Transcranial magnetic stimulation-electroencephalography (TMS-EEG) is a powerful technique to study the neuropathology and biomarkers of major depressive disorder (MDD). This study investigated cortical activity and its relationship with clinical symptoms and cognitive dysfunction in MDD patients by indexing TMS-EEG biomarkers in the dorsolateral prefrontal cortex (DLPFC).
Methods133 patients with MDD and 76 healthy individuals participated in this study. Single-pulse TMS was performed on the left DLPFC to obtain TMS-evoked potential (TEP) indices. TMS-EEG waveforms and components were determined by global mean field amplitude. We used the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) to measure participants’ cognitive function.
ResultsPatients with MDD had a lower excitatory P180 index compared to healthy controls, and P180 amplitude was negatively correlated with the severity of depressive and anxiety symptoms in patients with MDD. In the MDD group, P30 amplitude was negatively associated with RBANS Visuospatial/ Constructional index and total score.
ConclusionsTMS-EEG findings suggest that abnormal cortical excitation and inhibition induced by TMS on the DLPFC are associated with the severity of clinical symptoms and cognitive dysfunction in patients with MDD. P180 and P30 have the potential to serve as neurophysiological biomarkers of clinical symptoms and cognitive dysfunction in MDD patients, respectively.
Major depressive disorder (MDD) is a prevalent mental illness defined by persistent affective, behavioral, and cognitive dysfunction (Subhas et al., 2023). The World Health Organization estimates that approximately 3.8 % of the global population suffers from depression (World Health Organization, 2023). MDD involves complex neuropathological mechanisms, including changes in neuronal structure, neurotransmission, and brain network connectivity. Notably, it has been demonstrated that the dorsolateral prefrontal cortex (DLPFC) is essential to the pathophysiology of MDD. Hypofunction of the left DLPFC in individuals with MDD has been found in different study modalities, such as neurotransmitter (Tran et al., 2023), functional imaging (Shen et al., 2015), and electroencephalography (EEG) (Kamishikiryo et al., 2022) studies. A recent meta-analysis showed that the left DLPFC is a node where placebo effect mechanisms and neuromodulatory anti-depressant mechanisms overlap, indicating that changes in the left DLPFC play a vital role in the treatment of MDD (Burke et al., 2022). In addition, MDD patients often present with anxiety symptoms, which may have a profound impact on the condition. It is also suggested that the DLPFC is involved in anxiety regulation through working memory processes (White et al., 2023). How the DLPFC contributes to the neuropathology of MDD patients warrants further exploration.
Recently, cognitive dysfunction has been recognized as one of the core features of MDD patients, involving abnormalities in the DLPFC, genomic variation, brain-derived neurotrophic factor (BDNF), and inflammatory mediators (Fossati, 2018; Morozova et al., 2022). The main domains of cognitive deficits in MDD patients include executive function, attention, and memory (Kriesche et al., 2023). Specifically, the DLPFC is considered part of the central executive network and plays a vital role in cognitive dysfunction. An imaging study found reduced functional connectivity in the left DLPFC and enhanced functional connectivity in the right DLPFC, suggesting that an imbalance between the left and right DLPFC is linked to cognitive dysfunction in MDD patients (Zhang et al., 2022). Another mechanism by which the DLPFC is involved in cognitive deficits in MDD patients is an imbalance between excitatory and inhibitory neurotransmission. Numerous studies have shown that the imbalance between excitation and inhibition in the DLPFC cerebral cortex is related to the severity of depressive symptoms (Biermann et al., 2022; Dhami et al., 2023). Notably, higher levels of activity in the left DLPFC in MDD patients may be a compensatory mechanism for the inactivation of the default mode network (DMN), which has been linked to impaired working memory and emotion regulation (Chen et al., 2023). However, further study is needed to elucidate the implications of excitatory and inhibitory modes of the DLPFC on symptoms and cognitive functioning in patients with MDD.
Transcranial magnetic stimulation (TMS) is a non-invasive brain stimulation therapy effective for MDD patients. TMS combined with EEG (TMS-EEG), enables the assessment of cortical evoked activation in the DLPFC. TMS-EEG is a powerful tool for investigating the causal role of cortical activation in mental processes. TMS evoked potentials (TEPs), including P30, N45, P60, N100, and P180, offer a window for exploring the localized neural assemblies of the DLPFC's electrical and organizational properties (Kallioniemi & Daskalakis, 2022). These components are associated with the excitatory glutamate system and the inhibitory γ-aminobutyric acid (GABA) system, reflecting the inhibitory-excitatory balance in neural circuits (Kallioniemi et al., 2022). For example, pharmacological studies have shown that P30, N45, and P180 amplitudes are associated with voltage-gated sodium channels (VGSCs) (Darmani et al., 2019; Kallioniemi & Daskalakis, 2022). P30 is thought to be mediated by GABAA receptors rather than glutamatergic N-methyl-d-aspartate (NMDA) receptor-mediated neurotransmission (Ferreri et al., 2011; Rogasch et al., 2020). P60 is thought to be linked to glutamatergic activity (Belardinelli et al., 2021). N45 and N100 are associated with inhibition involving GABAergic neurotransmission, reflecting GABAA and GABAB receptor activation, respectively (Premoli et al., 2014a; Rogasch et al., 2015). In addition, Global Mean Field Amplitude (GMFA) has been used to acquire the TEP component of the whole scalp to reduce the bias caused by limiting electrodes (Poorganji et al., 2023).
TMS-EEG has attracted increasing attention as a well-validated technique to study neuroplasticity in the DLPFC of MDD patients (Farzan, 2023). A cross-sectional study using GMFA showed that N45 amplitude holds tremendous potential as a neurophysiological biomarker of the DLPFC in detecting depression (Voineskos et al., 2019). The N100 component of the right DLPFC was also significantly predictive of MDD in adolescents (Dhami et al., 2020). Longitudinal studies have also utilized TMS-EEG biomarkers to predict the efficacy of various physical therapies for depression and to explain their neuroplasticity mechanisms, such as magnetic seizure therapy (Hadas et al., 2020) and intermittent theta pulse stimulation (Strafella et al., 2023). In addition, TMS-EEG has been used to explore neural biomarkers of cognitive function associated with the prefrontal cortex. Previous studies have found that TMS-evoked local cortical responses following stimulation of the left PFC are positively correlated with working memory and reasoning ability (Redondo-Camos et al., 2022). TMS-EEG studies have demonstrated abnormal excitability and functioning of the DLPFC in Alzheimer's disease (AD) patients, which have been associated with overall cognitive and executive functioning (Casarotto et al., 2011; Di Lazzaro et al., 2021; Joseph et al., 2021). However, there is still a lack of TMS-EEG biomarkers to predict cognitive deficits in MDD patients (Ferrarelli & Phillips, 2021). Identifying TMS-EEG biomarkers that predict symptom severity and cognitive deficits in MDD patients is vital for clinical diagnosis and treatment.
Given the critical role of DLPFC abnormalities in clinical symptoms and cognitive impairment of MDD patients, it is valuable to explore their neurophysiological mechanisms and biomarkers using TMS-EEG. To date, there is limited literature that simultaneously explores the relationship between TMS-EEG markers and symptom severity and cognitive impairment of patients with MDD. The present study aimed to (1) investigate the differences in DLPFC activity between MDD patients and healthy controls as measured by TMS-EEG, and (2) identify neurophysiological markers predicting clinical symptoms and cognitive function in MDD patients. We hypothesized that (1) TMS-EEG indexes would reflect hypofrontality in patients with MDD, and (2) TMS-EEG biomarkers would relate with the severity of clinical symptoms and cognitive deficits in MDD patients.
MethodsParticipantsBetween January 2022 and June 2023, 133 patients with MDD were recruited at Ningbo Kangning Hospital. All patients were right-handed. Inclusion criteria were (a) age 16 to 65 years; (b) fulfillment of DSM-5 diagnostic criteria for MDD; and (c) score ≥ 20 points on the Hamilton Depression Rating Scale-24 (HDRS-24). Exclusion criteria included (a) comorbidity with other psychiatric illnesses or neurological impairments; (b) the presence of serious medical conditions such as cardiovascular disease, immune system disorders, and infectious diseases; and (c) a history of substance dependence other than nicotine dependence. Two trained psychiatrists assessed all patients to determine whether they met the study criteria.
We also recruited 76 healthy individuals from the local community as a control group. Inclusion (16–65 years old) and exclusion (those with any history of psychiatric disorders, physical diseases, and substance or alcohol addiction) of healthy controls were done by trained researchers.
The study was authorized by the Ethics Committee of Ningbo Kangning Hospital and complied with the Declaration of Helsinki regarding informed consent and confidentiality. All participants voluntarily took part in this study and completed an informed consent form after the researchers introduced the study procedure in plain language.
Clinical assessmentThe HDRS-24 was used to measure patients' current depressive episodes (Schwab et al., 1967). The Hamilton Anxiety Rating Scale-14 (HARS-14) was applied to assess the patients' anxiety symptoms (Hamilton, 1959). In addition, we used the Chinese edition of the Repeated Battery for the Assessment of Neuropsychological Status (RBANS) to measure participants' cognitive functioning. The RBANS consists of five subscales: immediate memory, delay memory, language, attention, and visuospatial/ constructional (Randolph et al., 1998). The scale was translated by our team and has shown adequate psychometric properties in Chinese populations (Zhang et al., 2008). In the present sample, the Cronbach's α for this scale was 0.81. Two psychiatrists conducted these assessments after consistent training for reliability, with the Intra-class Correlation Coefficient (ICC) exceeding 0.8.
TMS procedureThe left DLPFC region was stimulated with 100 single-pulse TMS stimuli by connecting a figure-of-8 coil and a TMS stimulator (Magstim Rapid2, UK). To locate the left DLPFC, the coil was fixed on F3. The handle of the splay lock was 45° slanted back and perpendicular to the scalp. The TMS pulse waveform used in our study conformed to the standard biphasic waveform typically applied in TMS studies (Supplementary Fig. S1). This waveform consisted of an initial phase followed by a second phase of opposite polarity, ensuring balanced stimulation and minimal residual charge. The direction of the induced current during stimulation is shown in Supplementary Fig. S2. The current generated by the coil flows in a specified direction, ensuring precise targeting of the cortical area. Before the TMS-EEG session, a resting motor threshold (RMT) test was performed on the left motor cortex to measure stimulus intensity. The RMT is the lowest intensity of stimulus that elicits a significant motor response in the right abductor pollicis brevis. While the participant was fully relaxed, we gradually adjusted the TMS stimulation intensity. Typically, the RMT elicited a motor-evoked potential (MEP) greater than 50 mV on at least 50 % of the trials (5 out of 10). We employed a stepwise approach, starting at a lower intensity and increasing by 1-2 % until the threshold was reached. The interval between stimuli was 5 s ± 10 % jitter, and the stimulus intensity was 110 % of RMT.
EEG recording and analysisTMS-evoked potentials (TEPs) were recorded in a soundproofed, temperature-regulated, and electrically shielded room with a TMS-compatible 64-channel cap (Easycap, Germany) and a BrainVision Recorder (BrainProducts, Germany). The electrodes were grounded to AFz and referenced to FCz. The electrode impedance was kept lower than 5 kΩ during the recording, and the sampling rate was set to 25 kHz. During stimulation, participants kept their eyes open and wore earmuffs to prevent the appearance of associated auditory evoked potentials. We also placed a foam layer between the coil and the head to reduce noise (ter Braack et al., 2015).
TMS-EEG processing was conducted with EEGLAB (Delorme & Makeig, 2004), FieldTrip (Oostenveld et al., 2011), and customized MATLAB scripts (R2022b, The MathWorks, Inc.). Data containing TMS pulses (-5 to 15 ms) were first removed and recovered with linear interpolation. Later, the data were downsampled to 1 kHz, baseline corrected (-550 to-200 ms), and optimized for the TMS stimulus pulse (-2000 to 2000 ms). Extreme noise in the data was visually inspected (e.g., muscle movement, electrode damage, etc.) (Rogasch et al., 2013). We initially performed Fast Independent Component Analysis (FastICA) to automatically eliminate TMS tails and significant attenuation artifacts. In the first round of ICA, the average number of components removed per participant was 15.8. Bandpass filtering (1–100 Hz) was used to remove high-frequency noise and drift in the data. Then a band reject (48, 52 Hz) filter removed 50 Hz alternating current line artifacts. A second phase of ICA was conducted to remove remaining artefacts (blinks, eye movements, sustained muscle artefacts). The average number of components removed per participant in the second ICA round was 8.2. The cleared data were re-referenced to the mean for further investigation. EEG data analysis and artifact elimination were referred to Rogasch et al. (2017).
GMFA analysisGMFA was computed for each participant using an equation developed by Lehmann and Skrandies (1980).
GMFA determines the largest amplitude of evoked electric fields and quantifies the whole brain's neurophysiological responses to TMS-EEG (Farzan et al., 2013). We calculated the amplitude of the GMFA component peaks for each participant. The time window for each component was determined based on previous studies (P30: 25 to 35 ms; N45: 40 to 50 ms; P60: 50 to 70 ms; N100: 80 to 120 ms; P180: 160 to 200 ms). The area under the GMFA curve (GMFA-AUC) was applied to probe the late N100-P180 complex. It was determined by summing the amplitudes 150–210 ms following the TMS pulse.
Statistical analysisEach generated data was analyzed using SPSS for Windows (IBM Corporation, Armonk, NY, version 19.0). Descriptive statistics were used to show demographic and clinical assessment results. We used χ2 tests for categorical variables and the Kolmogorov-Smirnov test to check for the normality of continuous variables. Then, we logarithmically transformed non-normally distributed variables into normally distributed variables. Analysis of covariance (ANCOVA) was conducted to examine for between-group differences in demographic, GMFA components, and clinical variables, using years of education as a covariate. We also performed Pearson correlation analyses between GMFA components and clinical variables, including HDRS, HDRA, and RBANS. Further multiple linear regression analyses were performed to predict the clinical features of the MDD group. Specifically, these analyses used the HDRS, HDRA, and RBANS total or index scores as dependent variables and multiple variables as predictors, including TMS-EEG markers, age at onset, and years of education. The variance inflation factor (VIF) was introduced to evaluate multicollinearity between independent variables. All analyses applied a two-sided significance level (p < 0.05).
ResultsDemographic and clinical characteristicsAfter EEG cleaning, 124 patients with MDD and 71 healthy controls were retained. Table 1 presents the demographic information and clinical results of these study participants. The healthy group had higher years of education than the MDD group (P < 0.001). Gender, age, and BMI were not considerably different between the two groups. For the clinical tests, before controlling for years of education, the MDD group had worse cognitive functioning than the healthy group concerning the RBANS total score and all subscale scores (P < 0.001). However, the difference of immediate memory was insignificant after controlling for years of education (p = 0.13, see Table 1). After Bonferroni correction, only the differences in RBANS language and total scores remained significant. The MDD group exhibited significantly higher HDRS and HARS scale scores than the healthy group (P < 0.001). The current medications of the MDD patients are summarized in Table 1.
Demographics and clinical information of healthy controls and MDD patients, and current medications of MDD patients.
| Healthy Controls(n = 71) | MDD Patients(n = 124) | F/χ2 | p | |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 32.55 ± 9.33 | 30.58 ± 15.02 | 0.994 | 0.320 |
| Female | 43(60.56 %) | 82(67.21 %) | 0.87 | 0.351 |
| Education (years) | 14.42 ± 2.71 | 10.89 ± 3.19 | 60.98 | <0.001 |
| Body Mass Index (BMI) | 22.19 ± 2.86 | 23.03 ± 11.95 | 0.311 | 0.578 |
| Clinical | ||||
| RBANS score | ||||
| Immediate Memory | 92.45 ± 14.51 | 81.90 ± 16.24 | 2.246 | a0.136 |
| Visuospatial/ Constructional | 103.43 ± 12.02 | 94.77 ± 15.31 | 4.037 | a0.046 |
| Language | 101.48 ± 10.74 | 92.37 ± 14.06 | 8.758 | a0.003 |
| Attention | 113.49 ± 12.75 | 101.18 ± 15.07 | 4.558 | a0.034 |
| Delayed Memory | 96.80 ± 11.08 | 88.07 ± 14.97 | 4.484 | a0.036 |
| Total score | 101.48 ± 10.93 | 88.64 ± 13.29 | 13.237 | a < 0.001 |
| HDRS score | 4.38 ± 1.91 | 24.67 ± 4.89 | 768.15 | a < 0.001 |
| HARS score | 3.39 ± 1.39 | 15.60 ± 6.37 | 161.51 | a < 0.001 |
| Episode Duration (months) | / | 47.69 ± 55.80 | ||
| Age at Onset (years) | / | 24.56 ± 13.32 | ||
| History of Suicide | / | 42(34.7 %) | ||
| Medications | ||||
| Escitalopram | / | 72 | ||
| Fluoxetine | / | 9 | ||
| Sertraline | / | 17 | ||
| Venlafaxine | / | 21 | ||
| Duloxetine | / | 5 |
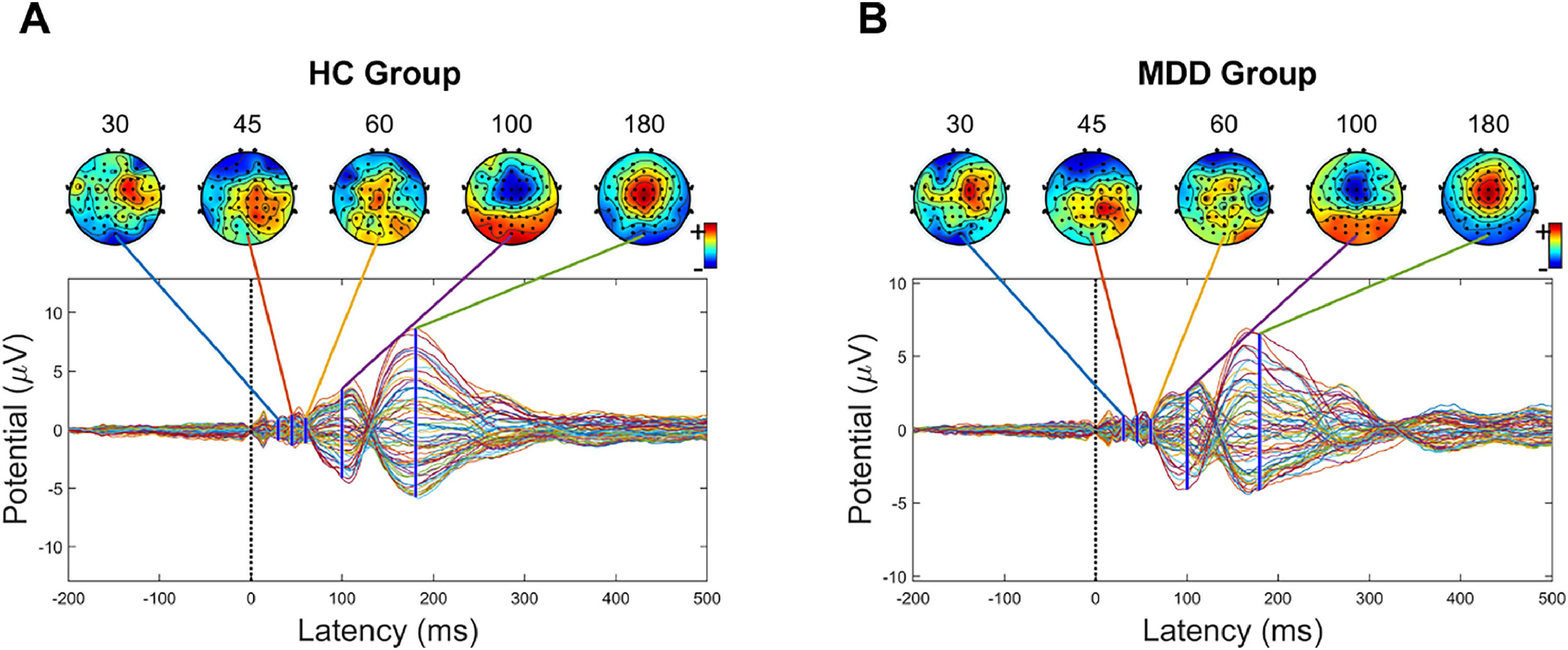
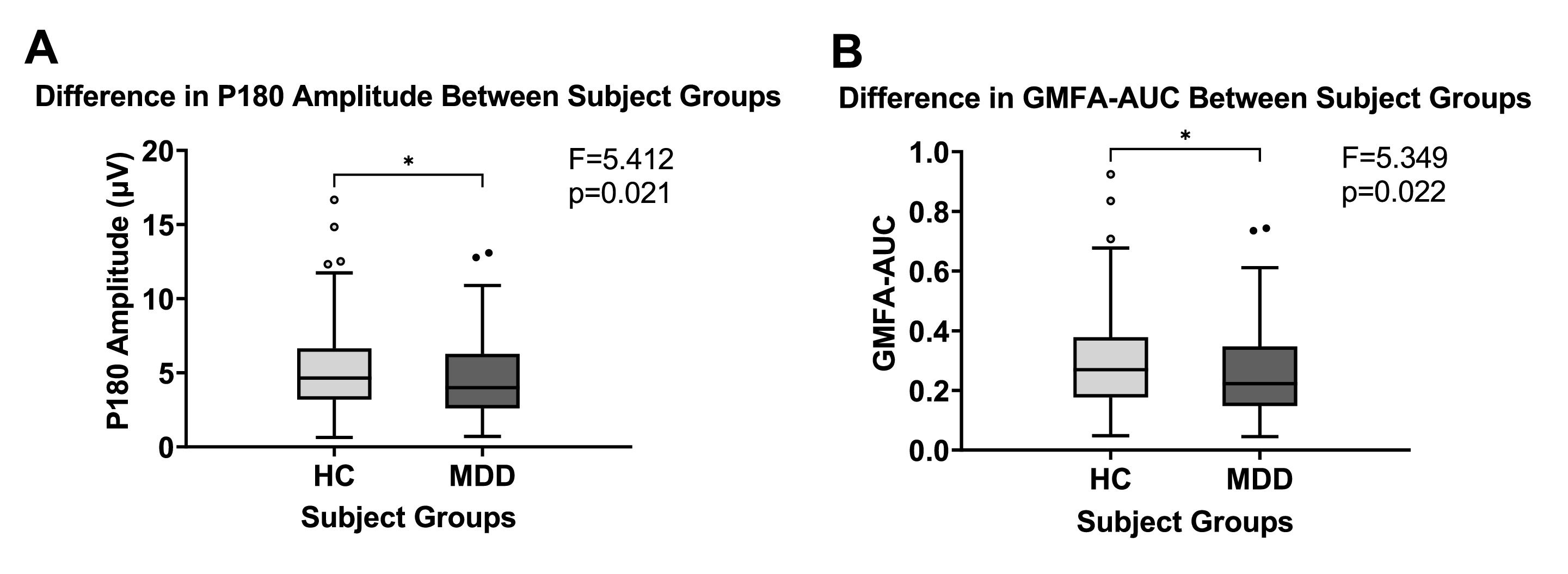
Fig. 1 shows the butterfly plot of TEPs waveform from TMS-EEG for healthy controls and MDD patients, respectively. Fig. 2 depicts the mean GMFA curves of the healthy and MDD groups. The mean P180 amplitude was considerably larger in the healthy group than the MDD group (F = 5.412, p < 0.05, η2 = 0.027; Fig. 3A). GMFA-AUC was also considerably greater in the healthy group than in the MDD group (F = 5.349, p < 0.05, η2 = 0.027; Fig. 3B). However, these results were not significant after Bonferroni correction. The P30, N45, P60, and N100 components showed no considerable difference between the two groups.
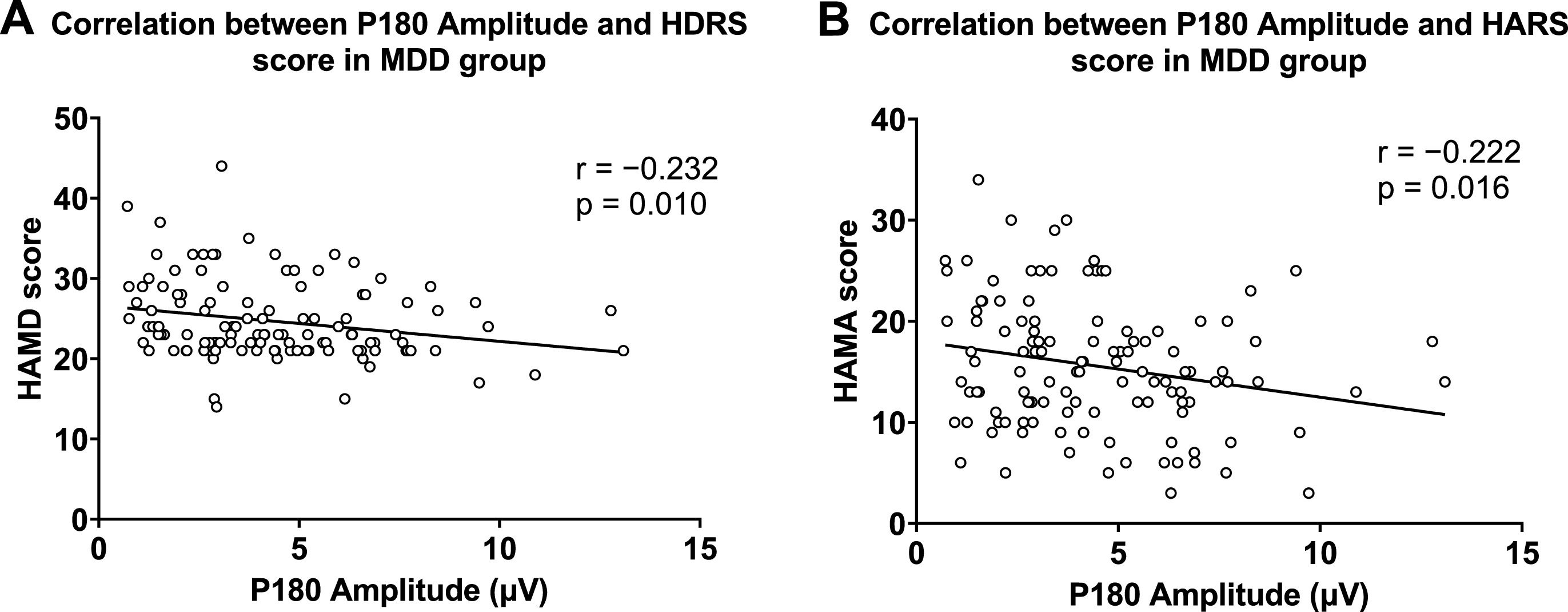
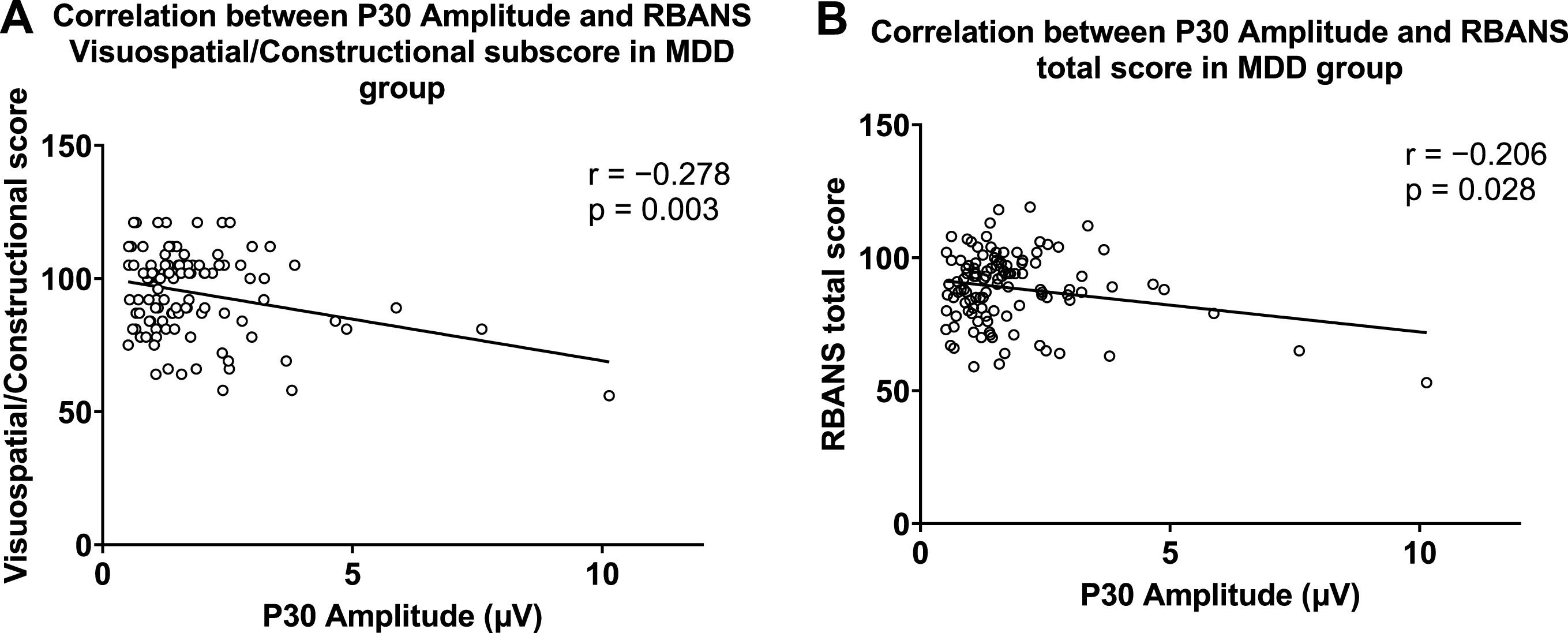
In correlation analysis, P180 amplitude was negatively correlated with HARS and HDRS scores in the MDD group (Fig. 4). In the MDD group, P30 amplitude was negatively associated with Visuospatial/ Constructional and total scores of the RBANS (Fig. 5). These results remained significant after Bonferroni correction. In the healthy group, no significant correlation was observed between GMFA components and cognitive function.
We further used linear regression analyses to explore the association between the GMFA components, cognitive function, and clinical symptoms in the MDD group. In terms of cognitive function, P30 amplitude (β = −0.21, t = −2.22, p = 0.02), age at onset (β = −0.36, t = −3.52, p = 0.001), and years of education (β = 0.33, t = 3.23, p = 0.002) were correlated with the total RBANS score. P30 amplitude (β = −0.28, t = −3.04, p = 0.003), age at onset (β = −0.35, t = −3.14, p = 0.002), and years of education (β = 0.25, t = 2.73, p = 0.007) were also correlated with the Visuospatial/ Constructional score. After Bonferroni correction, the association between P30 and RBANS total score was not significant.
For clinical symptoms, P180 was independently associated with HDRS score (β = −0.23, t = −2.58, p = 0.011) and HARS score (β = −0.22, t = −2.45, p = 0.016). The GMFA-AUC was independently associated with HDRS score (β = −0.22, t = −2.54, p = 0.012) and HARS score (β = −0.22, t = −2.44, p = 0.016). However, after Bonferroni correction, these results were not significant.
DiscussionIn our previous study, we found that P60 was lower in MDD patients than in healthy controls and was negatively correlated with the severity of depression (Li et al., 2023). Based on these important results, we independently recruited a larger sample using GMFA measures, focusing on exploring the relationship between TMS-EEG markers, clinical symptoms, and cognitive deficits in MDD patients. As the previous study did not collect any data on cognitive deficits, we did not use the overlapping dataset.
To our knowledge, this study is the first to examine the association between TMS-EEG components and cognitive function in MDD patients. This study confirmed the abnormalities of cortical excitability in MDD patients and provided evidence for its correlation with clinical symptoms and cognitive function. The key findings of our study were as follows: (1) P180 amplitude was lower in MDD patients than healthy controls. (2) In the MDD group, P180 amplitude was negatively correlated with depressive and anxiety symptoms. (3) Patients with MDD showed significant neurocognitive deficits than the healthy group. In the MDD group, P30 amplitude was negatively correlated with RBANS Visuospatial/ Constructional and total scores.
Our study found that P180 was smaller in MDD patients compared to healthy controls. Although the physiological mechanisms of P180 are unknown, several studies have suggested that it may be modulated by axonal excitability (Premoli et al., 2017b). One study found that P180 late activity was especially susceptible to VGSC blockade (e.g., carbamazepine) (Darmani et al., 2019). A recent review has also shown that antiepileptic and excitability-lowering drugs significantly reduce P180 component amplitude in epileptic patients and healthy individuals (Gefferie et al., 2023). Indeed, the Na+ channel system plays a crucial role in glutamate release, and there is growing evidence that levels of glutamate metabolites are decreased in the prefrontal cortex and medial frontal cortex of MDD patients (Kantrowitz et al., 2021; Moriguchi et al., 2019). Lower levels of P180 may indicate abnormal glutamatergic neurotransmission in patients with MDD. In addition, GMFA-AUC was also smaller in the late component of the MDD group, suggesting reduced cortical excitability associated with P180. However, we failed to find significant results for N45, P60, and N100, which is inconsistent with previous studies (Dhami et al., 2020; Voineskos et al., 2019). The reason for this discrepancy could be differences in sample size, medication regimen and disease duration.
Furthermore, our findings showed that P180 amplitude was negatively correlated with depression and anxiety symptoms in MDD patients. Prior studies have shown that long-interval intracortical inhibition (LICI) mediated by GABAB receptors significantly reduces P180 amplitude (Premoli et al., 2014b). Based on the above evidence, P180 can be used to indicate cortical excitability. Our findings suggest that low excitability of the DLPFC is correlated with depression and anxiety symptoms in MDD patients, which aligns with recent studies (Pilisi et al., 2020; Yosephi et al., 2019). It was suggested that the similar clinical phenotypic of MDD and anxiety symptoms may depend on shared prefrontal alterations (Eleonora et al., 2019). These alterations may be related to glutamatergic and GABAergic-mediated excitation-inhibition balance. However, one study found that P180 did not respond to GABAergic drugs, suggesting that P180 may not be under the direct control of GABAergic neurons (Premoli et al., 2017a). Due to the lack of neuropharmacological studies on P180, we could not determine its neural mechanisms.
More importantly, our findings demonstrated that cognitive dysfunction was prevalent in patients with MDD and found that P30 amplitude was negatively associated with the level of cognitive functioning. The main types of cognitive dysfunction in MDD patients included attention, language, memory, and visuospatial/constructional dysfunction. Recent studies have shown that high P30 amplitude predicts cognitive and memory decline in AD patients, suggesting that the strength of connectivity between the left DLPFC and the right superior parietal cortex is associated with low cognitive function (Bagattini et al., 2019). Our findings on P30 and visuospatial functioning in patients with MMD appear to support this view, as the parietal cortex plays a crucial role in spatial cognitive functioning (Husain & Nachev, 2007). Enhanced prefrontal-to-parietal connections may be the result of a compensatory mechanism for the decline in parietal connectivity and function; however, this compensation is considered pathological and not effective in avoiding spatial cognitive deficits in patients (Bagattini et al., 2019; Pievani et al., 2014). Notably, recent research have found that short-range positive functional connectivity is reduced in the right superior parietal cortex in patients with MDD (Zhang et al., 2023). This evidence suggests that there may be some similarities in the pathology of visuospatial cognitive deficits in patients with MDD and AD.
In addition, previous studies have suggested that GABAA receptors may mediate the formation and regulation of P30 (Ferreri et al., 2011). As one of the major inhibitory neurotransmitters, GABAA controls most of the rapid inhibitory neurotransmission in the brain. Therefore, the negative correlation between P30 and cognitive function may support the idea that cognitive deficits in MDD patients are related to abnormal inhibitory mechanisms. Recent studies have highlighted that low GABAergic inhibition in the prefrontal cortex contributes largely to cognitive impairments in patients with MDD (Luscher et al., 2023). It has been suggested that enhanced dendritic inhibition via α5-GABAA receptor potentiation may have therapeutic effects in patients with memory impairment, age-related cognitive deficits, and depression (Jacob, 2019; Koh et al., 2020). We also found that age at onset was negatively correlated with cognitive function in patients with MDD, which can be linked to abnormalities in the GABA system in the ventromedial prefrontal cortex (Hasler et al., 2005). Thus, our findings may suggest that GABAergic deficits and hyperexcitability of the prefrontal cortex are related with cognitive dysfunction in MDD patients.
The current study has several limitations. First off, due to the cross-sectional design, we were unable to determine causal relationships between GMFA biomarkers and clinical variables. Second, patients were treated with antidepressant medication during the TMS-EEG test, so we cannot rule out the potential effect of antidepressant medication on these results. Future studies should include patients who have not received medication, which may help to address this potential confounder definitively. Third, in this study, patients with MDD and healthy controls were not matched in terms of sample size and education level. Future studies need tighter controls to eliminate the effects of demographic differences. Fourth, computing GMFA does not capture the polarity of the TEP components. Although some studies have used GMFA to determine the polarity of components (e.g., N45, N100) with significant results (Strafella et al., 2023; Voineskos et al., 2021, 2019), this is only an extrapolation based on the TEPs component shown in the butterfly plot and should be viewed with caution. Fifth, because GMFA was used and TMS targeted the DLPFC, the results may reflect a general difference in cortical activity between MDD patients and healthy controls. We were unable to draw conclusions about the specificity of DLPFC. Future studies need to limit the area of interest and include stimulation of a control area to better understand the role of the DLPFC. Finally, as a component of the N100-P200 complex in the TMS-EEG waveform, a portion of P180 is considered an auditory evoked activity evoked by TMS "clicks" (Conde et al., 2019). Based on this view, both groups underwent the same TMS-EEG procedure, performed with auditory masking to eliminate the effects of the 'click'. However, further studies are required to clarify the physiological basis of P180.
In summary, our study using TMS-EEG technology provides evidence for the relationship between abnormal TMS-EEG measurements, clinical symptom severity, and cognitive functioning in patients with MDD. P180 and P30 have the potential to serve as neurophysiological biomarkers of clinical symptoms and cognitive dysfunction, respectively, in MDD patients. This research also demonstrates that cortical excitability, associated with neurotransmission and cortical connectivity, is critical in the pathological process of MDD patients. Nevertheless, further studies are needed to investigate the neurophysiological mechanisms and clinical significance of P30 and P180 in MDD patients.
FundingThis study was funded by STI2030-Major Projects2021ZD0202102, the Basic Public Welfare Project of Zhejiang Province (LGF22H090055), the Medical Health Science and Technology Project of the Zhejiang Provincial Health Commission (2019RC079), and the CAS Key Lab of Mental Health.
CRediT authorship contribution statementDeyang Li: Data curation, Methodology, Formal analysis, Writing – original draft. Xingxing Li: Data curation, Resources, Investigation. Jiaxin Li: Formal analysis, Data curation. Junyao Liu: Formal analysis, Data curation. Ruichenxi Luo: Formal analysis, Data curation. Yanli Li: Formal analysis, Data curation. Dongmei Wang: Conceptualization, Project administration, Writing – review & editing. Dongsheng Zhou: Resources, Supervision. Xiang-Yang Zhang: Project administration, Funding acquisition, Writing – review & editing.
The authors would like to thank all the participants who participated in this study. We would also like to thank the clinical psychiatrists for their significant contributions to this study.