Detecting and responding to target objects in the visual environment is a key factor in goal-directed behavior. Exposure to chronic stress often results in alterations of prefrontal cortex (PFC) function, which may impact PFC-dependent selective attention process. This study aimed to investigate the effect of chronic academic stress on attentional control process.
MethodBoth the stress group and the control group performed an arrow-based version of the Eriksen Flanker task. Event-related potentials (ERP) were recorded while the participants performed the task.
ResultsThe behavioural results exhibited decreased Flanker RT effect for the stress group compared to the control group, suggesting a reduced interference under stress. The ERP results showed that stress group showed decreased frontal N2 but increased early P3 and late P3/LPC activities compared to the control group. These results suggest reduced conflict monitoring but increased conflict resolution process under stress.
ConclusionsThe chronic academic stress improves attentional control by reducing the conflict monitoring and enhancing conflict resolution processes.
Chronic stress is usually caused by long-term life events and may lead to psychological and physiological changes. Students who prepared for a major examination for more than a month may suffer from chronic academic stress (Chrousos & Gold, 1992). Previous studies have shown that prolonged or excessive stress responses increase the individual's susceptibility to physical and mental diseases (Kuehl et al., 2020; Moon et al., 2022), leading to a decline and dysfunction of the prefrontal cortex (PFC), hippocampus, and a variety of cognitive prefrontal functions (Arnsten, 2015; Jung et al., 2020; Shi & Wu, 2020). However, increasing evidence indicates that the impact of chronic stress on PFC-mediated cognitive functions might be beneficial, particularly in well-rehearsed or simple tasks (Kofman et al., 2006; Lewis et al., 2008). The inconsistent stress effects on cognitive functions might be related to stressor type, stress intensity or time interval between stressor and cognitive task (Kofman et al., 2006; Plessow et al., 2011; Sandi, 2013).
The cortisol awakening response (CAR) has been seen as an important marker of the hypothalamo-pituitary-adrenocortical (HPA) activity under chronic stress and it might be regulated by the PFC (Fries et al., 2009). Specifically, the cortisol concentrations increased rapidly within 30 min following morning awakening and returned to the baseline level about 1 hour later (Stalder et al., 2016). Compared with the controls, the CAR is decreased for the students under chronic academic stress (Duan et al., 2013; Law & Clow, 2020).
Selective attention includes two mechanisms, i.e., perceptual selection mechanism, which functions to reduce the perception of distractor; and attentional control mechanism, which functions to maintain the attentional selection of relevant stimuli. The Eriksen Flanker task was often used to investigate attentional control in selective attention process (Cloutier et al., 2020; Shields et al., 2019; Wei & Zhou, 2020). In this task, a central target stimulus is simultaneously presented with the distractor stimuli (flankers), which are either congruent or incongruent with the target stimuli. The participants are asked to judge the target while ignoring the flankers. Numerous studies have found longer response times (RTs) and higher error rates for the incongruent than congruent trials, reflecting the interference effect (Cloutier et al., 2020; Eriksen & Eriksen, 1974; Shields et al., 2019; Wei & Zhou, 2020).
Cognitive control is well documented to be impaired under chronic stress (Arnsten, 2015; McEwen et al., 2016; Wirkner et al., 2019). Accordingly, a model of chronic stress weakening cognitive control is established. Exposure to a stressful situation can lead to the activation of HPA axis and release of glucocorticoids, with their receptors mainly distributed in the PFC and the amygdala (Marin et al., 2011). Chronic stress has been shown to impair PFC functioning and PFC regulation of the amygdala, but to increase activation of the amygdala, thus accentuating the switch from thoughtful top-down control based on task-relevance to bottom-up control based on salience (Arnsten, 2015; McEwen et al., 2016; Wirkner et al., 2019). However, this model could not accommodate other findings that chronic stress improves response inhibition (Gao et al., 2022; Shields et al., 2016). Herman (2013) reported that stress habituation can be seen as an important adaptive response to cope with repeated or prolonged challenges. Specifically, repeated exposure to a given stressor can reduce the activation of the HPA axis and thus reduce the physiological burden. The adaption model proposes that chronic stress could reallocate limited cognitive resources in adaptive ways, driven by goal-directed behavior (Park et al., 2012; Wu et al., 2014; Shields et al., 2016).
Prior studies have demonstrated that the chronic academic stress can affect the attention processing. Yuan et al. (2016) reported increased frontal P2 amplitude in the 1-back and 2-back tasks for participants in the exam group compared to the control group. The P2 reflected an early processing of target, with increased P2 amplitude indicating a greater allocation of attentional resources to the target (Yang et al., 2012). Yuan et al. (2016) suggested that chronic academic stress exerted a positive impact on early attention in working memory processing.
The Attention Network Test (ANT) is widely used to investigate the selective attention process, which comprises an attentional cuing paradigm and a Flanker task (Fan et al., 2002). The cues can predict the onset and/or corresponding location of the target in subsequent Flanker task. Liu et al. (2020) investigated the effect of chronic academic stress on selective attention by adopting ANT. The ERP components (i.e., N1, N2, and P3) evoked by both the cue and target were examined. They found that the cue evoked decreased anterior N1 and N2 amplitudes for the stress group compared to the control group. Previous ANT studies have linked anterior cue–N1 and cue–N2 components with alerting process (Williams et al., 2016). Liu et al. (2020) suggested that chronic stress might impair the alerting process. In addition, they also found increased frontal cue–P3 amplitude for the stress group than the control group. Cue–P3 was related to the attentional orientation (Kratz et al., 2011). This suggests that the stress group may require a greater allocation of cognitive resources for processing and estimating the cue compared to the control group (Liu et al., 2020). In the Flanker task, the target evoked larger frontal N2 but reduced frontal P3 amplitudes for the stress relative to the control groups. Target–N2 has associated with conflict monitoring process, and target–P3 linked to conflict resolution process (Larson et al., 2014; Neuhaus et al., 2010; Wei & Zhou, 2020). They suggested that chronic academic stress might promote conflict monitoring but impair conflict resolution, as evidenced by slower response time and lower accuracy in the stress group. These results suggest that chronic academic stress may exert dissociable effects on the processing of the cue and target, with facilitation of cue processing but impairment of target processing.
By adopting ANT, Liu et al. (2020) aimed to investigate the influence of chronic academic stress on the efficiency of attention network, i.e., alerting, orienting, and executive control. However, the selective attention process reflected by the target processing was modulated by the cue processing. Specifically, when the participants were alerted by a warning cue, the efficiency of selective attention process decreased as evidenced by a larger flanker effect (de Souza Almeida et al., 2021). Due to cue-target interaction in the ANT, it is unclear whether stress directly affects selective attention to the target or through influencing cue processing in the study of Liu et al. (2020). The purpose of this study was to assess the effect of chronic academic stress on the selective attention process using a Flanker task, in which participants were required to respond to the target stimulus, without any accompanying cues.
The National Postgraduate Entrance Exam (NPEE) is one of the most important and competitive examinations in China (Duan et al., 2013; Wu et al., 2014). Students usually spend more than six months preparing for this examination for approximately eight hours every day. During this preparation time, they will experience great chronic academic stress, especially in the months before the examination (Wu et al., 2014; Yuan et al., 2016). Because of its high importance and low passing rate (around 33 %), the NPEE has been widely used as a chronic academic stressor (Duan et al., 2013; Gao et al., 2022; Liu et al., 2020; Qi et al., 2023), and it can steadily increase negative mood and salivary cortisol levels in individuals like other chronic academic stress (e.g., Duan et al., 2013; Gao et al., 2022; Qi et al., 2023). In this study, the NPEE was employed as a chronic academic stressor. The participants under chronic stress (stress group) and non-stressed participants (control group) performed a Flanker task. The selective attention process was assessed by the Flanker task.
Previous studies have found that the CAR was disrupted under chronic academic stress (Duan et al., 2013; Law & Clow, 2020). Accordingly, a decreased CAR would be expected for the stress than the control group. In the Flanker task, the N2 component was thought to reflect the conflict monitoring process, with a larger N2 amplitude indicating an enhancement of conflict monitoring (Larson et al., 2014; Lin et al., 2021; Wei et al., 2022). The P3 component was associated with the attentional resources allocation and the conflict resolution processes. Alguacil et al. (2013) categorized the P3 component into the early P3 and the late P3/LPC in the Flanker task. The early P3 exhibited larger amplitudes for congruent trials compared to incongruent trials, indicting increased allocation of attentional resources to target processing (Alguacil et al., 2013; Neuhaus et al., 2010; Polich, 2007; Wei & Zhou, 2020). The late P3 (also termed as LPC) was associated with the conflict resolution process. Incongruent trials evoked more positive late P3/LPC amplitudes compared to congruent trials, suggesting that larger late P3/LPC amplitudes may reflect an enhanced conflict resolution process (Alguacil et al., 2013; Larson et al., 2009; McKay et al., 2017). We hypothesized that if the chronic academic stress improved the attentional control process, less intensive conflict would be experienced (enhanced N2), and enhanced conflict resolution process (increased early P3 and late P3/LPC) would be expected for the stress than the control group.
Materials and methodsParticipantsThe participants were screened via an online survey. Specifically, the Beck Depression Inventory-II (BDI-II) was adopted to assess severity of depression and excluded participants with moderate or higher depressive symptomatology (BDI score ≥ 14). The Life Events Scale (LES, Tennant & Andrews, 1976) was adopted to ensure that participants were not exposed to any other major stressors in the last three months. Participants who had experienced any event in the LES would be excluded.
Some studies concerning the Flanker effect under stress or stress-related disorders (e.g., Chang et al., 2015; Zinchenko et al., 2017) reported relatively large effect sizes (ηp2 > 0.14). For a 2 Congruency × 2 Group mixed design, a power analysis (MorePower Version 6.0) indicated that to obtain a moderate statistical power (0.80), a sample size of 26 per group was necessary based on a large effect size (Partial eta squared, ηp2) of 0.14 (α = 0.05). Consistent with previous studies (Gao et al., 2022; Lines et al., 2021), the recruitment and data collection were finished roughly 2–4 weeks prior to the NPEE. The time for data collection was relatively short (only 3 weeks). A total of 60 participants passed the prescreening measure. Five participants were excluded due to excessive artifacts during electroencephalography (EEG) recordings, and only data from participants whose average ERP contained more than 50 % of the trials per condition were included. Therefore, data from 55 participants were included in the analyses, including 28 students in the stress group (17 males, meanage = 21.6 years, SD = 1.61), and 27 students in the control group (10 males, meanage = 20.1 years, SD = 1.96). The participants in the stress group spent more than 8 h every day preparing for this examination. Their average preparation time was over 7 months. The participants in the control group did not participate in any important major examinations/interviews in past month.
All participants had reported being right-handed, having normal or corrected-to-normal vision. None of the participants reported mental or physical disorders. All participants had not previously participated in similar studies. Before starting the experiment, all participants signed a written informed consent form, and were paid for their participation. This study was approved by the Research Ethics Committee of local university, and followed the ethical guidelines of the Declaration of Helsinki.
Design and materialsIn this study, a 2 (Group: stress, control) × 2 (Congruency: congruent, incongruent) mixed-design was used. The participants in both groups performed a Flanker task. In each trial, the target stimulus was presented simultaneously with the interfering stimuli, and the direction of the interfering stimuli might be either congruent or incongruent with the target stimulus. In each trial, the participants were instructed to focus on a centrally located arrow (target stimulus), ignore flanking arrows, and respond quickly and accurately according to the direction of the central arrow. The participants were asked to press the "F" key with their left index finger if the central arrow pointed to the left, and press the "J" key with their right index finger if the central arrow pointed to the right.
ProcedureAfter participants arrived at the laboratory, Cohen's Perceived Stress Scale (PSS, 10-item version, Cohen, 1988) was used to assess their chronic academic stress levels. Both the Positive and Negative Affect Scale (PANAS, Watson et al., 1988) and State version of State-Trait Anxiety Inventory (SAI, Spielberger, 1983) were used to assess their emotional states and anxiety levels.
For the Flanker task, each trial started with a 500-ms fixation cross (+) which appeared in the center of the screen. After a 500 ms blank screen, the target arrow flanked by the distractor arrows was displayed for 1000 ms, then followed by a 1500 ms blank screen.
Before the formal experiment, the participants practiced 20 trials. The formal experiment consisted of 160 trials (80 trials per block). Participants took a 3 min rest between blocks. During the experiment, participants wore electrode caps and sat comfortably, approximately 80 cm from a computer screen, in an electrically shielded room. All stimuli were presented in black on a silver gray background.
Salivary cortisol sampling and cortisol analysisSaliva samples were collected with Salivette collection devices (Sarstedt, Germany) immediately upon awakening (S1), 30 min (S2), 45 min (S3) and 60 min (S4) on the day of the experiment and the next day of the experiment. Four samples were collected per day, resulting in a total of eight samples for each participant. We collected cortisol across two days for achieving reliable trait data on the CAR. Participants were required to enter sleep before 24:00 and complete the collection between 06:00 and 09:00. They were asked to refrain from brushing teeth, smoking, drinking, eating or excessive exercise before saliva collection. To ensure the reliability and validity of the cortisol measures, participants were informed to record waking time and the exact time for each sample. All participants self-reported that they entered sleep before 24:00 and their sleep duration was approximately 6–8 h. The saliva was stored at −80 °C until assaying. Saliva cortisol concentrations (in nmol/L) were measured by ELISA (SLV-2930; DRG, Germany).
Cortisol levels at the four time points were obtained by averaging the data of 2 days. Then, a repeated measures analysis of variance (ANOVA) with time point (0 min, 30 min, 45 min and 60 min) as within-subjects factor and group (stress, control) as between-subjects factor was conducted. In addition, the R30 (the change in cortisol level 30 min after awakening; i.e., R30 = S2-S1) and AUCi (area under the curve with respect to the increase; i.e., AUCi = [(S1+S2)*0.5/2+(S2+S3)*0.25/2+(S3+S4)*0.25/2)]-S1) were used to estimate the dynamics of the CAR. Independent sample t-tests between groups were performed on the CAR parameters.
Behavioral data analysisIndependent t-tests were conducted for the stress and the control group on perceived stress (PSS score), state anxiety (SAI score), and emotional state (PANAS score, including positive affect and negative affect scores).
For the Flanker task, repeated measures ANOVAs with group (stress, control) as between-subject factor and congruency (congruent, incongruent) as within-subject factor were performed on mean accuracy and RT. Dunn (2021) suggests that the observations exceeding 3 standard deviations from the mean are seen to be unusual. In this study, for each participant, any incorrect responses and RTs that were deviated by three standard deviations from their individual mean RTs were excluded from the analysis. Nearly 2.2 % of the data were excluded because of deviant RTs. The magnitude of the Flanker effect on RT was calculated by subtracting the RTs of congruent trials from the RTs of incongruent trials in each group. Then, an independent t-test between groups was performed on Flanker effect on RT.
ERP recording and analysisBrain EEG activity was recorded from a 64-Channel EEG recording system (Brain Products, GmbH, Germany) according to the international 10–20 system, with references on a fronto-central midline electrode. A vertical electrooculogram (EOG) was measured with an electrode placed below the right eye. All impedances were maintained below 5 kΩ. EEG was amplified using a 0.05–100-Hz bandpass filter and sampled continuously at 500 Hz for offline analysis.
EEG data were analyzed with BrainVision Analyzer 2.1 (Brain Products, Germany). ERPs time locked to the onset of the stimuli in Flanker task were re-referenced to the average of the left and right mastoids. The EEGs were segmented into 1000-ms epochs surrounding the onset of the stimulus, and baseline-corrected with respect to the 200-ms prestimulus. After ocular correction using independent component analysis algorithms, EEGs were digitally filtered with a 30 Hz low-pass filter. Trials contaminated with EOG artifacts (mean voltage exceeding ± 80 µV) or those with artifacts due to amplifier clipping, bursts of electromyographic activity were excluded from averaging. EEGs recorded in each kinds of trials (congruent vs. incongruent) were averaged separately for each participant, and only trials with correct responses were included in ERP averages. The mean numbers of trials retained after artifact rejection were as follows, control-congruent, mean = 73.6, range = 60–79; control-incongruent, mean = 72.1, range = 60–79; stress-congruent, mean = 73.9, range = 57–80; stress-incongruent, mean =72.4, range = 63–80.
Preliminary inspection of the data indicated that the maximum voltage and the maximum difference across different conditions for N1 component were shown over parietal-occipital scalp. Four parietal-occipital scalp electrode sites (P7, P8, PO7 and PO8) were selected for analysis of the N1 component. The N1 was measured by the mean amplitudes of 140–220 ms time window, and this corresponds to the typical latency range of the N1 (Fu et al., 2005). Repeated measures ANOVA with group (stress, control) as between-subject factor, and congruency (congruent, incongruent) and electrode sites (P7, P8, PO7 and PO8) as within-subject factors was performed on mean amplitudes of N1 (140–220 ms) time windows.
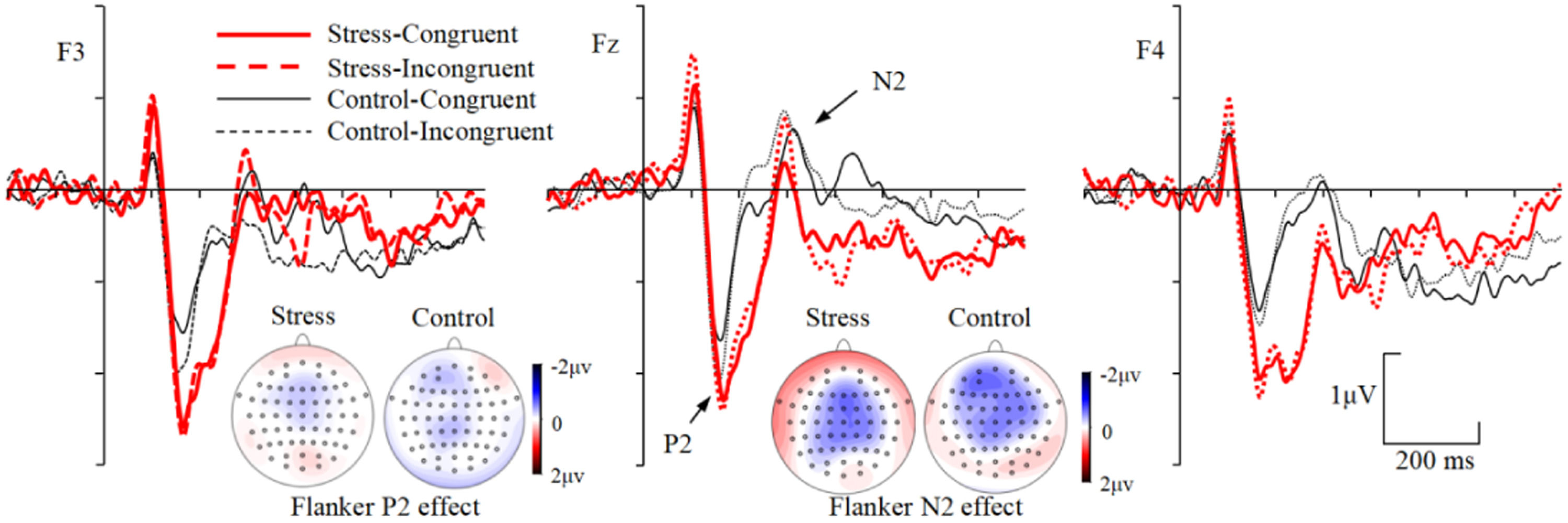
In addition, the maximum voltage and the maximum difference across different conditions for P2 and N2 components were shown over frontal scalp. Therefore, three frontal electrode sites (F3, Fz, F4) were selected for analysis of the P2 and N2 components. The P2 was measured by the mean amplitudes of 150–250 ms time window, and this corresponds to the typical latency range of the P2 (Luck & Hillyard, 1994). The N2 was measured by the mean amplitudes of 250–400 ms time window, which corresponds to the typical latency range of the N2 (Larson et al., 2014; Venetacci et al., 2018; Kopp et al., 1996). Repeated measures ANOVAs with group (stress, control) as between-subject factor, and congruency (congruent, incongruent) and electrode sites (F3, Fz, F4) as within-subject factors were performed on mean amplitudes of P2 (150–250 ms) and N2 (250–400 ms) time windows, respectively.
The maximum voltage and the maximum difference across different conditions for early P3 and late P3/LPC components were shown over central-parietal scalp. Three centro-parietal scalp electrode sites (CP3, CPz, CP4) were selected for analysis of early P3 and late P3/LPC components. The early P3 was measured by the mean amplitudes of 300–400 ms time window, and this corresponds to the typical latency range of the P3 (Polich, 2007). The late P3/LPC was measured by the mean amplitudes of 400–600 ms time window, which corresponds to the typical latency range of the LPC (McKay et al., 2017). Repeated measures ANOVAs with group (stress, control) as between-subject factor, and congruency (congruent, incongruent) and electrode sites (CP3, CPz, CP4) as within-subject factors were performed on mean amplitudes of early P3 (300–400 ms) and late P3/LPC (400–600 ms) time windows, respectively.
ERP Flanker effects were calculated by subtracting the mean amplitudes of congruent trials from those of incongruent trials in each component. Specifically, the Flanker effect of N1 component = N1incongruent – N1congruent. Flanker effect of P2 component = P2incongruent – P2congruent. Flanker effect of N2 component = N2incongruent – N2congruent. Flanker effect of early P3 component = early P3incongruent –early P3congruent. Flanker effect of late P3/LPC component = late P3/LPCincongruent –late P3/LPCcongruent. Repeated measures ANOVAs with group (stress, control) as a between-subject factor and electrode sites as within-subject factors were performed on these ERP Flanker effects.
Pearson's correlation analyses were conducted between the stress variables (PSS, R30 and AUCi) and the Flanker task performance (behavioral Flanker effect and ERP Flanker effects) across all participants.
All effects with more than one degree of freedom were adjusted for sphericity violations using the Greenhouse-Geisser correction. Main effects were followed by Fisher's LSD-corrected pairwise comparisons.
ResultsQuestionnaires and cortisol resultsFor the PSS scores, the levels of perceived stress were higher for the stress than the control group, t(53) = 3.631, p = 0.001, d = 0.983 (Fig. 1a). The levels of positive affect showed no differences between the stress and the control group, t(53) = −0.711, p = 0.480 (Fig. 1b). The levels of negative affect were higher for the stress than the control group, t(53) = 3.027, p = 0.004, d = 0.822 (Fig. 1c). The stress group reported a higher level of state anxiety compared with the control group, t(53) = 3.049, p = 0.004, d = 0.827 (Fig. 1d).
Mean subjective ratings of PSS (a), positive affect (b), negative affects (c), and state anxiety (d) for both the stress and control groups. Mean awakening cortisol levels (e), values of R30 and AUCi (f) for both the stress and control groups. The error bars indicate standard error of the mean. ⁎⁎p < 0.01, ⁎⁎⁎p < 0.001.
For the salivary cortisol concentrations, a significant Group × Time Point interaction was significant, F(3, 159) = 5.492, p = 0.001, ηp2 = 0.101. Simple effects analysis revealed that, 1) for the stress group [F(3, 51) = 10.289, p < 0.001, ηp2 = 0.377], the cortisol level was higher at S2 than that at S1, S3, S4 (ps < 0.012); the levels of cortisol at S3 was higher than that at S1 (p = 0.048) and S4 (p < 0.001); no cortisol level difference was found between S1 and S4 (p = 0.618); 2) for the control group [F(3, 51) = 34.464, p < 0.001, ηp2 = 0.670], higher cortisol level was found for S2 than S1, S3, and S4 (ps < 0.001); the cortisol level was higher for S3 than S1 and S4 (ps < 0.001); the cortisol level was higher for S4 than S1 (p = 0.007); 3) the levels of cortisol were decreased for the stress relative to control group at S2 [F(1, 53) = 14.244, p < 0.001, ηp2 = 0.212], S3 [F(1, 53) = 11.293, p = 0.001, ηp2 = 0.176], S4 [F(1, 53) = 9.718, p = 0.003, ηp2 = 0.155], but not at S1 [F(1, 53) = 0.153, p = 0.697] (Fig. 1e). Compared to the control group, both the R30 [t(53) = −3.995, p < 0.001, d = 1.076] and AUCi [t(53) = −3.545, p = 0.001, d = 0.954] were decreased for the stress group (Fig. 1f).
Behavioral resultsFor the RT of the Flanker task, a main effect of congruency was revealed, F(1, 53) = 406.667, p < 0.001, ηp2= 0.885. The Group × Congruency interaction was also significant, F(1, 53) = 4.977, p = 0.030, ηp2= 0.086. Simple effect analysis revealed that RTs were faster for the congruent than that for the incongruent trials in both control [F(1, 53) = 264.334, p < 0.001, ηp2 = 0.823] and stress groups [F(1, 53) = 163.810, p < 0.001, ηp2 = 0.756]. RTs were faster for the stress (433.98 ms) than the control group (465.59 ms) in incongruent trials, F(1, 53) = 2.357, p = 0.029, ηp2 = 0.086; but no RTs difference were found between the stress and the control groups in congruent trials, F(1, 53) = 5.013, p = 0.131 (Fig. 2). The main effect of group was not significant, F(1, 53) = 3.667, p = 0.061. In addition, the magnitude of the Flanker RT effect was larger for the control (51.27 ms) than the stress group (41.04 ms), t(53) = 2.231, p = 0.030, d = 0.602.
Electrophysiological resultsDuring the N1 (140–220 ms) time window, the main effect of group was significant, F(1, 53) = 7.527, p = 0.008, ηp2 = 0.124, with more negative ERPs for the stress than the control group (Fig. 3). Neither the main effect of congruency nor the interactions including congruency and/or group were significant, Fs < 1.662, ps > 0.203. For the Flanker N1 effect, neither the main effect of group [F(1, 53) = 0.627, p = 0.432] nor the Group × Electrode site interaction [F(3, 159) = 0.661, p = 0.577] were significant.
During the P2 (150–250 ms) time window, the main effect of group was significant, F(1, 53) = 5.011, p = 0.029, ηp2 = 0.086, with more positive ERPs for the stress than the control group (Fig. 4). Neither the main effect of congruency nor the interactions including group and congruency factors were significant, Fs〈 3.167, ps 〉 0.081. For the Flanker P2 effect, neither the main effect of group [F(1, 53) = 0.003, p = 0.957] nor the Group × Electrode site interaction [F(2, 106) = 0.266, p = 0.767] was significant.
During the N2 (250–400 ms) time window, the Group × Congruency × Electrode site interaction was significant, F(2, 106) = 3.149, p = 0.047, ηp2 = 0.056. Simple effects analysis revealed that, 1) for the control group, more negative ERPs were evoked for incongruent than congruent trials at F3 [F(1, 53) = 13.732, p = 0.001, ηp2 = 0.206], Fz [F(1, 53) = 8.306, p = 0.006, ηp2 = 0.135], and F4 [F(1, 53) = 14.334, p < 0.001, ηp2 = 0.213] electrode sites. 2) For the stress group, ERPs were more negative for incongruent than congruent trials at Fz electrode site [F(1, 53) = 11.503, p = 0.001, ηp2 = 0.178], but not at F3 [F(1, 53) = 0.753, p = 0.390] and F4 [F(1, 53) = 1.659, p = 0.203] electrode sites. 3) In incongruent trials, ERPs were more negative for control than stress group at F4 electrode site [F(1, 53) = 4.747, p = 0.034, ηp2 = 0.082], but not at F3 [F(1, 53) = 0.234, p = 0.630] and Fz [F(1, 53) = 1.100, p = 0.299] electrode sites. 4) In congruent trials, no ERP differences were found between groups at all electrode sites [Fs < 1.823, ps > 0.183] (Fig. 4). For the Flanker N2 effect, the Group × Electrode site interaction was significant, F(2, 106) = 3.149, p = 0.047, ηp2 = 0.056. Simple effects analysis revealed that the Flanker effect was increased for the control than stress group at F3 [F(1, 53) = 4.166, p = 0.046, ηp2 = 0.073] electrode site, but not at Fz [F(1, 53) = 0.099, p = 0.754] and F4 [F(1, 53) = 3.253, p = 0.077] electrode sites.
During the early P3 (300–400 ms) time window, the main effect of congruency was significant, F(1, 53) = 6.856, p = 0.011, ηp2 = 0.115, with more positive ERPs for congruent than incongruent trials. The main effect of group was significant, F(1, 53) = 4.330, p = 0.042, ηp2 = 0.076, with more positive ERPs for the stress group than the control group (Fig. 5). No interactions including group and congruency factors were found, Fs 〈 1.929, ps 〉 0.150. For the Flanker P3 effect, neither the main effect of group [F(1, 53) = 0.300, p = 0.586] nor the Group × Electrode site interaction [F(2, 106) = 0.715, p = 0.491] was significant.
During the late P3/LPC (400–600 ms) time window, the Group × Congruency interaction was significant, F(1, 53) = 6.544, p = 0.013, ηp2 = 0.110. Simple effects analysis revealed that, 1) ERPs were more positive for incongruent than congruent trials for stress group [F(1, 53) = 23.033, p < 0.001, ηp2 = 0.303], but not for control group [F(1, 53) = 1.275, p = 0.264]. 2) ERPs were more positive for stress group than the control group in the incongruent trials [F(1, 53) = 4.401, p = 0.041, ηp2 = 0.077] but not in the congruent trials [F(1, 53) = 1.603, p = 0.211] (Fig. 5). For the Flanker LPC effect, the main effect of group was significant, F(1, 53) = 6.544, p = 0.013, ηp2 = 0.110, with larger Flanker effect for the stress than the control group. No Electrode site × Group interaction was found, F(2, 106) = 1.987, p = 0.142.
Correlation analysis revealed that PSS was positively correlated with the Flanker LPC effect at the CP3 electrode (r = 0.271, p = 0.046). Both the R30 and AUCi were positively correlated with the behavioral Flanker effect (R30: r = 0.507, p < 0.001; AUCi: r = 0.592, p < 0.001). No other significant relationships between the stress variables and the Flanker task performance were found (ps > 0.056).
DiscussionIn this study, we investigated the effect of chronic academic stress on attentional control by adopting the Flanker task, while recording behavioral and EEG data. In line with the findings of previous studies (Gao et al., 2022; Wu et al., 2014; Yuan et al., 2016), the stress group showed increased scores of PSS, SAI and negative affect for the stress than the control group, suggesting increased levels of perceived stress, state anxiety, negative affect for the stress group. For the CAR, the levels of cortisol were decreased for the stress relative to the control group. In addition, decreased R30 and AUCi were observed for the stress group compared to the control group. This is in line with the finding of Duan et al. (2013) who found decreased CAR for participants under chronic stress than controls. CAR might be suppressed under chronic stress. These results suggested that the NPEE can induce chronic academic stress.
Consistent with the previous studies (Qi & Gao, 2020; Shields et al., 2019), faster RTs were observed in congruent than that in incongruent trials (i.e., distractor interference) in both the stress and the control group. The RTs were faster for the stress than the control group in incongruent trials, while no RTs differences were found in congruent trials, resulting in a decreased Flanker effect for the stress group. Some studies suggested that smaller Flanker effect on RT might be associated with decreased response conflict (conflict monitoring and resolution), which is achieved through enhanced top-down attentional control (Eriksen & Eriksen, 1974; Wei & Zhou, 2020). In this study, the decreased Flanker effect on RT for the stress than the control group indicated that the attentional control might be improved under chronic academic stress. Furthermore, the Flanker effect on RT was positively correlated with the CAR. The participants with decreased CAR showed enhanced attentional control. Chronic academic stress was found to disrupt the CAR, and a decreased CAR predicts an increased stress level (Duan et al., 2013). The present result further indicated that attentional control may be improved under chronic academic stress.
The ERP results showed that the posterior N1 was more negative for the stress compared to the control group in both congruent and incongruent trials. The N1 component has been associated with the operation of a discrimination process within the focus of attention, and was thought to reflect orienting and discrimination processes that operate via enhancement of incoming information (Fu et al., 2005; Hamilton et al., 2014; Rojas-Thomas et al., 2023). In line with this view, the increased N1 amplitude for the stress group than the control group indicated that the general attentional vigilance might be enhanced under chronic academic stress. The P2 amplitude was more positive for the stress than the control group in both congruent and incongruent trials. The P2 component has been associated with the selective attention process triggered by the task-relevant stimulus, and the enhanced P2 indicated that more amount of attentional resources were allocated to the target stimuli (Li et al., 2023; Luck & Hillyard, 1994; Zhou et al., 2019). The present results reflected that more amount of attentional resources might be allocated on processing of the visual stimuli under stress.
The N2 component was associated with the conflict monitoring process (Larson et al., 2014; Lin et al., 2021; Wei et al., 2022). A larger N2 amplitude has been associated with a more effective focus on task-irrelevant information than task-relevant information, as well as a higher cognitive demand required for the task (Danielmeier et al., 2009; Dubreuil-Vall et al., 2019). The N2 amplitude was more negative for the incongruent than the congruent trials in both control and stress groups, indicating an increased conflict monitoring process for the incongruent than congruent trails. Importantly, the N2 amplitude was less negative for the stress than the control group in the incongruent trials but not in the congruent trials, resulting in a reduced Flanker N2 effect for the stress group. These results indicated that a reduced conflict might be detected for the stress compared to the control group.
Polich (2007) posited that the modulation of P3 is attributed to inhibitory processes involved in avoiding irrelevant information and in focusing attentional resources on the relevant information. Larger early P3 amplitude has been associated with greater cognitive resource allocation for target processing (Alguacil et al., 2013). Compared to the congruent trials, it is more difficult to resolve the conflict in the incongruent trials, and less cognitive resources available for the target processing. Consequently, the incongruent condition generates smaller early P3 amplitude (Alguacil et al., 2013; Neuhaus et al., 2010; Wei & Zhou, 2020). In this study, the early P3 amplitude was decreased for the incongruent than the congruent trials in both the control group and the stress group, indicating less attentional resources were allocated to the target during processing incongruent trials. The early P3 amplitude was more positive for the stress than the control group in both congruent and incongruent trials, suggesting a potential increase in attentional resources available for target processing under stress.
The late P3/LPC amplitudes were more positive for the incongruent than the congruent trials in the stress group, but not in the control group. The late P3/LPC component has been termed as conflict sensitive slow potential, and larger late P3/LPC amplitudes might reflect enhanced conflict resolution process (Alguacil et al., 2013; Larson et al., 2009; McKay et al., 2017). The present results might indicate that an increased conflict resolution process for the incongruent relative to the congruent trials under stress, but conflict resolution was weak for both the incongruent and congruent trials in the control group. The late P3/LPC was more positive for stress than control group in the incongruent but not in the congruent trials, resulting in a larger Flanker late P3/LPC effect for the stress group. In this study, the conflict resolution process might be increased for the stress relative to the control group. Additionally, the correlation analysis revealed that PSS was positively correlated with the Flanker LPC effect, further suggesting that conflict resolution was heightened with increasing stress levels. The N2, early P3, and late P3/LPC results suggested that stress group showed low but effective level of conflict processing compared to the control group. This is consistent with the behavioral performance showing that the Flanker RT effect was decreased in the stress group than the control group. The distractor interference was attenuated under chronic stress.
Sandi (2013) suggested that the chronic stress improved performance of simple tasks, or when the cognitive load is not excessive. In this study, the Flanker task was relatively simple for the participants, and superior performance was found for the stress group than the control group. The present results could be explained by the adaptation model that chronic academic stress might reallocate limited cognitive resources in adaptive ways. Specifically, cognitive resources are allocated to the task-relevant cognitive processing, such as target processing and conflict resolution process, rather than to task-irrelevant information under chronic academic stress.
Previous studies suggested that the participants might have experienced increases in motivation during the period of examination preparation and chronic academic stress arises when positive motivation is needed (Qi et al., 2023; Wu et al., 2014). Consistent with this view, Park et al. (2012) reported that academic motivation is one the key factors contributing to chronic academic stress. Therefore, chronic academic stress and academic motivation might be affected by each other and it might be difficult to disentangle them from one another (Wu et al., 2014). The superior performance for the stress group might also be associated with the higher motivation (Qi et al., 2023; Wu et al., 2014). Future studies could investigate the interaction between chronic academic stress and motivation on attentional control.
It is worth to note that the intensive studying may also be a potential confounding factor in evaluating the effects of stress on attentional control. The stress group may have been studying harder to pass the NPEE. Motes et al. (2014) have found that one-month cognitive strategy training improves the inhibitory control for middle school students. Sari et al. (2016) found that a three-weeks working memory training improved high trait anxious participants’ distractor inhibition ability particularly when they were performing the task under stress. In our study, the stress group could be considered as engaging in intensive cognitive training through the prolonged studying periods each day, which may account for their improved performance in attentional control. While higher level of stress was found to be positively correlated with enhanced conflict resolution process, there is a need for further investigation into potential correlations between other factors (e.g., motivation level or study intensity) and the task performance under chronic academic stress.
Some limitations have to be mentioned in our study. First, the participants in the stress group were senior students who were preparing the NPEE. Considering that senior students may face other stressors (i.e., job interview), the participants in the control group was comprised mostly of sophomores and senior students. As a result, the participants in the control group were about a year younger than those in the stress group. Second, to avoid the influence of sleep duration on CAR, we asked participants to sleep 6–8 hour the night before saliva collection. However, the participants self-reported their sleep duration without the use of additional monitoring devices.
In conclusion, the present study found that the stress group showed a higher level of self-reported stress and decreased CAR compared to the control group, indicating chronic academic stress was induced by the NPEE. For the Flanker task, the chronic academic stress promotes the vigilance and selective attention to the target (increased N1 and P2 activity), and improves attentional control (decreased Flanker RT effect) by reducing the conflict monitoring (decreased Flanker N2 effect) and enhancing the conflict resolution process (increased early P3 activity and Flanker late P3/LPC effect).
Open practices statementThe data are accessible online (via OSF) at https://doi.org/10.17605/OSF.IO/8WNDS
CRediT authorship contribution statementMingming Qi: Funding acquisition, Writing – original draft, Writing – review & editing. Ru Gai: Formal analysis, Writing – review & editing. Yuxi Wang: Formal analysis, Writing – review & editing. Heming Gao: Funding acquisition, Investigation, Supervision, Writing – review & editing.
This work was supported by the Humanities and Social Science Fund of Ministry of Education of China (23YJC190018), and the Basic Scientific Research Foundation of the Educational Department of Liaoning Province (LJKMZ20221421), and National Natural Science Foundation of China (32100849).