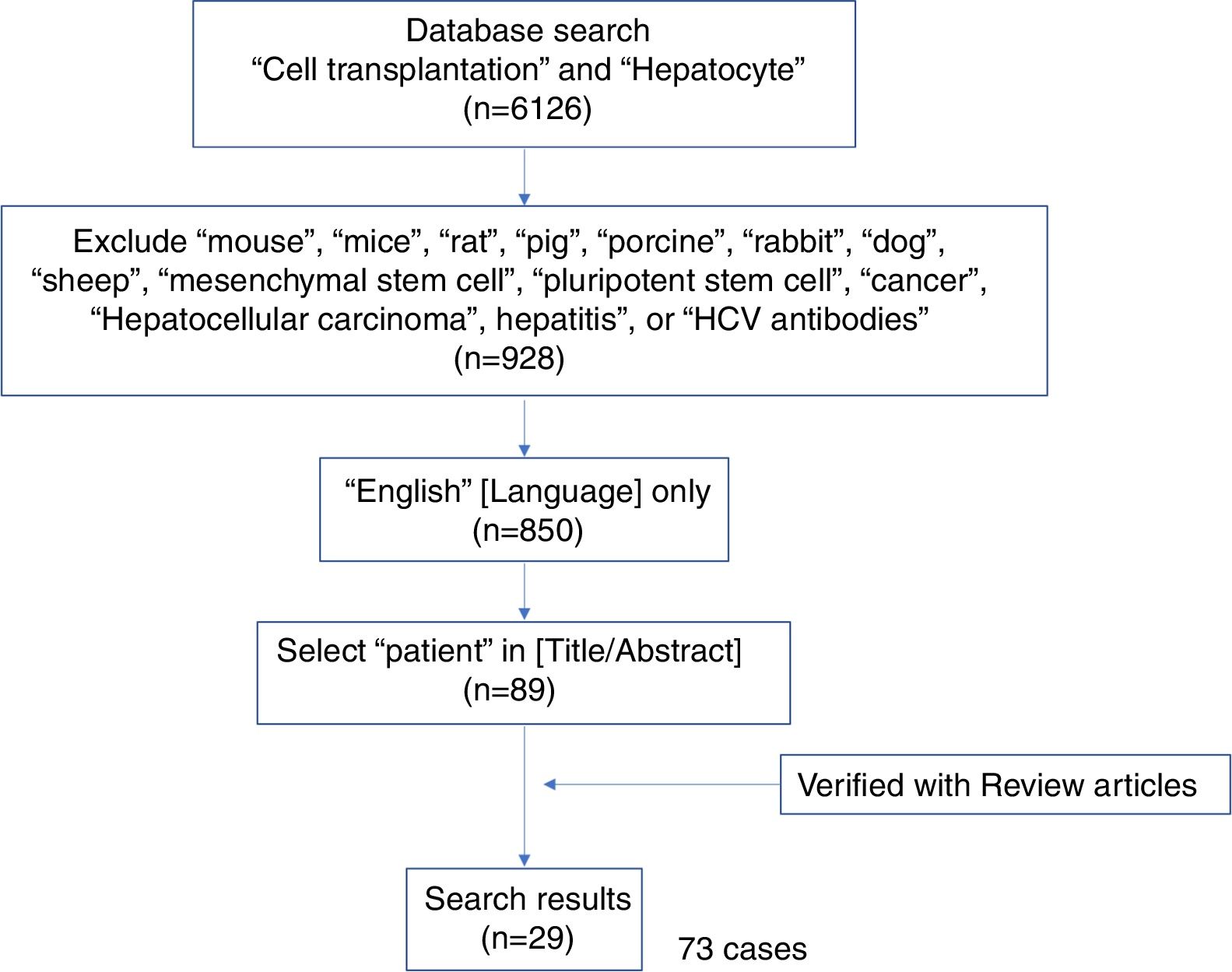
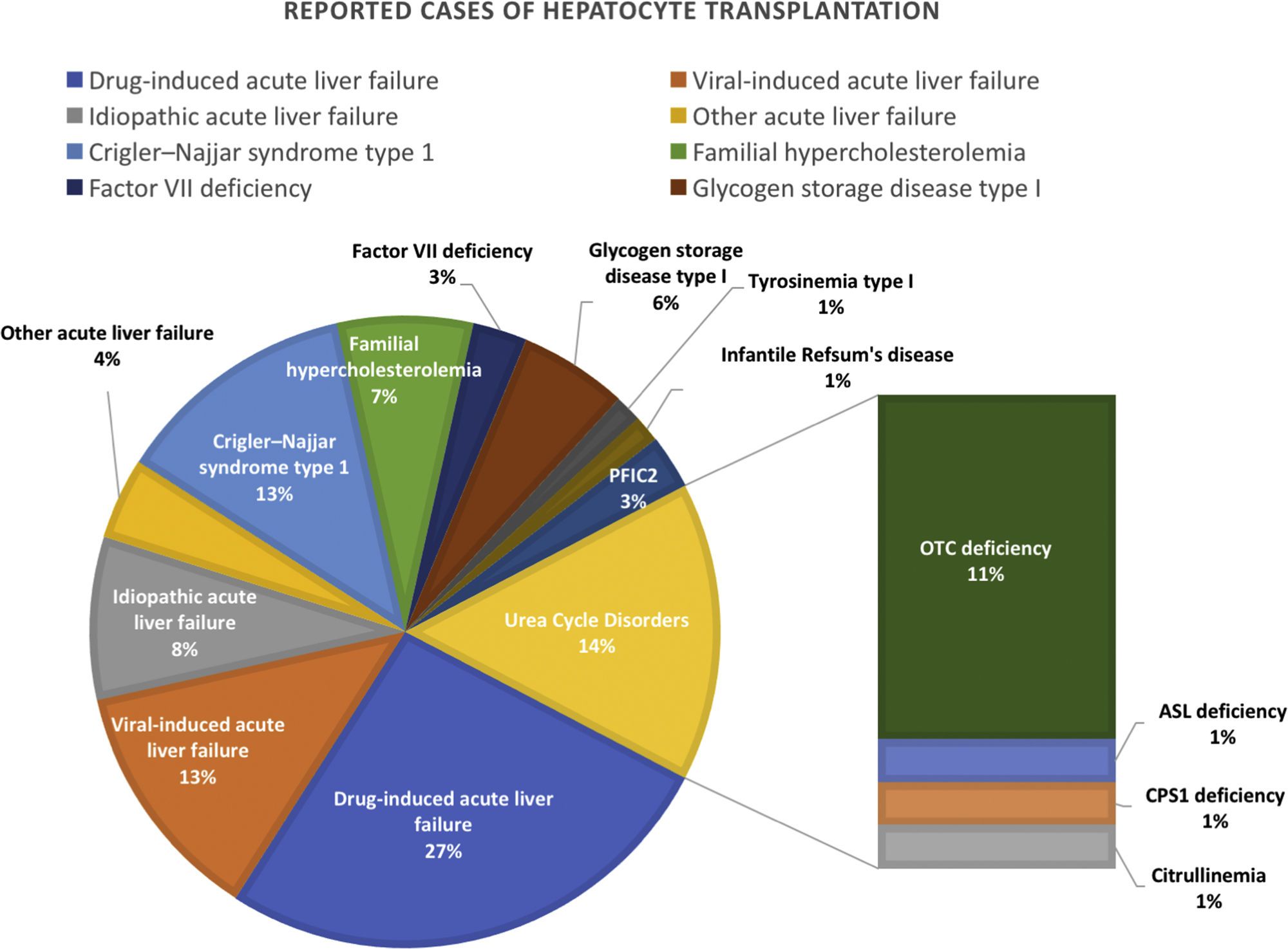
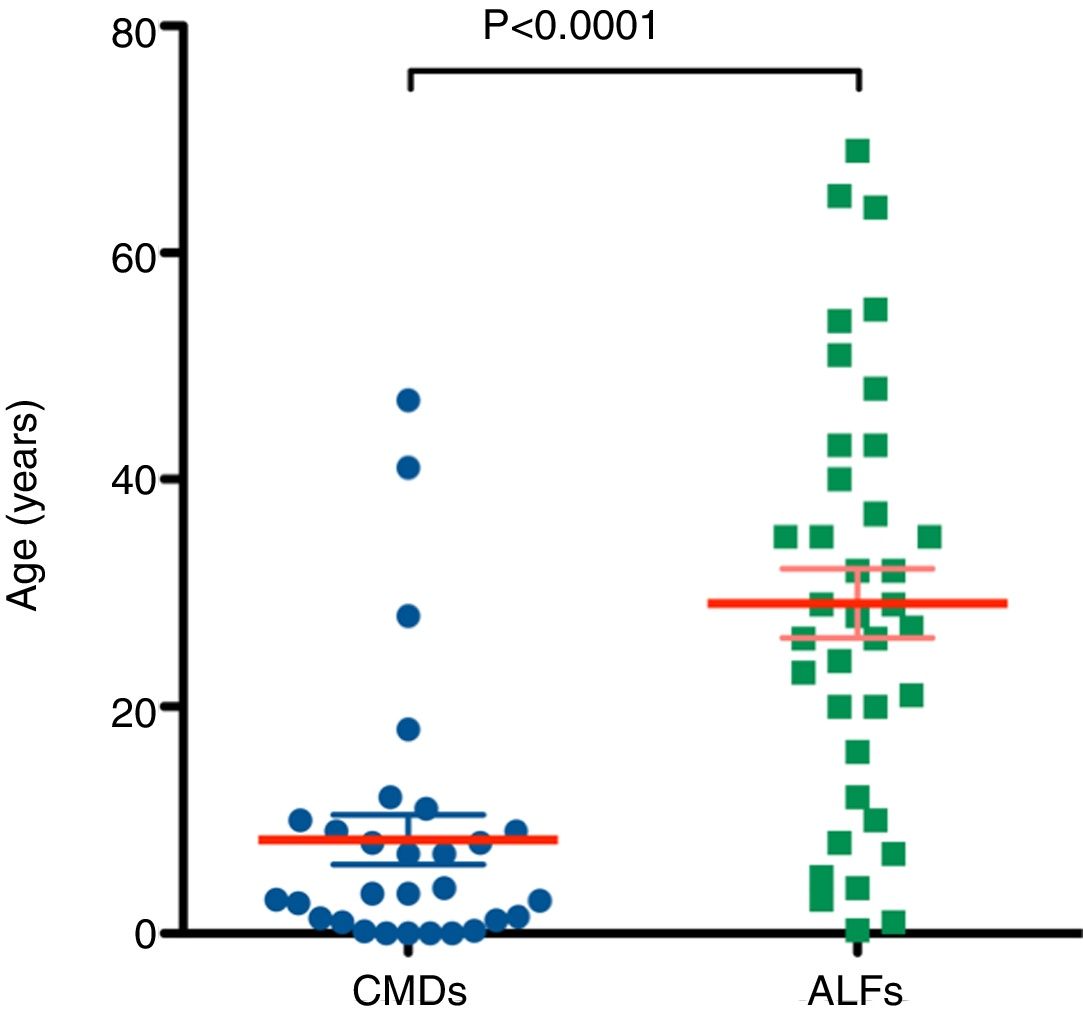
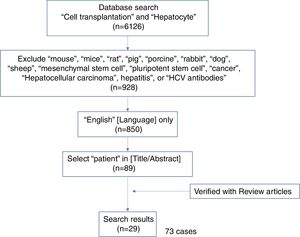
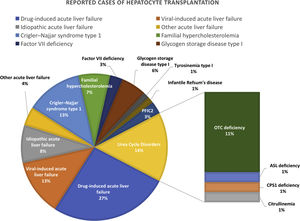
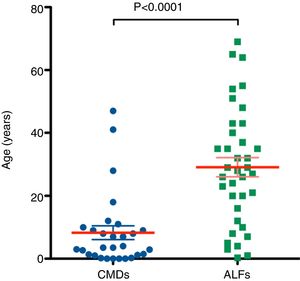
A systematic literature search was performed as shown in the literature search strategy flowchart (Fig. 1). MeSH terms “cell transplantation” and “hepatocyte” were used to initially identify 6126 publications. To obtain reports of clinical hepatocyte transplantation, MeSH terms related to animals, stem cells, cancer, and hepatitis were used to exclude unrelated publications. After including only those publications written in the English language, publications using the term “patient(s)” in the title or abstract were selected. The search results were cross-referenced with previously published review papers.8–13 A total of 29 publications with 73 clinical hepatocyte transplantation case reports were identified. The reported clinical hepatocyte transplantation cases can be categorized into those involving either congenital metabolic disorders or acute liver failure (Fig. 2). Due to the epidemiologic differences between these two disease categories, the patients’ ages are significantly disparate (Fig. 3). In most CMD cases except in one case with progressive familial intrahepatic cholestasis type 2,13 the therapeutic efficacies were partial but satisfactory.
The source of primary human hepatocytes is from livers unused for OLT. As the extended donor criteria has increased the usage of marginal-suboptimal donor organs for OLT, the availability of organs for cell isolation has been diminished. The quality of available donor organs for cell isolation is often poor. The degree of steatosis affects the yield, viability, and overall hepatic function.14 Hepatocytes can be obtained from livers removed from OLT recipients with congenital metabolic disorders. These hepatocytes can be used to treat different types of congenital metabolic disorders.15 The donor criteria can be extended to advanced-age donors and non-heart-beating donors.16 However, the cell quality is highly variable. Therefore, these cell sources are not reliable and insufficient to overcome the primary human hepatocyte shortage problem. Hepatocyte isolation requires a unique and well-established enzymatic digestion technique. The standard protocol was established based on Seglen's two-step collagenase perfusion technique for rat hepatocyte isolation. The protocol was slightly modified for human hepatocyte isolation.17 Hepatocytes should be transplanted as soon as possible, preferably within 24h of isolation, as their hepatic functions deteriorate when kept at 4°C.
Cell dose and route of administrationAlthough 100% replacement of diseased hepatocytes with healthy functional hepatocytes is ideal, the practical goal for replacement will be 10–15%, which may improve enzyme functions from the most severe types of congenital metabolic disorders to the mild phenotypes. It is assumed that 2×108 cells per kg of body weight may be the upper limit of hepatocytes that can be safely infused during transplantation. The currently proposed optimized dose is 30–100×106cells/kg of body weight at an infusion rate of 5–10ml/kg per hour, and a concentration of 1–10×106cells/ml.18 To achieve the estimated number for cell transplantation, therefore, multiple infusions are necessary with defined interval periods.19
Cell transplantation is performed to target either the liver or the spleen. Although cell engraftment in the liver is physiologic, the spleen could be a feasible alternative destination in case the recipient's liver suffers from severe fibrosis (cirrhosis). Regardless, the route of administration must be through intraportal injection, by direct injection into the intrahepatic portal vein, inferior mesenteric vein, umbilical vein, or via the spleen. Systemically injected cells will be trapped in the lung and may cause the pulmonary thromboembolism. On the other hand, cells injected into portal vein do not pass beyond the liver.20 Although the mechanism of cell integration in the recipient's hepatic lobule structure is not well studied, it is speculated that the intraportal infusion causes cell accumulation at the intrahepatic portal capillaries and increases the portal pressure. The portal hypertension and mechanical expansion stimulate intercellular signaling exchange between the nonparenchymal cells which increases the vascular permeability.21
Recipient liver preconditioning treatmentsPreconditioning treatments are common strategies used in preclinical studies to enhance engraftment and proliferation of donor cells.22 The aims of the preconditioning treatments can be classified into four categories: (1) to decrease recipient's immunoreaction; (2) to disrupt the native liver structure; (3) to stimulate liver regeneration signaling; and (4) to suppress the native hepatocyte proliferation. The most common treatment is a partial hepatectomy combined with radiation or drugs, such as retrorsine.23
Although many of these approaches are not clinically acceptable, Fox et al. demonstrated a significant increase of cell engraftment with partial radiation in clinical hepatocyte transplantation.24 Regional liver-directed irradiation using intensity modulated radiation therapy may meet all four previously-stated aims.25 The irradiation inhibits the phagocytic activity of Kupffer cells, transiently disrupts the sinusoidal endothelial barrier, induces apoptosis of native hepatocytes to stimulate liver regeneration, and inhibits native hepatocyte proliferation. In addition, these effects can be controlled by optimizing the radiation dose. A total dose of 10Gy for patients greater than 3 years of age, and 5Gy for patients less than that age was used in the clinical trial.24 The donor cells injected into the portal vein were guided to the irradiated right lobe by left branch occlusion.
One of the advantages of hepatocyte transplantation is that the native liver serves as a back-up to the therapy. Unlike with OLT, the patient's condition only returns to the pre-transplantation state in the event of cellular graft failure. A major concern of this preconditioning treatment is losing this advantage. The eligible recipients must be evaluated carefully. For example, severe acute liver failure patients are often functionally immunosuppressed with impaired cell-mediated immunity, complement levels, and phagocytosis. Therefore, the pre-conditioning strategy could be harmful and may not be necessary for these patients.
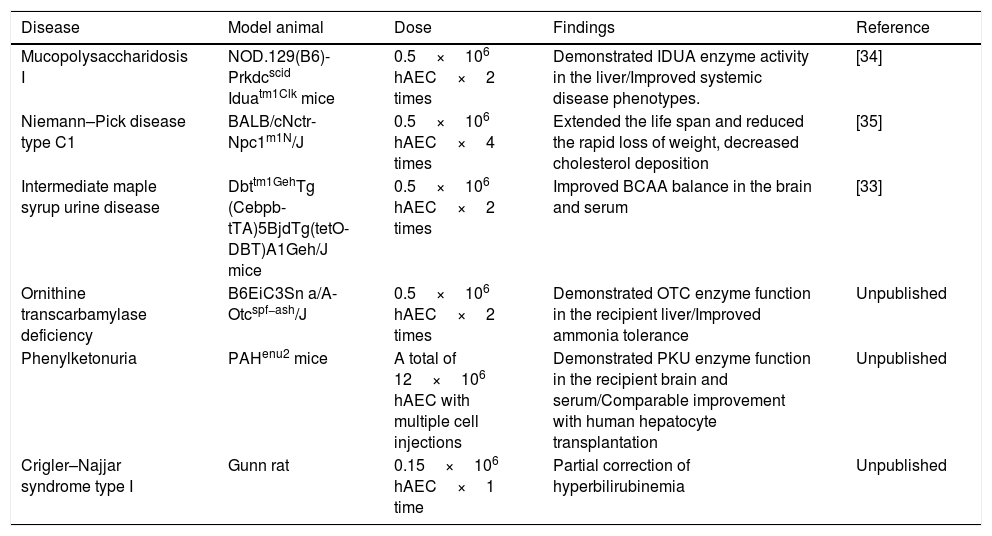
Limitations/obstacles and future directionsThere are several obstacles to providing this promising therapy to patients as an alternative to standard therapies. The shortage of donor organs limits the availability of livers for hepatocyte isolation. In an endeavor to increase the opportunities to obtain primary human hepatocytes, researchers have extended the donor criteria to advanced-age donors and non-heart-beating donors. Fetal hepatocytes or immortalized hepatocytes were also considered as alternative cell sources.26 However, none of these cell sources were sufficient alternatives due to their limited availability, concerns for safety, and functional insufficiency. Recent advancements in stem cell research have demonstrated that hepatocyte-like cells can be derived from human stem cells. Pluripotent stem cells such as embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) possess tremendous differentiation potential, however, their developmental capability is a double-edged sword, associated with the risk of tumorigenicity.27 It is almost impossible to guaranty the safety of injecting over two billion ESC or iPSC-derived hepatocytes with the current technology. Other stem cells such as mesenchymal stem cells were also proposed in that they possess the capability for hepatic differentiation. However, their efficiency is still open to debate. One of the placental stem cells, human amnion epithelial cells (hAEC), has been getting attention as an alternative for hepatocyte transplantation.28 hAECs possess multi-lineage differentiation potential including the ability to differentiate toward the hepatic lineage, which allows them to express the desired enzymatic functions.29 Unlike other components of the placenta, the human amniotic epithelium is derived from pluripotent epiblasts.30 Studies showed that the embryonic stem cell surface markers TRA1-60, TRA1-81, SSEA3, and SSEA4 are present on most of the fetal amniotic epithelium and some of these stem cell marker positive cells are retained in term placental amniotic epithelium.31 Primary hAECs respond to exogenous stimuli in vitro to induce differentiation, changing their morphological and transcriptional profile. This demonstrates the developmental plasticity of the hAECs. Under appropriate culture conditions, hAECs exhibit the capability of differentiating into endoderm lineage tissues including hepatocytes in vitro and in vivo. Transcriptional analysis of hAECs transplanted into SCID/Beige mouse livers indicate that transplanted hAECs terminally differentiated into mature hepatocytes in mouse liver and expressed functional marker genes including cytochrome P450 genes at equivalent levels to human primary hepatocytes.32 Several preclinical studies have demonstrated that the hAEC-derived hepatic cells acquire the desired enzyme function for the treatment of congenital metabolic disorders using disease model animals.32–35 The outcomes of these pre-clinical studies are summarized in Table 1. Other preclinical studies also demonstrated significant therapeutic properties of hAECs for cirrhosis.36 Importantly, upon transplantation into the livers of mice, undifferentiated hAECs have been shown to engraft, display hepatocyte-like morphology, and express various hepatic enzymes without tumorigenicity.
Pre-clinical studies using human amniotic epithelial cells as an alternative cell source of hepatocyte transplantation.
| Disease | Model animal | Dose | Findings | Reference |
|---|---|---|---|---|
| Mucopolysaccharidosis I | NOD.129(B6)-Prkdcscid Iduatm1Clk mice | 0.5×106 hAEC×2 times | Demonstrated IDUA enzyme activity in the liver/Improved systemic disease phenotypes. | [34] |
| Niemann–Pick disease type C1 | BALB/cNctr-Npc1m1N/J | 0.5×106 hAEC×4 times | Extended the life span and reduced the rapid loss of weight, decreased cholesterol deposition | [35] |
| Intermediate maple syrup urine disease | Dbttm1GehTg (Cebpb-tTA)5BjdTg(tetO-DBT)A1Geh/J mice | 0.5×106 hAEC×2 times | Improved BCAA balance in the brain and serum | [33] |
| Ornithine transcarbamylase deficiency | B6EiC3Sn a/A-Otcspf−ash/J | 0.5×106 hAEC×2 times | Demonstrated OTC enzyme function in the recipient liver/Improved ammonia tolerance | Unpublished |
| Phenylketonuria | PAHenu2 mice | A total of 12×106 hAEC with multiple cell injections | Demonstrated PKU enzyme function in the recipient brain and serum/Comparable improvement with human hepatocyte transplantation | Unpublished |
| Crigler–Najjar syndrome type I | Gunn rat | 0.15×106 hAEC×1 time | Partial correction of hyperbilirubinemia | Unpublished |
The lack of direct monitoring/tracking technology after cell transplantation is another obstacle that prevents cell therapy from becoming one of the standard therapies. Currently histological evaluation is the only valid diagnostic test to detect and grade acute T-cell and antibody-mediated rejection after liver transplantation. Elevations of serum bilirubin and peripheral blood eosinophils count can be used as suboptimal surrogate markers of rejection. However, in the case of hepatocyte transplantation, the relatively low number of donor cells in the liver is a limiting factor to determine cell rejection from histological analysis and these biomarkers. Molecular biology approaches such as detecting Y chromosome sequences with qRT-PCR may be able to demonstrate the presence of donor cells, however, it will not indicate the cell viability. If the target enzyme function is restored or improved, that could be an indirect evidence of cell engraftment. However, these parameters may not be sensitive enough to control immunosuppression in a practical manner. Development of novel methods to label cells will be required that can implement a detection/tracking technology with single cell level resolution. Novel biomarkers that correspond to cell rejection will be helpful to optimize the immunosuppression regimen.37 It is critical to monitor the status of transplanted cells in order to optimize and design the immunosuppression regimen for each patient. Currently, a similar immunosuppression regimen used for OLT or islet transplantation is used for hepatocyte transplantation, however, it could be reduced because of the immune privileged nature of the hepatocytes. HLA compatibility has been considered when performing hepatocyte transplantation. However, the impact of the immunogenic characteristics of donor hepatocytes is under debate. The necessity of immunotype matching should be studied immunologically with consideration for the unique characteristics of the hepatocyte and the conditions of cell transplantation. As clinical hepatocyte transplantation is still an experimental therapy, current trials have recruited patients solely based on the severity of disease or the quality of life. In the future, it will be necessary to establish a standardized scoring system to evaluate the recipient prior to hepatocyte transplantation, including the immunotype compatibility.
In summary, clinical hepatocyte transplantation studies have clearly demonstrated that this therapy is a suitable treatment for patients with CMDs. However, there are some obstacles to providing this promising therapy to patients as an option for standard therapies. The obstacles are the insufficient supply of donor cells and the lack of a direct monitoring/tracking technology after cell transplantation, which subsequently creates difficulty with optimizing immunosuppression protocols. Further studies on stem cell-derived hepatic cells and finding novel biomarkers are required to translate hepatocyte transplantation into the clinic.
Funding source informationThis work was supported by California Institute for Regenerative Medicine (CIRM) grant TR3-05488 (TM).
Conflicts of interestThe author owns stock in Noveome Biotherapeutics, Inc. The author has received no payment for the preparation of this manuscript and states no other financial and non-financial conflicts of interest.
The author would like to thank Mr. Brandon Blau and Dr. Jose C. Fernandez-Checa for their comments and feedback for the review.