There is limited scientific evidence available to stratify the risk of developing metachronous colorectal cancer after resection of colonic polyps and to determine surveillance intervals and is mostly based on observational studies. However, while awaiting further evidence, the criteria of endoscopic follow-up needs to be unified in our setting. Therefore, the Spanish Association of Gastroenterology, the Spanish Society of Family and Community Medicine, the Spanish Society of Digestive Endoscopy, and the Colorectal Cancer Screening Group of the Spanish Society of Epidemiology, have written this consensus document, which is included in chapter 10 of the “Clinical Practice Guideline for Diagnosis and Prevention of Colorectal Cancer. 2018 Update”.
Important developments will also be presented as regards the previous edition published in 2009. First of all, situations that require and do not require endoscopic surveillance are established, and the need of endoscopic surveillance of individuals who do not present a special risk of metachronous colon cancer is eliminated. Secondly, endoscopic surveillance recommendations are established in individuals with serrated polyps. Finally, unlike the previous edition, endoscopic surveillance recommendations are given in patients operated on for colorectal cancer. At the same time, it represents an advance on the European guideline for quality assurance in colorectal cancer screening, since it eliminates the division between intermediate risk group and high risk group, which means the elimination of a considerable proportion of colonoscopies of early surveillance. Finally, clear recommendations are given on the absence of need for follow-up in the low risk group, for which the European guidelines maintained some ambiguity.
La evidencia disponible para estratificar el riesgo de presentar un cáncer colorrectal metacrónico tras la extirpación de pólipos colorrectales y determinar los intervalos de vigilancia es limitada y se basa en estudios observacionales. No obstante, a la espera de nuevas evidencias, es necesario unificar los criterios del seguimiento endoscópico en nuestro medio. Por ello, desde las principales sociedades científicas involucradas en el manejo de estos pacientes, como son la Asociación Española de Gastroenterología, la Sociedad Española de Medicina Familiar y Comunitaria, la Sociedad Española de Endoscopia Digestiva y el Grupo de Cribado de Cáncer Colorrectal de la Sociedad Española de Epidemiología, se ha creado este documento de consenso, que se encuentra incluido en el capítulo 10 de la «Guía de Práctica Clínica de Diagnóstico y Prevención del Cáncer Colorrectal. Actualización 2018».
A continuación, se presentarán importantes novedades respecto a la edición previa publicada en 2009. En primer lugar, se establecen situaciones que requieren y no requieren vigilancia endoscópica y se elimina la necesidad de realizar seguimiento en individuos que no presentan un riesgo especial de cáncer de colon metacrono. En segundo lugar, se establecen recomendaciones de vigilancia endoscópica en individuos con pólipos serrados. Finalmente, a diferencia de la edición anterior, se dan recomendaciones de vigilancia endoscópica en individuos intervenidos por cáncer colorrectal. Paralelamente, supone un avance sobre la guía europea de calidad en el cribado del cáncer colorrectal, ya que elimina la división entre grupo de riesgo medio y grupo de riesgo alto, lo que supone la eliminación de una proporción considerable de colonoscopias de vigilancia precoz. Finalmente, se dan recomendaciones claras sobre la ausencia de necesidad de seguimiento en el grupo de riesgo bajo, para el que la guía europea mantenía cierta ambigüedad.
The gradual implementation of colorectal cancer (CRC) population screening programmes throughout Spain's autonomous communities is prompting a considerable increase in the number of colonoscopies performed, resulting from positive faecal occult blood (FOB) tests and indications for the endoscopic surveillance of lesions detected in previous colonoscopies. It is estimated that 20–25% of colonoscopies performed on the over 50s correspond to endoscopic surveillance indications,1–3 resulting from both population screening programmes and the assessment of patients with gastrointestinal symptoms. These entail a significant cost for the health system and use up a significant number of the limited colonoscopies on offer, with indications that are not always correct. Specifically, in 21.99‰ of population screening candidates, a colorectal lesion will be detected, resected and subsequently require endoscopic surveillance.4 Conversely, the rate of progression from advanced adenoma to CRC cannot be accurately determined. It is estimated to be low and to range from 2.6% in the 50–59 age group to 5.6% in the population aged ≥80 on an annual basis.5 It is essential for endoscopic surveillance to be targeted to patients who reap real benefits from the procedure, with the minimum frequency required in order to achieve optimum CRC prevention, thereby avoiding colonoscopy discomfort and complications and limiting the number of explorations with a dubious degree of efficacy.
The objective of this review is to establish a suitable stratification for the risk of metachronous CRC following the removal of colorectal polyps or those overlooked in previous investigations and to determine suitable surveillance intervals based on the available scientific evidence.6 At present, the available evidence is based on observational studies in which the indicated interval for a repeat follow-up colonoscopy is arbitrary.7,8 Most studies assess the efficacy of follow-up colonoscopy based on intermediate endpoints (advanced adenoma detection rate) rather than final endpoints (incidence and mortality).9,10 The results of current studies involving Spanish sites will help to bring forth new evidence to determine which are high-risk situations following the removal of colorectal polyps and which are the most suitable surveillance intervals in these different contexts, as well as to understand the carcinogenic pathways involved, based on the associated precursor lesion and the potential use of molecular markers in the stratification of CRC risk.11
MethodsThis document was prepared after a consensus meeting between professionals from the main scientific societies involved in endoscopic surveillance: the Spanish Association of Gastroenterology, the Spanish Society of Digestive Endoscopy, the Spanish Society of Family and Community Medicine and the Spanish Society of Epidemiology.
The objective of said meeting was to unify the endoscopic follow-up criteria in our setting in order to reduce variability in decision making, to establish a reference in the routine clinical practice of the professionals involved and to extend the recommendations to CRC population screening programmes.
The starting document for the review of the evidence and established recommendations listed below was the European Society of Gastrointestinal Endoscopy Guideline, published in 2013,12 as well as the British Society of Gastroenterology 2017 position statement on the follow-up of serrated polyps.13 An exhaustive literature search was performed based on the scientific evidence available up to December 2017, initially focusing on systematic reviews, meta-analyses and clinical practice guidelines published on MEDLINE, EMBASE and the Cochrane Library. However, the selection was later broadened to include clinical studies from the above-mentioned information sources. The references noted in the documents consulted were also assessed. The search strategy used was:
- •
#1 POPULATION SURVEILLANCE [MeSH] OR Surveillan*
- •
#2 DISEASE PROGRESSION [MeSH]
- •
#3 TIME FACTORS [MeSH]
- •
#4 #1 OR #2 OR #3
Finally, the quality of the evidence and strength of recommendations were established based on the Grading of Recommendations, Assessment Development and Evaluation (GRADE).
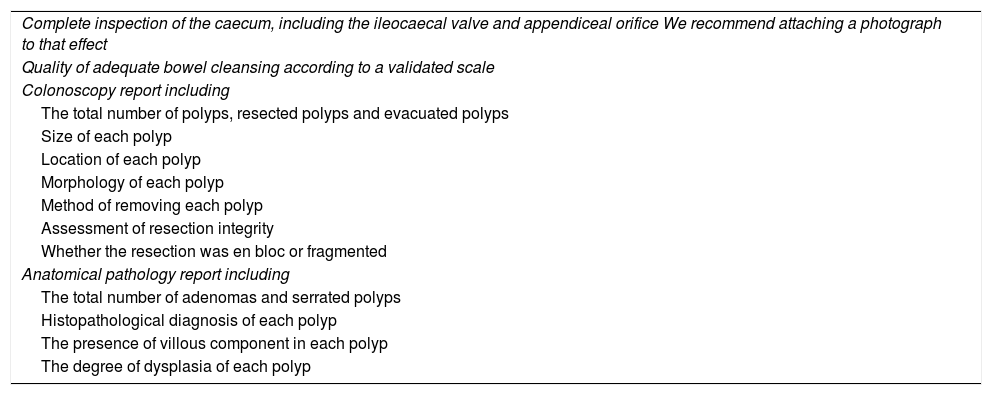
Quality of baseline colonoscopySurveillance recommendations are established based on the findings of the baseline colonoscopy, so this exploration must be of the highest possible quality. Regarding colonoscopy quality, there are three fundamental elements required to establish surveillance recommendations: complete examination of the colon, adequate bowel cleansing and complete resection of the detected lesions. Table 1 shows the minimum quality requisites that must be met during the baseline colonoscopy, established by an expert panel from the World Endoscopy Organization.14
Quality criteria that the baseline colonoscopy must meet before surveillance recommendations are provided.
| Complete inspection of the caecum, including the ileocaecal valve and appendiceal orifice We recommend attaching a photograph to that effect |
| Quality of adequate bowel cleansing according to a validated scale |
| Colonoscopy report including |
| The total number of polyps, resected polyps and evacuated polyps |
| Size of each polyp |
| Location of each polyp |
| Morphology of each polyp |
| Method of removing each polyp |
| Assessment of resection integrity |
| Whether the resection was en bloc or fragmented |
| Anatomical pathology report including |
| The total number of adenomas and serrated polyps |
| Histopathological diagnosis of each polyp |
| The presence of villous component in each polyp |
| The degree of dysplasia of each polyp |
In terms of morphology, polyps can be flat, sessile and pedunculated. As regards histology, they are classified as adenomatous (60–70%), serrated (10–30%) and other (10–20%), which includes hamartomatous, juvenile and inflammatory polyps, as well as other non-mucosal lesions.
Adenomatous lesionVarious studies and meta-analyses have shown that the main risk factors for metachronicity are the size and number of lesions removed in the baseline colonoscopy. A size greater than 10mm carries a 2–3 times higher risk of advanced adenoma or CRC in follow-up,9,10,15 with an even higher risk when the lesion is greater than 20mm.10,16 Regarding the number of lesions, the existence of three or more is also associated with increased risk of an advanced colorectal lesion or CRC.10,15 However, these data come from studies performed in the 1990s. Technological advances in endoscopy, as well as the introduction of a colonoscopy quality policy, are likely to have given rise, on the one hand, to an increase in the number of subjects in whom multiple adenomas are detected and, on the other, a reduction in the real metachronous cancer risk.17,18
As for histology, there is less evidence associating risk with advanced histological findings, particularly in lesions smaller than 10mm. Lesions with a villous component (>20%) or high-grade dysplasia (HGD) are at a slightly higher risk of an advanced colorectal lesion in relation to predominantly tubular lesions, which have an exclusively tubular component (≥80%) or low-grade dysplasia (LGD).10,15,19
In light of the above, an advanced adenomatous lesion is considered to be an adenoma with a villous component (>20%), a diameter of 10mm or more or HGD, and a non-advanced lesion, a tubular adenoma (≥80%) with LGD which is smaller than 10mm (Table 2).
Classification of advanced and non-advanced colorectal lesions.
| Adenomatous lesion | |
|---|---|
| Non-advanced | Tubular adenoma, <10mm with LGD |
| Advanced | Adenoma with villous component, ≥10mm or HGD |
| Serrated lesion | |
|---|---|
| Non-advanced | Serrated polyp <10mm without dysplasiaa |
| Advanced | Serrated polyp ≥10mm or with dysplasia |
HGD: high grade dysplasia; LGD: low grade dysplasia.
Most CRCs develop through the traditional adenoma-carcinoma sequence. However, around 20–30% of CRCs do so by means of the so-called “serrated carcinogenesis pathway”, where the precursor lesion is a serrated polyp. Serrated polyps are classified as hyperplastic polyps, sessile polyps and traditional serrated adenomas.20,21 Several observational studies have shown the presence of small hyperplastic polyps in the rectum or sigmoid colon to not be associated with a risk of metachronous advanced lesions.22,23
Some studies have assessed the risk of synchronous or metachronous lesions following the removal of serrated lesions. The characteristics of such lesions which seem to entail an increased risk are the presence of dysplasia and a size greater than 10mm,22,24–29 hence they are deemed advanced (Table 2). Non-advanced lesions, on the other hand, are deemed to be serrated polyps smaller than 10mm without dysplasia.
Risk groupsRisk of patients with adenomatous polyps developing colorectal cancerMultiple studies show the presence of non-advanced adenomas to not be associated with a risk of metachronous CRC (Appendix 1). A retrospective study published in 1992 involving 1618 patients30 observed that those with non-advanced adenomas had a risk of developing CRC that was similar to the general population, despite not undergoing endoscopic follow-up, a result which was subsequently corroborated by another study with a greater number of participants: 5779.7 Later, in the study by Løberg et al.,31 which assessed mortality due to CRC, it was detected that patients with non-advanced adenomas (1–2 tubular adenomas with LGD) who underwent polypectomy in the baseline colonoscopy were 25% less likely to die from CRC compared to the general population. These findings confirm the protective effect of polypectomy versus endoscopic surveillance in this group of patients, who had a lower risk of CRC mortality than the general population.
In contrast, various studies show an association between advanced adenomas and metachronous CRC (Appendix 1). In 1992, patients with advanced lesions were observed to have a CRC risk that was 3.6–6.6 times greater than the general population.30 These results were confirmed in the study by Cottet et al.7, where patients with advanced adenomas who did not undergo endoscopic follow-up had a standardised incidence rate of CRC of 4.26 (95% CI 2.89–6.04). Meanwhile, Løberg et al.31 showed that patients with high-risk adenomas (at least 3, villous histology or HGD) had a 16% increase in the risk of CRC mortality compared to the general population. Likewise, Atkin et al.16 show, in a retrospective study which included around 12,000 intermediate-risk patients (1–2 adenomas ≥10mm or 3–4 adenomas <10mm), that endoscopic surveillance reduces the incidence of CRC and that a series of variables define a group of patients in whom the risk increase and benefit of endoscopic follow-up is greatest: low-quality colonoscopy, size greater than 20mm and proximal adenomas.
Metachronous advanced colorectal lesionThe incidence of metachronous advanced neoplasia in patients with non-advanced adenomas compared to the adenoma-free population has been analysed in various studies (Appendix 1). Two randomised controlled clinical trials,9,32 as well as three cohort studies,33–35 have compared the prevalence of such lesions in different follow-up intervals (2 versus 4 years; 3 versus 5 years; 3–5 versus 6–10 years), and did not detect statistically significant differences. Later, in 2014, a systematic review36 of seven observational studies (three retrospective, four prospective) was published which analysed the incidence of metachronous advanced neoplasia in patients with non-advanced adenomas and those without neoplasia in the baseline colonoscopy. The relative risk was 1.83 (95% CI 1.31–2.56), although the advanced neoplasia incidence rate was low in both groups: 1.6% in patients with no neoplasia and 3.6% in those with low-risk adenomas. Moreover, Gupta et al.19 analysed which factors allowed them to identify a group with the highest risk of a metachronous advanced colorectal lesion among these patients. In this sense, age (≥70 years), a history of prior polyps and the presence of proximal and distal adenomas could stratify the risk in these patients.
The available evidence regarding the risk of detecting a metachronous advanced colorectal lesion in patients with high-risk adenomas (advanced or more than two non-advanced adenomas) indicates that it is five to seven times higher (prospective cohort studies)9,35,37 and twice as high (pooled analysis of eight clinical trials and two meta-analyses)10,15,38 compared to subjects without adenomas (Appendix 1).
Risk of patients with serrated polyps developing colorectal cancerIn the case-control study published by Erichsen et al.,24 patients with serrated lesions without dysplasia had a CRC risk at 10 years of 2.56%. This result is very similar to that seen in patients with conventional adenomas, who had a 2.33% risk of CRC in the same period. Moreover, data from this same study show patients with hyperplastic polyps to have a lower risk (Appendix 2).
In contrast, the risk of advanced serrated lesions is higher. Thus, the hazard ratio (HR) of CRC at 10 years in patients with serrated lesions ≥10mm, compared to subjects with no polyps, is 4.2 (95% CI 1.3–13.3), which is similar to that of patients with advanced adenomas (HR 3.3, 95% CI 2.1–5.2).26 Moreover, the study by Erichsen et al.24 defines the risk of CRC in patients with dysplastic serrated lesions (serrated sessile polyps with dysplasia and traditional adenomas) to be 4.43%. Conversely, there is no information on the risk of patients with multiple non-advanced serrated lesions developing CRC.
Metachronous advanced colorectal lesionTwo observational studies22,23 have shown the presence of small hyperplastic polyps to not be associated with an increased risk of metachronous advanced adenomas. Likewise, the coexistence of hyperplastic polyps and adenomas does not entail an increased risk of metachronous adenomas or advanced adenomas when compared to those that only present adenomas (Appendix 2).25,39,40
Moreover, with respect to advanced serrated polyps, there are no solid studies assessing the incidence of metachronous advanced neoplasia. However, the presence of dysplasia, a size >10mm and a proximal location have been observed to be predictors of synchronous advanced colorectal lesions.22,26–28 In this sense, a recent study shows that the synchronous appearance of advanced adenomas and serrated lesions >10mm or with dysplasia increases the risk of both metachronous advanced lesions and metachronous serrated lesions >1cm. In contrast, the synchronous appearance of small serrated lesions without dysplasia does not increase the risk in patients with non-advanced adenomas.29
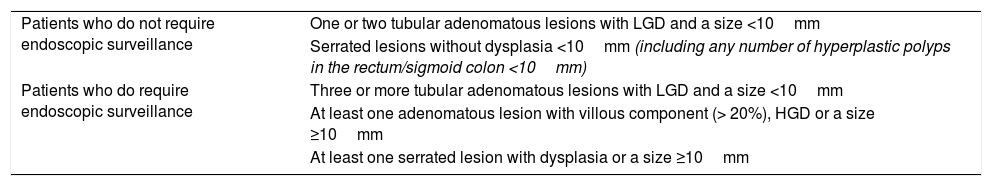
Classification according to risk groupsBased on the criteria for advanced and non-advanced colorectal lesions set forth in the previous section and in the available evidence on the risk of patients developing CRC or an advanced colorectal lesion, two risk groups are proposed: patients who do not require endoscopic surveillance and patients who do require endoscopic surveillance (Table 3).
Classification of risk groups into patients who do and do not require surveillance.
| Patients who do not require endoscopic surveillance | One or two tubular adenomatous lesions with LGD and a size <10mm |
| Serrated lesions without dysplasia <10mm (including any number of hyperplastic polyps in the rectum/sigmoid colon <10mm) | |
| Patients who do require endoscopic surveillance | Three or more tubular adenomatous lesions with LGD and a size <10mm |
| At least one adenomatous lesion with villous component (> 20%), HGD or a size ≥10mm | |
| At least one serrated lesion with dysplasia or a size ≥10mm |
HGD: high grade dysplasia; LGD: low grade dysplasia.
According to the aforementioned studies, patients with lesions deemed non-advanced or low risk have a slightly increased risk of an advanced colorectal lesion compared to those with no lesions, but lower CRC mortality than the general population, so the benefit of endoscopic surveillance is residual in this group. We therefore propose a return to population screening with the FOB test, or indicating a colonoscopy at 10 years in case there is no CRC population screening programme available. As regards this return to the population screening programme, since the risk is similar to that of the population with a normal colonoscopy, it is recommended that this population be reincorporated at 10 years. However, there is currently no evidence either in favour or against reincorporating these patients at this time or within shorter intervals (2, 5 years).
Patients with advanced lesions have an increased risk of developing an advanced colorectal lesion and of CRC mortality, so we propose performing the first endoscopic surveillance three years after the baseline colonoscopy. This interval has been established based on limited scientific evidence, since there are no studies in this regard, so longer intervals may well report similar results. There is only one randomised controlled clinical trial published in 199341 comparing two follow-up intervals in patients who underwent the removal of colon adenomas: at one and three years versus three years only, with no statistically significant differences obtained as regards the rate of advanced adenomas in follow-up.
Moreover, the European and UK guidelines42,43 divide the high-risk group into intermediate risk and high risk (one adenoma measuring at least 20mm or five or more adenomas). For the former, they recommend a surveillance colonoscopy at three years, and for the latter, at one year. In Spanish population screening programmes, 44% of patients with adenomas fall into the first category and 21% the second.44 Various studies have compared both strategies.45,46 In the study by Martínez et al.46 the rate of advanced colorectal lesions in the high-risk group one year after the baseline colonoscopy was found to be more than twice that of the intermediate-risk group, with no differences in the CRC rate. Cubiella et al.,45 on the other hand, in a retrospective study performed on Spanish screening programmes, observed that the incidence of advanced neoplasia at three years in the high-risk group was 16% versus 12% in the intermediate-risk group (HR 1.5, 95% CI 1.2–1.8). Said study found no differences in CRC incidence (0.5% in the high-risk group and 0.4% in the intermediate-risk group; HR 1.6, 95% CI 0.6–3.8), although it was not designed for that purpose. Therefore, based on these data, we feel that there is insufficient evidence to warrant performing the first surveillance colonoscopy at one year in patients who meet the high-risk criteria of the European guidelines.42 On the other hand, we only have the aforementioned data available to warrant removing said risk group from CRC population screening programmes. Consequently, for patients with five or more adenomas or an adenoma ≥20mm who make up the high-risk group in the European guidelines,42 there is currently no evidence either for or against shortening the follow-up interval to one year.
Finally, it is worth highlighting that the promising multicentre European Polyp Surveillance (EPoS) study is currently underway, which comprises three randomised controlled clinical trials assessing the incidence of CRC at 10 years in different follow-up arms of patients with low- and high-risk adenomas. In the latter group, the patients are randomised 1:1 to surveillance intervals of three and five years versus five years only.11 No results are available at the current time, although they will provide a greater degree of scientific evidence which will enable more suitable follow-up intervals to be established.
| Prior to making surveillance recommendations, it should be verified that the baseline colonoscopy was performed under high-quality conditions: complete examination with careful inspection of the mucous, adequate bowel cleansing and complete removal of the polyps. |
| Patients with 1–2 tubular adenomatous lesions with LGD and a size <10mm do not require endoscopic surveillance. They should be reincorporated into the population screening programme, preferably at 10 years, or a colonoscopy should be indicated at 10 years if there is no CRC population screening programme available. Moderate quality evidence, strong recommendation. |
| Patients with serrated lesions without dysplasia <10mm do not require endoscopic surveillance, regardless of the number of lesions. They should be reincorporated into the population screening programme, preferably at 10 years, or a colonoscopy should be indicated at 10 years if there is no CRC population screening programme available. Low quality evidence, strong recommendation. |
| Patients with hyperplastic polyps in the rectum/sigmoid colon <10mm do not require endoscopic surveillance. At 10 years, they should be reincorporated into the population screening programme or a colonoscopy should be indicated if there is no CRC population screening programme available. Low quality evidence, strong recommendation. |
| Patients with three or more tubular adenomatous lesions with LGD <10mm or at least one villous adenomatous lesion with HGD or a size ≥10mm should have their first endoscopic surveillance at three years. Moderate quality evidence, weak recommendation. |
| Patients with at least one serrated neoplastic lesion with dysplasia or a size ≥10mm should have their first endoscopic surveillance at three years. Low quality evidence, strong recommendation. |
| For patients with five or more adenomas or an adenoma ≥20mm, who make up the high-risk group in the European guidelines,42 at present there is no evidence either for or against shortening the follow-up interval to one year. Low quality evidence, weak recommendation. |
Surveillance recommendations should always be made after the complete resection of lesions found in the baseline colonoscopy. If complete resection cannot be achieved in a single colonoscopy, the procedure should be repeated until the goal of leaving the colon completely explored and free from neoplastic lesions is achieved. Factors such as incomplete colonoscopy16 and incomplete lesion resection are associated with interval cancer and a greater incidence of CRC.47,48
Fragmented resectionFragmented polyp resection is sometimes associated with incomplete resection, especially when the lesion is sessile or flat and measures ≥20mm.49 The recurrence rate at three to six months in non-pedunculated lesions >20mm may be up to 32%,50 and in a retrospective study published in 2016,51 where the endoscopic resection of this type of lesion was assessed, it was observed that fragmented removal increases the risk of residual tissue on the post-polypectomy scar. Therefore, given that the risk of recurrence and interval CRC may be increased,52 performing an endoscopic review within six months of the baseline colonoscopy is recommended in case of the fragmented resection of lesions ≥20mm, even if a complete resection has been performed, as is the collection of biopsies from the polypectomy scar.50,53,54 In such cases, the risk of recurrence remains sizeable,53 so once the absence of adenomatous scar tissue is confirmed, it is recommended that the first surveillance colonoscopy be performed one year after the eschar review.
Non-evacuated resected lesionsComplete evacuation of all resected lesions is recommended. However, this is not always possible, owing to various circumstances.55 In such cases, given that the histology of resected and non-evacuated lesions cannot be determined, lesions ≥10mm will be considered as advanced and lesions <10mm as non-advanced. To assess risk and establish the surveillance recommendation, these lesions will be added to those evacuated.
Tattooing of lesionsAfter the resection of lesions suspected of exhibiting invasive cancer or which may prove difficult to locate during successive endoscopic explorations, it is recommended that the area next to the lesion be tattooed (injection of a biocompatible liquid carbon marker) in order to facilitate identifying the lesion site for the purpose of follow-up or future surgery, unless said lesion is located in the caecum, adjacent to the ileocaecal valve or in the lower rectum.56
Special risk situationsReferral to high-risk or colorectal cancer prevention units- •
≥10 adenomas. An undefined number of patients with 10 or more adenomas present hereditary cancer syndromes57 and these cases should thus be dealt with individually. In these patients, an early repeat colonoscopy should be considered and the suitability of genetic counselling assessed. They should therefore be assessed at a high-risk unit (HRU)58 or a specialist gastroenterology consultation if no HRUs are available.
- •
≥5 serrated polyps or≥2 serrated polyps ≥10mm proximal to the sigmoid colon or ≥10 polyps with ≥50% serrated polyps. A number of these patients will be diagnosed with serrated polyposis syndrome (SPS) at subsequent colonoscopies.59 As regards patients with multiple serrated polyps, it has been observed that they and their relatives are at an increased risk of developing CRC.60 For this reason, they should also be individually assessed and followed up at HRUs.
- •
SPS criteria. Patients who meet the SPS criteria have an increased risk of CRC61 and should also be assessed and followed up at HRUs.
| Surveillance recommendations should always be made after the complete resection of lesions found in the baseline colonoscopy. |
| When resection is incomplete, colonoscopy should be repeated until the goal of leaving the colon completely explored and free of neoplastic lesions is achieved. Low quality evidence, strong recommendation. |
| In large sessile or flat lesions (≥20mm) which are resected in a fragmented manner, an endoscopic review of the scar should be performed within six months of the baseline colonoscopy. High quality evidence, strong recommendation. |
| In large sessile or flat lesions (≥20mm) which are resected in a fragmented manner, the first endoscopic surveillance should be carried out at one year after confirmation of complete resection. High quality evidence, strong recommendation. |
| Complete evacuation of all resected lesions is recommended. |
| Lesions ≥10mm which are resected and not evacuated will be considered as advanced and lesions <10mm as non-advanced. Lesions <10mm in the rectum/sigmoid colon which are resected and not evacuated will not be taken into account. |
| To establish the surveillance recommendation, non-evacuated advanced and non-advanced lesions will be added to those evacuated. |
| After the resection of lesions suspected of exhibiting invasive cancer or which may later prove difficult to locate, the lesion should be tattooed. Low quality evidence, strong recommendation. |
| Individuals at high risk of CRC (≥10 adenomas; ≥5 proximal serrated polyps; ≥2 serrated polyps ≥10mm; ≥10 polyps with ≥50% serrated polyps or criteria for SPS) require personalised investigation and should be referred to a specific HRU or for a specialist gastroenterology consultation. |
Some observational studies40,62–64 have assessed the risk of metachronous advanced neoplasia in the second surveillance colonoscopy based on the findings of the first, although the available evidence is limited. Similarly, the only existing studies in this regard are after two normal surveillance colonoscopies. All of these studies note that findings in the second surveillance colonoscopy are more dependent on what was found during the first surveillance colonoscopy rather than at the baseline one.40,62–64 However, since patients with advanced baseline lesions have around a 10% risk of an advanced colorectal lesion in the second colonoscopy, even in cases of low-risk lesions, we recommend a second surveillance colonoscopy at five years if no lesions requiring surveillance are found in the first colonoscopy, or at three years if any such lesions are detected. Although there is no evidence in this regard, after two normal surveillance colonoscopies or lesions which do not require surveillance, it seems reasonable for the patient to return to the screening programme at 10 years.
Cessation of surveillanceIt may be substantiated that the benefit of this activity in healthy individuals over a certain age may be limited by comorbidities, the patient's life expectancy or the risks and complications of an invasive procedure. The risk of developing CRC having not undergone follow-up in a cohort of patients with intermediate-risk adenomas (1–2 adenomas ≥10mm or 3–4 adenomas <10mm) over a mean period of 7.8 years is 3.3%.16 Therefore, the benefit of surveillance when life expectancy is less than 10 years is dubious. By way of example, the risk of death in Spain at 75 years of age is 21.03‰ and, at 80, 36.9‰.65 As a result, it seems unlikely that endoscopic surveillance will have any kind of effect and, in this sense, in candidates for endoscopic surveillance, we recommend ending said surveillance at 75 years of age or, exceptionally, at 80, in select patients with no comorbidities.
Adequacy of endoscopic surveillanceThere is a high rate of inadequate post-polypectomy surveillance,1,66 as well as a lack of adherence to guidelines.67,68 In order to ensure the adequacy and implementation of endoscopic surveillance, we recommend establishing strategies within CRC population screening programmes.1 If this is not possible, it is advisable to put in place reminder systems aimed at the patient or primary care physician. Outside of the screening programme, measures should be applied which promote the use of recommendations for colon polyp follow-up.
Onset of new symptomsSurveillance recommendations are made in asymptomatic individuals. If an individual who has undergone colon polyp removal experiences the onset of new symptoms, these must be assessed in a suitable context and the pertinent explorations indicated for investigation.
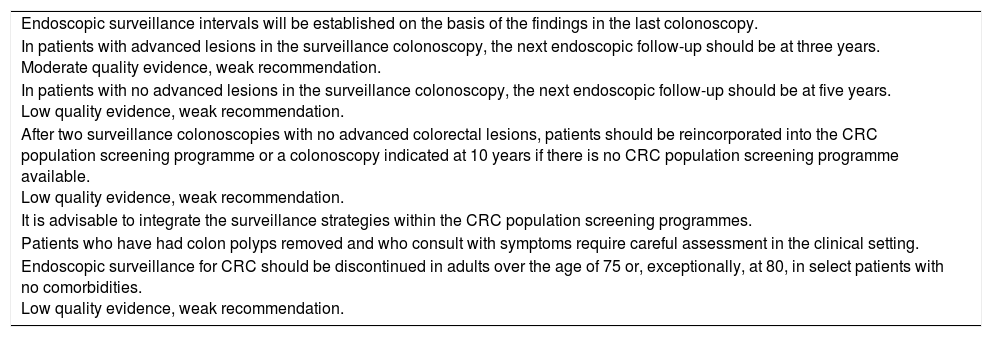
| Endoscopic surveillance intervals will be established on the basis of the findings in the last colonoscopy. |
| In patients with advanced lesions in the surveillance colonoscopy, the next endoscopic follow-up should be at three years. Moderate quality evidence, weak recommendation. |
| In patients with no advanced lesions in the surveillance colonoscopy, the next endoscopic follow-up should be at five years. Low quality evidence, weak recommendation. |
| After two surveillance colonoscopies with no advanced colorectal lesions, patients should be reincorporated into the CRC population screening programme or a colonoscopy indicated at 10 years if there is no CRC population screening programme available. Low quality evidence, weak recommendation. |
| It is advisable to integrate the surveillance strategies within the CRC population screening programmes. |
| Patients who have had colon polyps removed and who consult with symptoms require careful assessment in the clinical setting. |
| Endoscopic surveillance for CRC should be discontinued in adults over the age of 75 or, exceptionally, at 80, in select patients with no comorbidities. Low quality evidence, weak recommendation. |
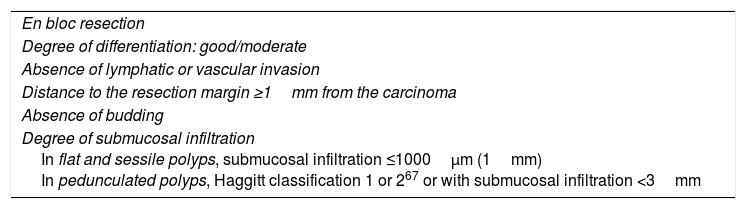
pT1 invasive cancer is considered to be when the lesion invades the muscularis mucosae and extends to the submucosa without reaching the muscularis propria.69 Once complete endoscopic resection of the lesion has been performed, allowing the margins thereof to be assessed, there is a risk of loco-regional lymph node metastasis of 6.8–17.8% of cases.70,71 Several meta-analyses have assessed the associated histological criteria and have determined that the degree of differentiation, lymphatic or vascular invasion, the presence of budding and the degree of submucosal infiltration are independent predictors of lymph node metastasis.72–76 In pT1 lesions, the risk of lymphatic invasion when the above-mentioned criteria are not met is 1.9%. During decision making, this risk must be balanced with the risk of surgery-related mortality, which stands at 0.5%. This means that, if the criteria for good prognosis (Table 4) are met, the number of interventions needed to prevent one CRC-related death is 208, so the benefit of surgery is residual.77 In contrast, if any of the above-mentioned criteria are not met, the risk of residual disease or lymphatic involvement increases. For this reason, it is recommended that patients with endoscopically-resected pT1 CRC undergo assessment at HRUs or specialist gastroenterology consultations, and that decisions are ultimately made by multidisciplinary committees.
Histological criteria for a good prognosis in adenocarcinomas arising from polyps and invading the submucosa.
| En bloc resection |
| Degree of differentiation: good/moderate |
| Absence of lymphatic or vascular invasion |
| Distance to the resection margin ≥1mm from the carcinoma |
| Absence of budding |
| Degree of submucosal infiltration In flat and sessile polyps, submucosal infiltration ≤1000μm (1mm) In pedunculated polyps, Haggitt classification 1 or 267 or with submucosal infiltration <3mm |
The objectives of a complete perioperative colonoscopy are to both detect synchronous lesions and resect precancerous lesions, since the prevalence of synchronous cancer is estimated at 0.7–7% in CRC patients.78–80 Therefore, in cases where the colonoscopy was incomplete due to the presence of a stenosing tumour, poor bowel preparation or incomplete resection of the neoplastic lesions observed in the baseline exploration (not present on the surgically removed piece or not resected prior to surgery), it is recommended that the colon preferably be explored perioperatively or, where this is not possible, postoperatively within three to six months, in order to ensure a safety margin after the intervention. In any case, we do not recommend performing an intraoperative endoscopic investigation.81 It should be highlighted that the colonoscopy must meet the standard quality criteria applied to other endoscopic procedures. In cases of stenosing neoplasia, computed tomography (CT) colonoscopy performed preoperatively will also be an option.82
Surveillance colonoscopyThe objective of surveillance colonoscopies following a curative-intent CRC resection is to detect new precancerous and CRC lesions as well as recurrences. The available evidence for establishing surveillance intervals is limited and not based on randomised clinical trials, although there are some studies assessing the risk of metachronous lesions in surveillance.
Various studies estimate that between 1.5% and 3% of patients will develop metachronous lesions within three to five years of the initial resection.73,74 Similarly, some observational studies79,83 have analysed the incidence of metachronous CRC in these patients, observing an increased incidence of CRC within the first few years of surgery. This may be due to the fact that pre-existing neoplastic lesions were not detected in the baseline colonoscopy, which highlights the importance of both quality in the baseline colonoscopy and a compete perioperative investigation of any synchronous lesions. Moreover, it is estimated that 80% of recurrences are detected in the first two and a half years.84,85 Thus, the first surveillance colonoscopy is recommended to take place at one year (Fig. 1), an interval that is also clinically efficient and a cost-effective strategy in terms of detecting cancer and preventing cancer deaths, as shown in the cost-effectiveness study by Hassan et al.86 However, after the first surveillance interval, the available evidence for establishing subsequent periods is weak and based primarily on the recommendations established for other advanced lesions.
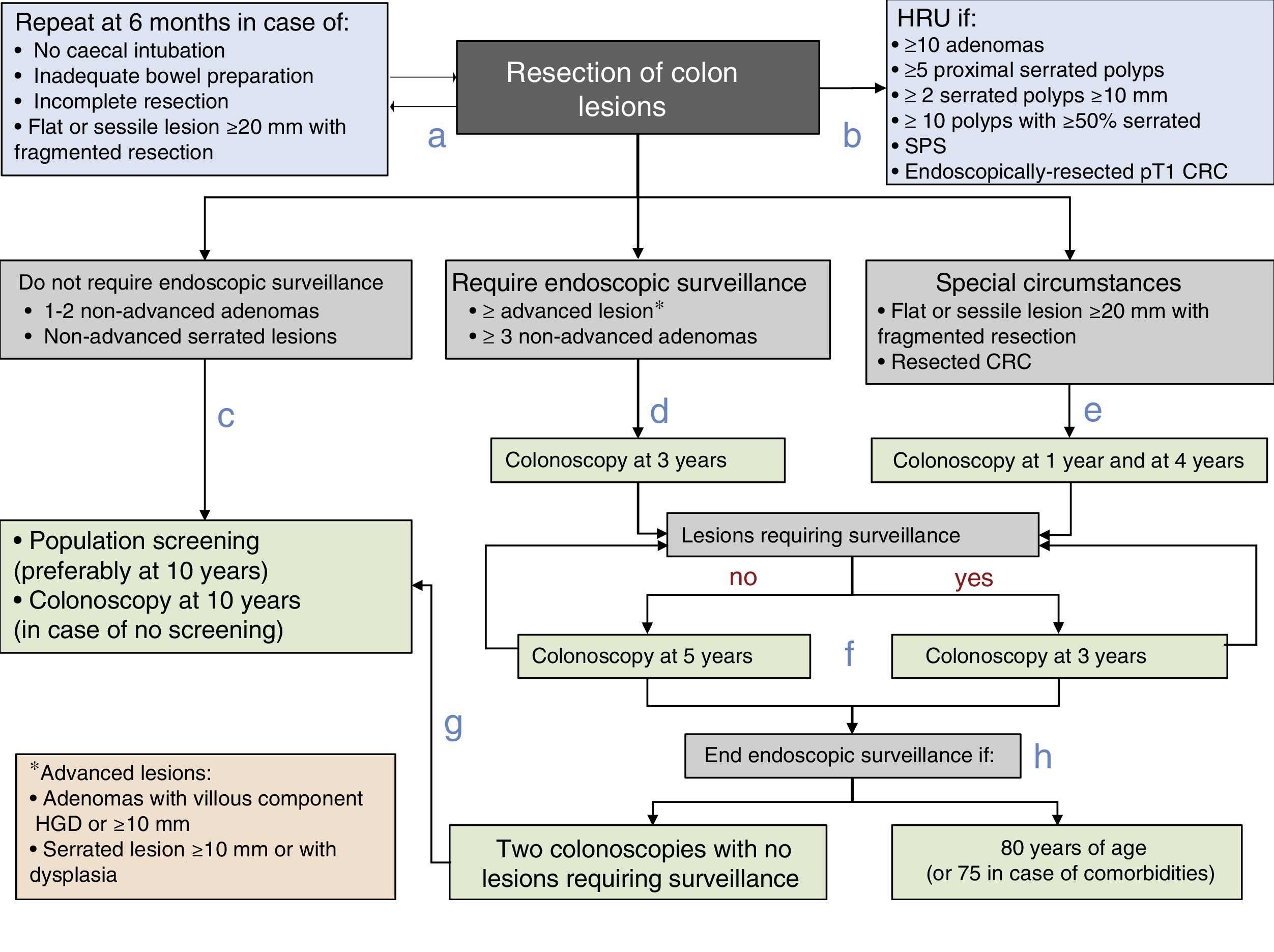
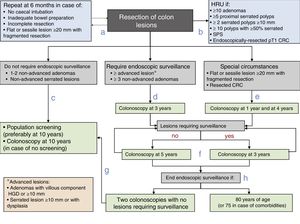
Post-polypectomy surveillance recommendations diagram of the Spanish Association of Gastroenterology, the Spanish Society of Digestive Endoscopy, the Spanish Society of Family and Community Medicine and the Spanish Society of Epidemiology. CRC: colorectal cancer; HGD: high grade dysplasia; HRU: high-risk unit; SPS: serrated polyposis syndrome.
Rectal cancer deserves special mention due to the increased likelihood of local recurrence. Some studies have observed that over 80% of anastomotic recurrences occur in patients with rectal cancer,87–89 although this in turn depends on the preoperative staging, neoadjuvant therapy and surgical technique. There is also little evidence regarding surveillance intervals in these patients.
Thus, in patients who undergo rectal surgery with total mesorectal excision associated, if required, with neoadjuvant therapy, specific rectal surveillance is not recommended, since the likelihood of recurrence is very low. Conversely, in patients who do not undergo total mesorectal excision, close endoscopic surveillance would prove adequate in the first two years after the resection by means of proctosigmoidoscopy or echo-endoscopy. There are no data in favour of one technique over the other (Fig. 1), nor is there clear evidence on the benefit of any specific strategy. Finally, in patients who have not received neoadjuvant therapy, based on their tumour stage, the surveillance strategy to be followed will be determined on an individual basis.90–94
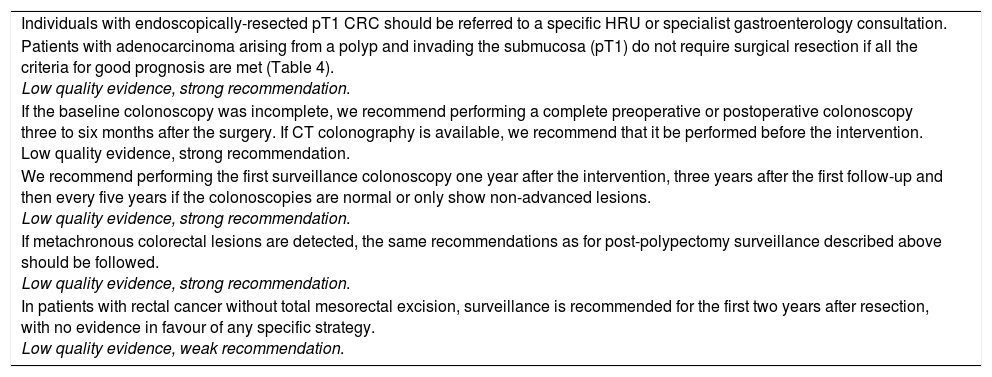
| Individuals with endoscopically-resected pT1 CRC should be referred to a specific HRU or specialist gastroenterology consultation. |
| Patients with adenocarcinoma arising from a polyp and invading the submucosa (pT1) do not require surgical resection if all the criteria for good prognosis are met (Table 4). Low quality evidence, strong recommendation. |
| If the baseline colonoscopy was incomplete, we recommend performing a complete preoperative or postoperative colonoscopy three to six months after the surgery. If CT colonography is available, we recommend that it be performed before the intervention. Low quality evidence, strong recommendation. |
| We recommend performing the first surveillance colonoscopy one year after the intervention, three years after the first follow-up and then every five years if the colonoscopies are normal or only show non-advanced lesions. Low quality evidence, strong recommendation. |
| If metachronous colorectal lesions are detected, the same recommendations as for post-polypectomy surveillance described above should be followed. Low quality evidence, strong recommendation. |
| In patients with rectal cancer without total mesorectal excision, surveillance is recommended for the first two years after resection, with no evidence in favour of any specific strategy. Low quality evidence, weak recommendation. |
- a.
Prior to establishing the surveillance strategy, the baseline exploration should be completed in the subsequent six months in case of incomplete colonoscopy, inadequate bowel preparation, incomplete lesion resection or the fragmented resection of a flat or sessile lesion with a diameter of 20mm or more.
- b.
Patients will be referred to a HRU to complete the assessment if they meet any of the following characteristics: ≥10 adenomas, ≥5 proximal serrated polyps, ≥2 serrated polyps ≥10mm, ≥10 polyps with ≥50% serrated, SPS or endoscopically-resected pT1 CRC.
- c.
Patients with lesions that do not require endoscopic surveillance (one to two non-advanced adenomas and/or non-advanced serrated lesions) will be monitored within the CRC population screening programme or undergo a colonoscopy at 10 years in case no such programme is available.
- d.
In patients with lesions that do require endoscopic surveillance (at least one advanced lesion or more than two non-advanced adenomas), endoscopic surveillance is recommended within three years of the baseline exploration.
- e.
Endoscopic surveillance will be recommended at one year and four years after the complete resection of a flat or sessile lesion initially resected in a fragmented manner is confirmed, or after a CRC resection.
- f.
Thereafter, the endoscopic surveillance interval will be established on the basis of the lesions detected: Three years if lesions requiring endoscopic surveillance are detected and five years if no such lesions are detected.
- g.
Patients will be reincorporated into the CRC population screening programme if no lesions requiring surveillance are detected in two consecutive colonoscopies.
- h.
Endoscopic surveillance will end when the patient reaches 80 years of age (or 75 in case of associated comorbidities).
This consensus document outlines significant developments with respect to the previous 2009 edition of the clinical practice guidelines. Firstly, it set outs which situations require and do not require endoscopic surveillance and eliminates the need to perform follow-up in individuals who are not particularly at risk of metachronous colon cancer. These individuals represent a high number of patients in whom post-polypectomy surveillance is still indicated. In such cases, the protocol to be followed should match the measures that would be adopted had their colonoscopy been normal, since their degree of risk is similar to individuals with normal colonoscopy results. Secondly, endoscopic surveillance recommendations are set out for individuals with serrated polyps, determining, based on the limited evidence available, which patients should be monitored from among those who have serrated polyps removed. Finally, unlike the previous edition, endoscopic surveillance recommendations are provided for individuals who have undergone CRC surgery.
These recommendations also constitute an advance on the European guidelines on quality in CRC screening,12 which are the recommendations currently accepted and applied by most CRC screening programmes in Spain. The main advances are, on the one hand, eliminating the division of patients into intermediate-risk and high-risk groups, which means abolishing a considerable number of early surveillance colonoscopies. This recommendation lacks sufficient evidence to support it and recent results, some of which were generated in Spain, seem to discourage it. On the other hand, clear recommendations are provided regarding the fact that follow-up is not required in the low-risk group, an aspect which was somewhat ambiguous in the European guidelines.
Finally, it is important to mention that this document is the result of the consensus reached by the main scientific societies involved in the management of these patients, such as in the fields of gastroenterology and gastrointestinal endoscopy, primary care and public health with CRC screening programmes. In these recommendations, the available evidence is brought up-to-date and we hope to rationalise one of the main colonoscopy indications in our setting. New evidence must be generated that rationalises the use of a resource as valuable as endoscopy in this indication, particularly regarding an understanding of which are the most suitable endoscopic surveillance intervals in various situations, which are situations of real risk following the removal of colon polyps, as well as the potential use of molecular markers that may go beyond size and number in the stratification of cancer risk following the resection of colon neoplasia.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Mangas-Sanjuan C, Jover R, Cubiella J, Marzo-Castillejo M, Balaguer F, Bessa X, et al. Vigilancia tras resección de pólipos de colon y de cáncer colorrectal. Actualización 2018. Gastroenterol Hepatol. 2019;42:188–201.