In inflammatory bowel disease (IBD) a high percentage of women are diagnosed during their reproductive age. IBD in remission is the ideal scenario when planning a pregnancy.
AimsTo describe the clinical characteristics of pregnancy/newborn and assess disease activity at the time of conception and throughout the pregnancy in patients with IBD treated at a tertiary centre in Chile.
MethodsWe retrospectively reviewed women diagnosed with IBD who were pregnant or delivered between 2017 and 2020. Demographic, clinical, obstetric and delivery data were obtained from the IBD registry, approved by the local IRB. Descriptive statistics and association tests were performed (χ2, p ≤ 0.05).
ResultsSixty women with IBD were included. At the beginning of pregnancy, 21 (35%) had active disease and 39 (65%) were in remission. Of those with active disease, 16 (66%) remained active and 6 had spontaneous abortions. In those who were in remission, 26 (69%) remained in this condition. Nine patients (15%) discontinued treatment, and 6 of these had inflammatory activity during pregnancy. Preconception counselling was performed in 23 of the 60 patients, being higher in the group that remained in remission during pregnancy (65% vs. 35%, p = 0.02). Patients who had a flare during pregnancy had more probability of preterm birth (<37 weeks) and newborn with lower weight compared with the group that always remained in remission (89% vs 74%, p = 0.161) and (2.885 vs 3.370 g; p = 0.0014).
ConclusionRemission presents better outcomes in pregnancy and preconception counselling would allow a better IBD control during pregnancy.
El diagnóstico de enfermedad inflamatoria intestinal (EII) se realiza, en un alto porcentaje, durante la edad reproductiva. La EII en remisión es el mejor escenario para planificar el embarazo.
ObjetivosDescribir las características clínicas del embarazo y del recién nacido, evaluando la actividad de la enfermedad en el momento de la concepción y en la evolución del embarazo en un centro terciario en Chile.
MétodosEstudio observacional, retrospectivo, incluyó a mujeres con EII que habían tenido un parto durante 2017–2020. Los datos demográficos, clínicos y obstétricos se obtuvieron del Registro de EII. Se realizó análisis estadístico descriptivo y de asociación (χ2, p ≤ 0,05).
ResultadosSe incluyeron 60 mujeres en el estudio. Al inicio del embarazo, 21 (35%) presentaban actividad inflamatoria y 39 (65%) estaban en remisión. Del grupo con actividad, 16 (66%) permanecieron activas y seis tuvieron un aborto espontáneo. Aquellas en remisión, 26 (69%) permanecieron en esta condición; nueve pacientes (15%) habían suspendido el tratamiento, seis de las cuales presentaron actividad durante el embarazo. El consejo preconcepcional fue realizado en 23/60 pacientes, siendo mayor en el grupo que permaneció en remisión durante el embarazo (65% vs. 35%, p = 0,02). Pacientes con brotes durante el embarazo tuvieron mayor probabilidad de embarazo pretérmino (<37 semanas) y recién nacido de menor peso comparado con el grupo que permaneció en remisión (89 vs. 74%; p = 0,161) y (2,885 vs. 3,370 g; p = 0,0014), respectivamente.
ConclusiónLa remisión durante el embarazo presenta los mejores resultados y el consejo preconcepcional permite un mejor control de la EII durante el embarazo.
The highest incidence of inflammatory bowel disease (IBD), mainly consisting of Crohn’s disease (CD) and ulcerative colitis (UC), is between the ages of 20 and 40,1 with 50% to 60% of patients diagnosed in this period.2,3 This coincides with reproductive age and obviously leads to questions about the impact of IBD and its treatment on fertility, maternal and foetal health during pregnancy, breastfeeding safety and child development.4,5 Misinformation about these points that can lead patients to avoid pregnancy or stop taking treatment if they become pregnant.6,7
Although the fertility rate reported in women with inactive IBD is similar to the general population,8 it is important to remember that total proctocolectomy with ileoanal pouch reduces fertility. This, however, has decreased with the use of the laparoscopic approach.9 Studies have shown that in women with IBD at the time of pregnancy, up to 35% will experience an increase in disease activity,10 being greater in patients with active disease at the time of conception.11 Controlling inflammatory activity and maintaining remission before and during pregnancy are the variables with the greatest impact on the progress of the pregnancy and effects on the foetus. Studies focusing on the role of IBD have shown that disease activity is associated with a significant increase in adverse outcomes, in particular a higher incidence of abortions, stillbirths, low birth weight, preterm deliveries and Caesarean sections.10–13 Therefore, when planning a pregnancy, it is recommended that the patient has been in clinical remission for three to six months, ideally with faecal calprotectin (FC) <250 µg/g).7,14,15
Most of the drugs currently used in the treatment of IBD, including five aminosalicylic acid (5-ASA), steroids, thiopurines and anti-tumour necrosis factor (anti-TNF) agents (infliximab, adalimumab, golimumab and especially certolizumab), have a good safety profile during pregnancy and breastfeeding (Category B, C, D and B respectively, according to the FDA). Thalidomide and methotrexate are contraindicated due to the risk of teratogenicity (FDA category X).16 As regards other biologic drugs such as anti-integrins (vedolizumab) and anti-p40 IL-12/23 (ustekinumab), the studies are limited so it is not possible to make a recommendation. Tofacitinib, a small molecule, has been associated with a risk of malformations in animal models and so should be avoided during pregnancy.3,4 It is therefore the obligation and responsibility of the treating team to provide optimal management of the patient from preconception, explaining the risks associated with disease activity, the need for sustained adherence to treatment, and the benefits of achieving and maintaining remission throughout the pregnancy.3,4
The aims of this study were to describe the clinical and demographic characteristics of the pregnancy and the newborn baby, analysing the disease activity at the time of conception, during the course of the pregnancy and with the newborn in a tertiary hospital in Chile in the period 2017–2020.
Patients and methodsThis was a retrospective, observational, descriptive single-centre study of female patients ≥18 years of age with a confirmed diagnosis of CD, UC or IBD unclassified, who were enrolled on the IBD programme at the Clínica Las Condes and became pregnant or delivered in the period 2017–2020.
We included demographic and clinical variables: diagnosis; Montreal classification; years since disease onset; treatment at the time of conception and during pregnancy, considering the use of 5-ASA, corticosteroids (prednisone and oral budesonide), immunomodulators (thiopurines and methotrexate), anti-TNF biologic therapy (infliximab or adalimumab); history of surgery for IBD; and disease activity at the time of conception and during the course of the pregnancy. For this last point, we used clinical and endoscopic indices obtained at the time each patient was assessed. FC was also used as a biomarker to measure disease activity. For clinical remission, the partial Mayo score was used for UC and the Harvey Bradshaw (HB) index for CD. Clinical remission was defined as a partial Mayo score <2 for UC and an HB index <5 for CD.7,17 To measure endoscopic remission, we used the Simple Endoscopic Score for CD (SES-CD) and Rutgeerts score in post-surgical CD, and Mayo endoscopic score in UC. In UC, endoscopic remission was defined as a Mayo endoscopic score of 0 or 1. In CD, endoscopic remission was defined as an SES-CD <2 in the case of luminal disease and a Rutgeerts i0 or i1 in patients who had undergone surgery.17 FC was used as a follow-up biomarker, considering biochemical remission with FC < 250 μg/g.14,15
In relation to the pregnancy, we analysed whether the patients received preconception counselling; if they had inflammatory activity periconception, defined as any clinical disease activity from eight weeks before conception to the first two weeks of pregnancy18; if they had a flare-up during pregnancy; if their therapy had to be optimised; how the pregnancy progressed (complications during pregnancy/delivery and type of delivery); weight, length and Apgar score for the newborn; and whether or not live virus vaccines (rotavirus) and/or BCG vaccine (attenuated bacillus) were administered to newborns whose mothers were on biologic therapy. Adverse pregnancy outcomes [preterm delivery (<37 weeks gestation), low birth weight (<2.5 kg), and small for gestational age (SGA)] were assessed as rates. SGA classification was performed for national reference19 and World Health Organization curves,20 Apgar <7 at 1 and 5 min and stillbirth.
This study was approved by the institution’s Ethics Committee and conducted in accordance with the principles of the Declaration of Helsinki.
Statistical analysisThe results of the study were analysed using the R Commander software program. Categorical variables were analysed through absolute frequencies and percentages. Continuous variables were described in terms of measures of central tendency and dispersion depending on data distribution (mean/standard deviation whether or not they had normal distribution, and median/interquartile range in case of non-normal distribution). Continuous variables were compared using the Mann-Whitney U test or Student’s t test, depending on the distribution. Percentage relative frequency was used for categorical variables, and the X2 test for comparative statistical analysis. A p value ≤0.05 was considered statistically significant.
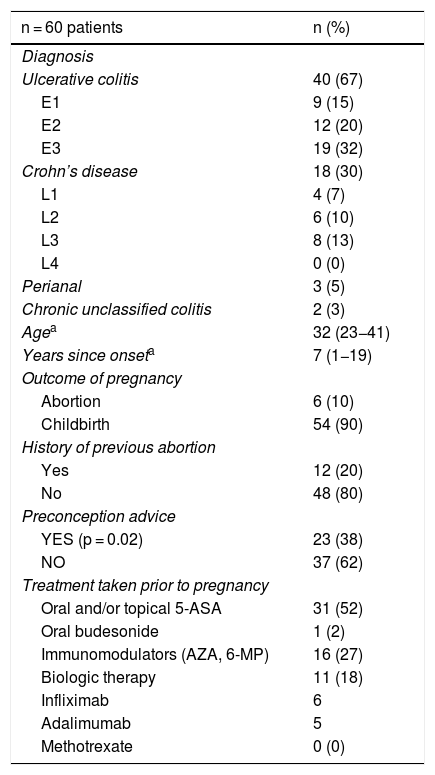
ResultsPatientsWe included 60 patients, 40 with UC, 18 with CD and two with IBD unclassified, with a median age of 32 (range 24–41) at the beginning of pregnancy, and a median time since disease onset of seven years (range 1−19). Twelve patients had been pregnant before, 10 of them once already diagnosed with IBD. None of the patients had onset of the disease during pregnancy. The patients’ demographic and clinical data are shown in Table 1. Twenty-three patients received preconception counselling. The most common treatments at the time of pregnancy were oral 5-ASA (52%) and azathioprine (AZA) (27%). Nine patients had discontinued treatment prior to pregnancy or in the first weeks of gestation, five as instructed by a doctor outside the IBD programme and four of their own volition. Our team did not discontinue or alter the dose of any drugs when learning of the pregnancies.
Clinical and demographic characteristics of pregnant patients with IBD.
| n = 60 patients | n (%) |
|---|---|
| Diagnosis | |
| Ulcerative colitis | 40 (67) |
| E1 | 9 (15) |
| E2 | 12 (20) |
| E3 | 19 (32) |
| Crohn’s disease | 18 (30) |
| L1 | 4 (7) |
| L2 | 6 (10) |
| L3 | 8 (13) |
| L4 | 0 (0) |
| Perianal | 3 (5) |
| Chronic unclassified colitis | 2 (3) |
| Agea | 32 (23−41) |
| Years since onseta | 7 (1−19) |
| Outcome of pregnancy | |
| Abortion | 6 (10) |
| Childbirth | 54 (90) |
| History of previous abortion | |
| Yes | 12 (20) |
| No | 48 (80) |
| Preconception advice | |
| YES (p = 0.02) | 23 (38) |
| NO | 37 (62) |
| Treatment taken prior to pregnancy | |
| Oral and/or topical 5-ASA | 31 (52) |
| Oral budesonide | 1 (2) |
| Immunomodulators (AZA, 6-MP) | 16 (27) |
| Biologic therapy | 11 (18) |
| Infliximab | 6 |
| Adalimumab | 5 |
| Methotrexate | 0 (0) |
E: extension; L: location; 5-ASA: 5-aminosalicylic acid; AZA: azathioprine; 6-MP: 6-mercaptopurine.
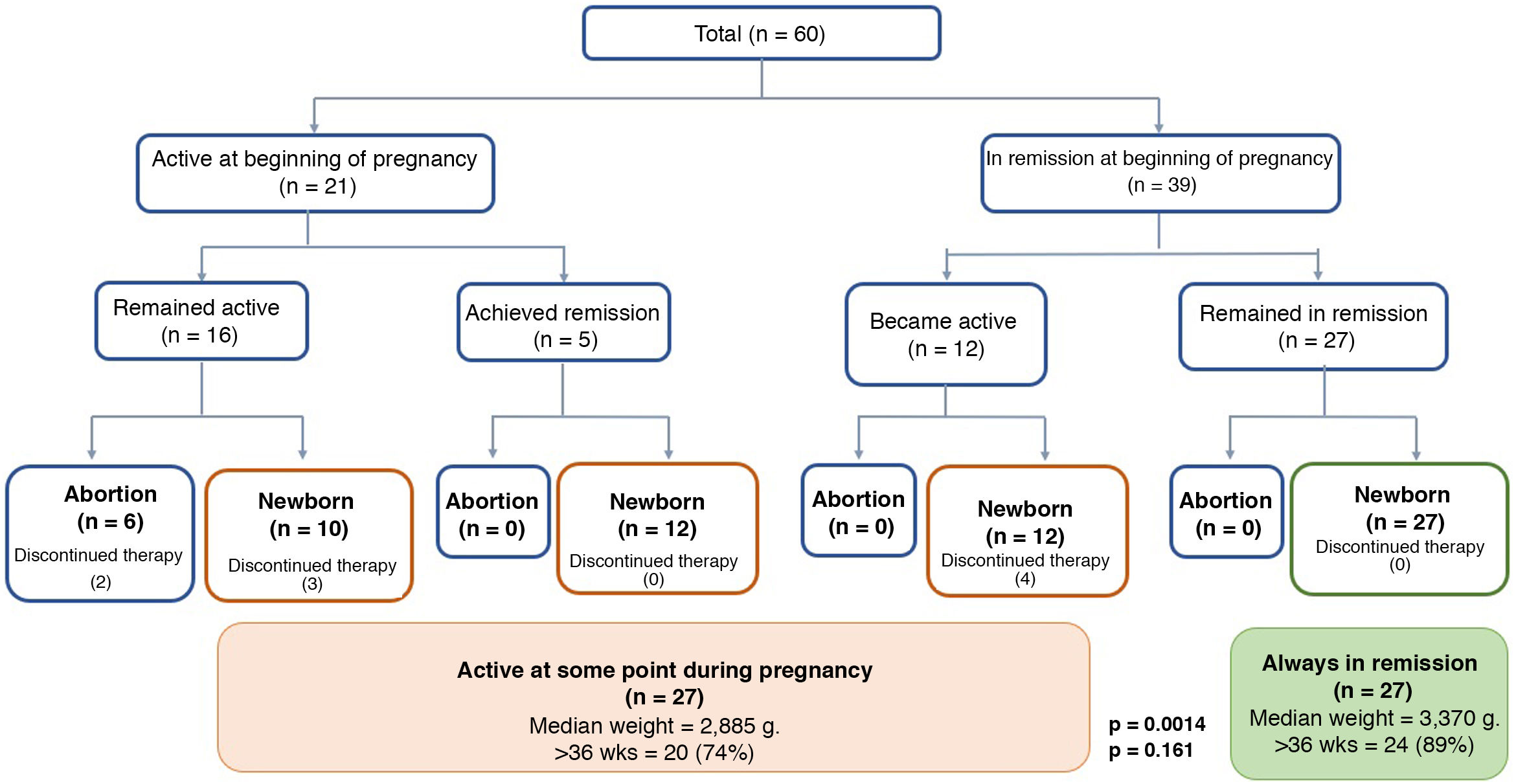
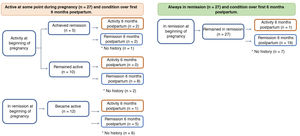
In terms of clinical activity at the beginning of pregnancy, 39 patients (65%) were in remission, 23 of whom had UC, 15 CD, and one IBD unclassified. There were no differences in clinical activity at the time of conception between UC and CD. Twenty-seven patients (69%) in remission at the beginning of pregnancy remained in remission. Six of the patients had a flare-up in the first trimester, five in the second trimester and one in the third trimester. Of the group with clinical inflammatory activity at the beginning of pregnancy (35%, 21 patients), 17 had UC, three had CD, and one had IBD unclassified. In 66% (16 patients), the active disease continued during their pregnancy (Fig. 1). None of the patients had a history of perianal fistula at the time of conception or developed one while pregnant.
In the group that remained in remission, a higher percentage had received preconception counselling (65% vs 35%, p = 0.02). Of the nine patients who discontinued IBD treatment, four experienced flare-ups in the first trimester (with one abortion at eight weeks) and two in the second trimester. The other three patients restarted their treatment with mesalazine and achieved clinical remission.
Inflammatory activity was assessed with FC during the pregnancy in 43 patients, with a total of 67 measurements (median 265 μg/g; range 9−1,700). In patients with flare-ups, the median FC was 496 μg/g (range 163−1,700) vs 35 μg/g (range 5–600) in patients in clinical remission (p < 0.0001). Four patients (3 UC and 1 CD) had an endoscopic study during their pregnancy, three had a colonoscopy and one a pouchoscopy. These tests were performed in the first trimester in one case and in the second trimester in the other three cases. Endoscopic inflammatory activity was confirmed in two patients (one in week 10 and the other in week 21 of their pregnancy). None of the patients had imaging studies.
In the 28 patients who had flare-ups while pregnant (16 from the beginning and 12 during the course of the pregnancy, Fig. 1), the drugs used were 5-ASA (64%), AZA (14%), infliximab (7%), oral budesonide (7%) and prednisone (4%). The three patients who started AZA during the first trimester had no adverse events. Only one patient was hospitalised for a flare-up of UC with biopsies positive for cytomegalovirus in the second trimester. She was treated with ganciclovir with a favourable response and was able to stay on her maintenance therapy (infliximab). There were no surgical interventions in this cohort.
PregnancyThe progress and outcomes of the disease activity and pregnancies are shown in Fig. 1 and Table 2, respectively. In the group with active disease during pregnancy, six patients had a spontaneous abortion, all of them with moderate or severe flare-ups. In comparison, none of the patients whose disease had been active but who achieved remission, or who were in remission from the start and remained so, had a spontaneous abortion (Fig. 1). Among those who remained in remission, only 11% had a preterm delivery (<37 weeks) vs 26% of those who had flare-ups during their pregnancy (p = 0.161). Comparing the route of delivery, vaginal delivery was more common among patients with UC than in CD (39% vs 6%, p = 0.016), with no instrumental deliveries. In contrast, elective caesarean section was more common in CD (75% vs 28%; p = 0.001). There were no differences between the two groups in the frequency of emergency caesarean sections (Table 2). In terms of the weight of the newborns, the median was 3,370 g (range 2,220−3,950) in the group that remained in remission compared to 2,885 g (range 1,860−4,000) in the group with flare-ups during pregnancy (p = 0.0014) (Fig. 1). In the group that remained in remission, two newborns were in the SGA range vs three in the group with disease activity during pregnancy. All the newborns had a normal Apgar score at 1 and 5 min.
Outcome of the pregnancy according to type of IBD.
| Ulcerative colitis | Crohn’s disease | Chronic unclassified colitis | |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Pregnancy outcome (total = 60) | |||
| Childbirth | 36 (60) | 16 (27) | 2 (3) |
| Abortion | 4 (7) | 2 (5) | 0 (0) |
| Type of birth (total = 54) | |||
| Vaginal | 14 (26) | 1 (2) | 2 (3) |
| Elective caesarean | 10 (19) | 12 (22) | 0 (0) |
| Emergency caesarean | 12 (22) | 3 (6) | 0 (0) |
| Weeks of gestation | 38 (31−40) | 38 (30−41) | 39.5 (39−40) |
| Weight (g)* median | 3,136 (1,860−3,990) | 3,123 (2,200−3,800) | 3,050 (2,880−3,020) |
| 3,200 | 3,380 | 3,050 | |
| Length (cm)* | 48.6 (40.5−53) | 48.8 (43.5−53) | 49.7 (49−50.5) |
None of the children of mothers on biologic therapy had a live virus vaccine (rotavirus and attenuated bacillus TB vaccine) administered before one year of life.
Biologic therapy plasma levels were measured in seven patients, four of them with infliximab with a mean of 6.8 μg/mL (range 4.7–10.3) and three with adalimumab (two with levels >12 µg/mL and the other with 6 μg/mL). No dose optimisation was necessary in these patients.
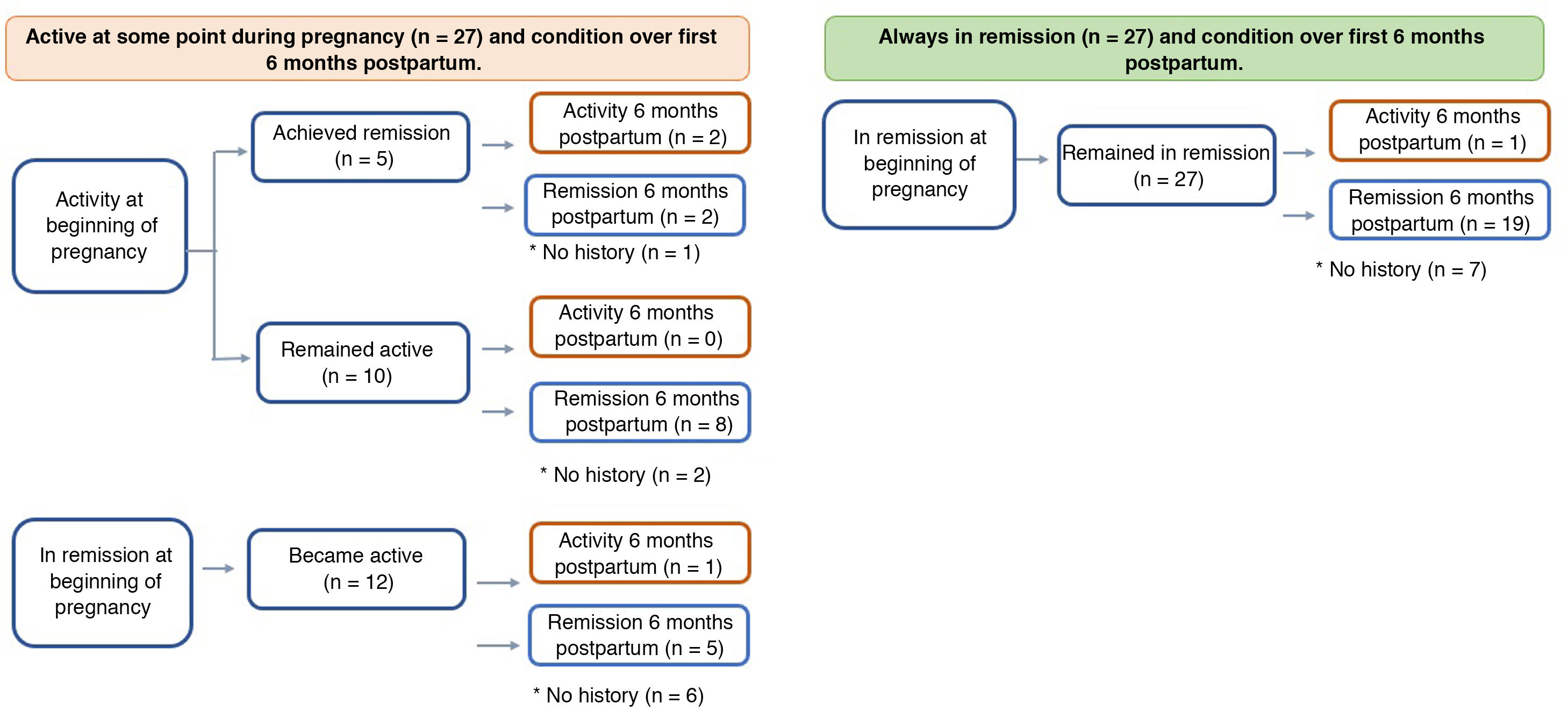
PostpartumIn the postpartum period, information was obtained for 80% of the patients, all of whom continued with their maintenance therapies (Fig. 2). Of those who had flare-ups during their pregnancy, 17% had postpartum inflammatory activity vs only 5% of those who started and remained in remission throughout their pregnancy (p = 0.241).
DiscussionA favourable outcome, not only of the pregnancy, but also of the IBD during pregnancy, depends on good collaboration between a cohesive and well-prepared multidisciplinary team and a well-informed and proactive patient. Our study confirms that controlling disease activity and maintaining remission before and during pregnancy have an impact on both how the pregnancy progresses and the development of the newborn.
Women with IBD frequently have concerns about the safety of continuing with drug treatment for IBD during pregnancy, particularly because of the risk of congenital malformations and other adverse foetal outcomes.21 This fear of the negative effect of drugs on pregnancy, mainly during the first trimester, is the main reason suggested by patients for a decrease in their adherence to treatment.6 In our study, nine patients discontinued treatment prior to their pregnancy or during the first weeks of gestation. It is quite worrying that five of these cases stopped treatment on instruction from a doctor, confirming the need to educate both patients and the healthcare team.4 Of the nine patients who discontinued treatment prior to pregnancy or during the first weeks of gestation, six had a flare-up.
Preconception counselling is essential for aiding safe decision-making about therapy and stressing the importance of achieving disease remission prior to conception, helping to improve outcomes for both the disease and the pregnancy.22 In our study, a higher percentage of the patients who remained in remission had received preconception counselling, confirming its importance in the course of IBD during pregnancy.
Inflammatory activity at the time of conception is an important predictor of how the disease will behave during a pregnancy. A meta-analysis which included 14 studies showed that patients with clinical activity at the time of conception had twice the risk of having some degree of activity during the pregnancy.9,23 This was also confirmed in a prospective study of 229 pregnant patients with IBD, where activity at the time of conception was associated with an increased risk of having a flare-up during the pregnancy with an odds ratio of 7.66.24 Our results are in line with the above studies. Of the patients in remission at the time of conception, 31% developed some degree of inflammatory activity vs 76% of those with disease activity at the beginning of their pregnancy (p = 0.002).
In women with IBD in remission at the time of conception, the risk of flare-ups during pregnancy is approximately 30%,25 similar to non-pregnant patients. It has been suggested that the likelihood of disease activity may be higher in the first and second trimesters of pregnancy, and within six months postpartum.26,27 In our study, 12 of the 39 patients in remission at the time of conception had some degree of inflammatory activity during pregnancy and, of the 27 who achieved remission during pregnancy, only one had a flare-up in the six months postpartum. These results once again confirm how important it is for the treating team to stress the need for adherence to drug therapy, not only before, but also during the pregnancy and in the puerperium.
Studies have shown that pregnancy does not affect FC, so it continues to be a useful biomarker for assessing inflammatory activity in this period.28–30 One study which included 219 pregnant women with IBD on anti-TNF therapy showed that FC levels were correlated with disease activity; patients with active disease had significantly higher concentrations of FC compared to those in clinical remission (p < 0.003).31 Our results also show that FC was significantly higher in patients with clinical activity than in those in remission (median 496 μg/g vs 35 μg/g; p < 0.0001), confirming its utility in monitoring disease activity during pregnancy.
There is sufficient evidence to support the safety of colonoscopy in pregnant patients with signs and symptoms related to a possible diagnosis of IBD or deterioration of an existing IBD.32 Sigmoidoscopy can be performed without sedation or preparation at any time during pregnancy.4 Therefore, when indicated, this procedure should not be deferred as it can directly affect decision-making to help achieve optimal obstetric and gastrointestinal outcomes. In our study, an endoscopic study was performed in four patients without complications, enabling two of them to have their treatment optimised after confirming the presence of endoscopic activity.
With regard to treatment, the literature supports the safety of continuing most IBD drugs during pregnancy,4,16 which is what happened with all our patients, including those who were on thiopurines and anti-TNFs. In terms of flare-ups during pregnancy, three patients started AZA in the first trimester. Although none had adverse events, recent reports suggest that treatment with thiopurines should not be started during pregnancy because of the long period of time it takes to work (eight to 12 weeks) and the potential adverse events in the mother, particularly myelotoxicity, hepatotoxicity and drug-induced acute pancreatitis.4 In contrast, anti-TNFs are safe drugs in the induction and maintenance phase in the event of disease during pregnancy,33 although it is recommended that the last dose should be at 30–32 weeks for infliximab, at 36–38 weeks for adalimumab, and at 34–36 weeks for golimumab.5 In our study, one patient started infliximab when 10 weeks pregnant without developing any complications during the rest of her pregnancy.
It has been suggested that measuring serum levels of biologic drugs before conception would enable therapy to be optimised and personalised, thus avoiding subtherapeutic levels, which would increase the likelihood of a flare-up during pregnancy, or supratherapeutic levels, which could increase the risk to both mother and foetus.4 One important variable that must be considered when interpreting these results is that the pharmacokinetics of these drugs may undergo changes during pregnancy.34 In our study, anti-TNF levels were measured in seven patients, but none of them had any changes made to their therapy.
As regards the outcomes of the pregnancies, 10% of our patients had spontaneous abortions, this figure being very similar to that published in the literature.13 The six cases in our study were in the group with active disease at the time of conception which remained active during the pregnancy. Three of these patients had a history of AZA use during the first trimester of pregnancy. Although such an association was suggested initially,35 recent studies have ruled out exposure to thiopurines during the first trimester being associated with an increased risk of spontaneous abortion or congenital malformations.36,37 There is no question that the most important risk factor for spontaneous abortion is moderate-to-severe intestinal inflammatory activity at the time of conception or during pregnancy,13 as was the case in our study.
In pregnant patients with IBD, the choice of delivery route, whether vaginal or caesarean, should be based primarily on obstetric indications.4 In patients with active perianal disease, rectovaginal fistula or an ileal pouch, caesarean section should be considered.38 Despite this, the rate of caesarean sections is twice as high in women with IBD as in the general population.39 In our study, 68% of the pregnancies resulted in caesarean section, 40% of which were performed as emergencies for obstetric reasons. These results are above the national average. A study recently published with the experience of two university hospitals showed the rate of caesarean sections in the general population to be in the region of 35.7–55.7%.39 Obviously, our higher percentage of caesarean deliveries should make us rethink the management of these patients.
The available information shows that 85% of women with IBD have an uncomplicated pregnancy with foetal adverse event rates similar to those in the general population.40 However, flare-ups during pregnancy have a negative impact on the foetus and can lead to premature delivery, low birth weight and even foetal death.41,42 Assessing the risk of preterm birth and foetal death, some studies have been unable to confirm these results,13,43,44 but this may be explained by differences in the extent and severity of disease activity in the populations studied.26,44 Our results confirm that having some degree of inflammatory activity during pregnancy significantly increases the likelihood of a lower birth weight.
In accordance with the immunisation schedule in newborn infants, it has been suggested that live virus or attenuated bacilli vaccines should be postponed for at least six months postpartum in newborns of mothers who have been exposed to anti-TNF therapy.45 In our study, none of the newborn babies of mothers receiving this biologic was given a live virus vaccine before the age of 12 months.
Our study has certain strengths. By showing the experience of a tertiary hospital with an established IBD programme, it provides insight into the routine clinical practice of pregnancy management in patients with IBD. Moreover, inflammatory activity was assessed with FC in 72% of the patients, which enables appropriate management according to objectives.1 Nevertheless, we know there are also limitations. First, our study was retrospective and lacked a control group with which to compare pregnancy and newborn outcomes. Second, some patients attended a different centre for the latter part of their pregnancy, which meant we did not have adequate information about the severity of their inflammatory activity prior to delivery or the decision to perform a caesarean section. Lastly, the lack of access to information for the six months postpartum in some patients may affect our results.
In conclusion, IBD affects women of reproductive age, raising important concerns for both the patient and her treating team. Appropriate preconception advice and good collaboration between obstetrician and gastroenterologist will help ensure access to information which is both clear and adequate. The main aims are to optimise treatment in order to achieve remission at the time of conception and then maintain that state throughout the pregnancy. Accomplishing these goals will certainly improve our chances of good outcomes for both the mother and the newborn baby.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Nuñez F. P, Quera R, Sepúlveda E, Simian D, Pizarro G, Lubascher J, et al. Embarazo en enfermedad inflamatoria intestinal: experiencia en una cohorte chilena. Gastroenterol Hepatol. 2021;44:277–285.