Background/Objective: Colorectal and gynecologic cancer survivors are at cardiovascular risk due to comorbidities and sedentary behaviour, warranting a feasible intervention to increase physical activity. The Health Action Process Approach (HAPA) is a promising theoretical framework for health behaviour change, and wearable physical activity trackers offer a novel means of self-monitoring physical activity for cancer survivors. Method: Sixty-eight survivors of colorectal and gynecologic cancer will be randomised into 12-week intervention and control groups. Intervention group participants will receive: a Fitbit Alta™ to monitor physical activity, HAPA-based group sessions, booklet, and support phone-call. Participants in the control group will only receive the HAPA-based booklet. Physical activity (using accelerometers), blood pressure, BMI, and HAPA constructs will be assessed at baseline, 12-weeks (post-intervention) and 24-weeks (follow-up). Data analysis will use the Group x Time interaction from a General Linear Mixed Model analysis. Conclusions: Physical activity interventions that are acceptable and have robust theoretical underpinnings show promise for improving the health of cancer survivors.
Antecedentes/Objetivo: Los sobrevivientes de cáncer tienen riesgo cardiovascular debido a la comorbilidad y al comportamiento sedentario, lo que justifica desarrollar una adecuada intervención para aumentar la práctica de actividad física. El Enfoque del Proceso de Acción de Salud (EPAS) constituye un marco teórico para el desarrollo de conductas saludables y los dispositivos electrónicos de actividad física son nuevas herramientas de automonitorización para los supervivientes de cáncer. Método: Sesenta y ocho sobrevivientes de cáncer colorrectal y ginecológico serán aleatorizados en grupos de intervención y control. Los participantes del grupo de intervención recibirán un Fitbit Alta™ para monitorizar la actividad física, sesiones grupales y aplicación de un folleto de EPAS, y una llamada telefónica de apoyo. Los participantes del grupo control únicamente recibirán un folleto basado en EPAS. Al inicio del estudio, a las 12 y 24 semanas, se evaluarán la actividad física (usando acelerómetros), la presión arterial, el Índice de Masa Corporal (IMC) y los constructos EPAS. El análisis de datos utilizará la interacción Grupo x Tiempo a partir de un análisis del Modelo Mixto Lineal General. Conclusiones: Las intervenciones de actividad física son factibles y tienen fundamentos teóricos que auguran mejorar la salud de los sobrevivientes de cáncer.
Cancer survivors are at increased risk of secondary cancers, cardiovascular disease (CVD) and other comorbidities compared to those without a cancer history (Rock et al., 2012). Despite cancer survival rates improving, survivors of colorectal and gynaecological cancers continue to be at cardiovascular risk due to their physical inactivity. Up to 70% of endometrial cancer survivors are obese (von Gruenigen et al., 2008), and these survivors are twice as likely to die from not meeting the government's physical activity guidelines of 150-minutes of moderate-intensity physical activity per week (Fisher, Smith, & Wardle, 2016). Fifty-eight percent of colorectal cancer survivors are overweight or obese, and 83% are insufficiently active (Grimmett, Bridgewater, Steptoe, & Wardle, 2011), putting survivors at CVD risk. Given that these two cancer types have a high survival rate, and a significant proportion of these individuals have comorbidities resulting in increased CVD risk (Loprinzi & Lee, 2014), interventions to increase physical activity in these patients are important.
Although cancer survivors are at increased CVD risk and recurrence, clinicians may be optimally positioned to capitalize on the ‘teachable moment’ (Demark-Wahnefried, Azid, Rowland, & Pinto, 2005) or post-traumatic growth (Ochoa, Casellas-Grau, Vives, Font, & Borràs, 2017) created by the cancer diagnosis and play a central role in guiding survivors toward positive health behaviours that improve overall health and physical well-being.
Interventions that incorporate behaviour change techniques including goal-setting, counselling and feedback to increase physical activity and improve quality of life in survivors have yielded promising findings (Bennett, Lyons, Winters-Stone, Nail & Scherer, 2007; De la Torre-Luque, Gambara, López, & Cruzado, 2016). Based on the effectiveness of these interventions and our recent qualitative work (Hardcastle, Glassey, Salfinger, Tan & Cohen, 2017; Hardcastle, Maxwell-Smith, et al., 2017; Maxwell-Smith, Zeps, Hagger, Platell & Hardcastle, 2017), addressing support needs and facilitating self-monitoring strategies for survivors are important components of successful interventions (Hardcastle et al., 2015).
Wearable trackersWearable activity technology (WAT) holds great potential as a self-monitoring tool to increase physical activity in survivors. WAT and associated ‘apps’ use many of the techniques employed in physical activity interventions (i.e., self-monitoring, feedback, goal-setting) (Lyons, Lewis, Mayrsohn y Rowland, 2014). Thus, WAT presents a feasible opportunity for widespread physical activity promotion (Sanders et al., 2016). Previous physical activity interventions for cancer survivors have used pedometers as self-monitoring tools (Bennett et al., 2007). WAT is hypothesised to be more effective than pedometers for increasing physical activity because it provides real-time feedback and prompts, links to mobile applications where users can monitor behaviour and create a network to promote accountability, and facilitates peer-support amongst other users.
The Fitbit™ has demonstrated effectiveness for increasing physical activity in overweight and obese adults (Wang et al., 2015). The first Fitbit™ trial in cancer survivors has recently been published, however the Fitbit™ and Facebook intervention targeted adolescent cancer survivors (Mendoza et al., 2017). To our knowledge, no study has assessed the effectiveness of the Fitbit™ to increase physical activity in adult survivors.
Health Action Process ApproachPhysical activity interventions that are based on theoretical underpinnings have been more successful for improving health-related outcomes compared to those without theoretical bases (atheoretical) (Bennett et al., 2007; Parschau et al., 2014). The Health Action Process Approach (HAPA) attempts to overcome the ‘intention-behaviour gap’ by proposing two phases that are required for behaviour change; motivation and volition (Schwarzer & Luszczynska, 2008). Motivational processes involve initial recognition of risk perception and positive outcome expectances associated with behavioural change. The individual must form an intention to change and graduate to volitional processes by acting on this intention. This requires planning and self-efficacy for the proposed behaviour and self-regulation to monitor and maintain the behavioural change (Schwarzer & Luszczynska, 2008). A recent intervention by Ungar, Sieverding, Weidner, Ulrich, and Wiskemann (2016) found survivors who received HAPA-based counselling to enhance self-regulation were significantly more active than a control group.
Given the promise of the HAPA model and the importance of self-regulation for successful behaviour change, physical activity interventions for survivors that involve monitoring and motivational tools are warranted. The use of a Fitbit™ as a motivational device to increase physical activity in cancer survivors is yet to be explored and is a novel aspect of the study.
We aim to determine whether a pragmatic intervention package using WAT, coupled with action-planning, goal-setting and coping planning is effective for increasing physical activity and reducing sedentary behaviour in gynecologic and colorectal cancer survivors at CVD risk. A secondary aim is to assess the acceptability of this intervention that could be incorporated into routine after-care for survivors.
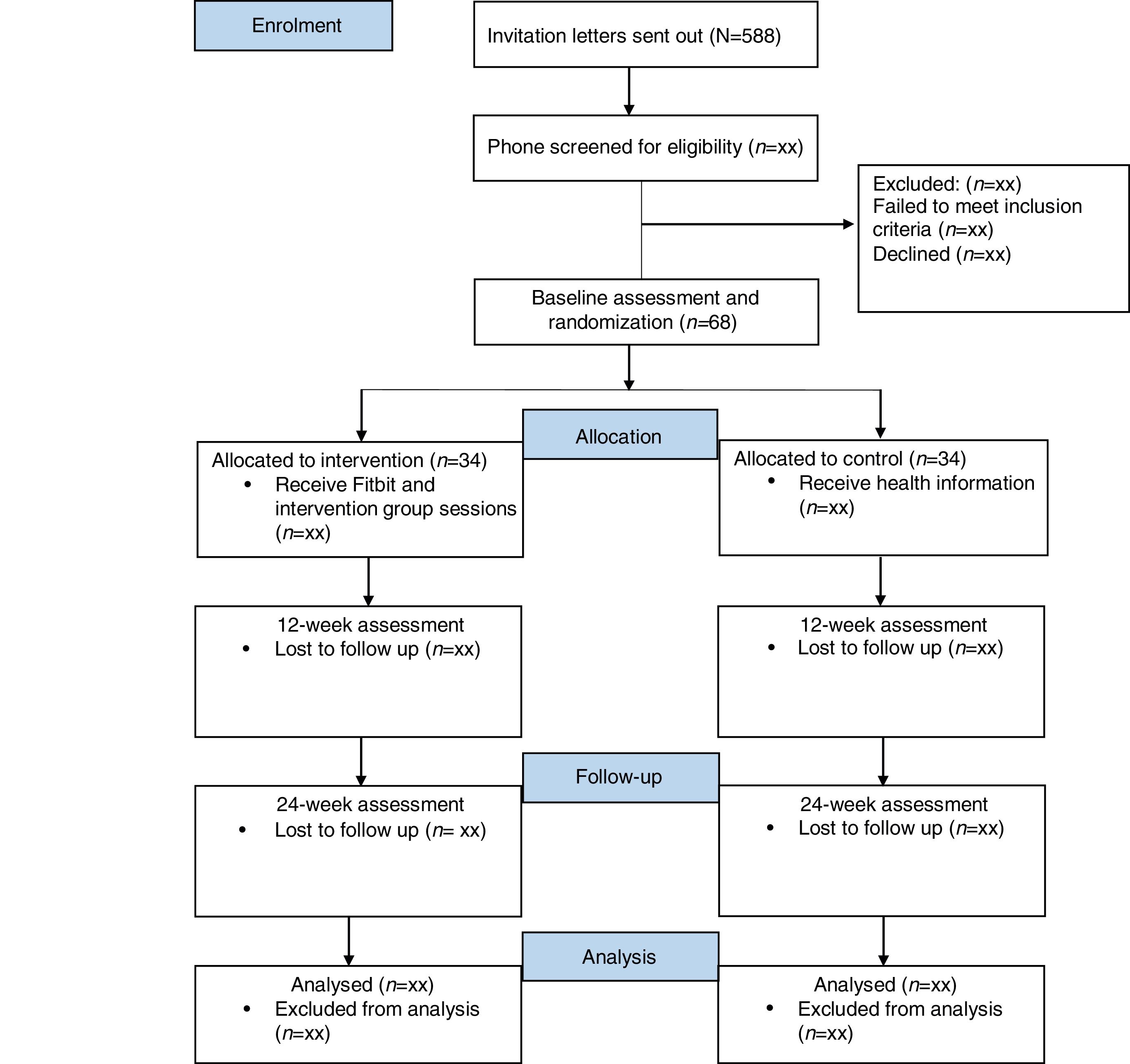
MethodDesignThe two-arm Randomised Controlled Trial (RCT) tests the efficacy of a self-monitoring intervention relative to an information only control group. Participants will complete data collection at baseline (T1), after the 12-week intervention (T2) and at 24-week follow-up (T3). Ethics approval was obtained from the St. John of God Hospital Human Research Ethics Committee (#1102), and reciprocal approval from Hollywood Private Hospital. The reporting of the study will adhere to the CONsolidated Standards Of Reporting Trials (CONSORT) and Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) guidelines for RCTs (Begg et al., 1996; Chan et al., 2013). The study flow chart is presented in Figure 1.
ParticipantsParticipants will be stage 1 and 2 colorectal and gynecologic cancer survivors aged 18-80, who have finished active treatment (surgery, chemotherapy, and/or radiotherapy) in the previous 5 years, and are completing less than 150-minutes of Moderate-Vigorous intensity Physical Activity (MVPA) per week (Rock et al., 2012). Participants must have comorbidities resulting in increased CVD risk identified through hospital records (i.e., on blood pressure medication or have blood pressure >150/90mmHg, BMI >28, hypercholesterolemia >5.2mmol/L) or an American Society of Anaesthesiologists (ASA) score of 2 or 3, in the absence of medical records.* Participants who are in remission at the time of recruitment, English-reading and speaking, live in the Perth Metropolitan area, and have no surgery planned for the 6 months following recruitment will be eligible to participate.
Exclusion criteria include those who (1) are meeting the physical activity guidelines (Rock et al., 2012); (2) have a current diagnosis of a severe psychiatric illness or cardiac abnormalities (those with minor psychiatric diagnoses will be eligible if they are willing and able to participate in the intervention); (3) severe disabilities including arthritis; (4) have ASA scores of 1 or 4; (5) already enrolled in a physical activity program/trial; (6) have been diagnosed with uterine carcinosarcoma (MMMT), uterine serous carcinoma, or ovarian cancer, as these cancer types are associated with a poor prognosis.
Individuals with an ASA score of 2 or 3 will be eligible for recruitment. An ASA score from 1-4 is assigned to patients upon admission to hospital for a surgical procedure. A low ASA score indicates minimal cardiovascular risk, and a higher ASA score suggests comorbidities that threaten a patient's life. Participants with ASA scores of 2 or 3 have comorbidities putting them at risk of CVD. The ASA score is globally recognised as an indicator of physical health status of patients prior to undergoing surgery (Owens, Felts & Spitznagel, 1978).
Participants will be recruited using purposive sampling methods, involving screening the hospital records of participating oncologists (N=8), to collate a pool of eligible survivors. The participating oncologists are based at St John of God Subiaco and Murdoch Hospitals, Hollywood Private Hospital, and the Women Centre in West Leederville, Western Australia. Eligible individuals (N=588) will be mailed an invitation letter and information sheet from their treating oncologist.
An independent statistician will generate the randomisation sequence using STATA v14 with a 1:1 allocation using blocks of 4. Following recruitment, participants will be allocated to either intervention or control groups by the statistician using the next consecutive randomisation code. Participants will be randomly sorted into blocks, with random assignment to group within each block. Upon randomisation, participants will be evenly split between treatment (N=34) and control groups (N=34). Assessors (post-baseline), clinicians and data analysts will be blinded to group allocation.
Statistical power and sample sizeFor each GLMM, the 2-way Group x Time interaction embodies the treatment effect. According to G*Power, for the primary outcome (MVPA), 28 participants in each of the two groups provides sufficient power for an 80% chance of detecting a ‘small to moderate’ (f=.17) group x time interaction at an alpha level of .05. A meta-analysis of physical activity interventions in cancer survivors reported a weighted mean effect size of 0.38 (95 CI: 0.22-0.54) for the difference between groups on physical activity outcomes. We anticipate a similar effect size (i.e., 0.40). We aim to recruit 68 participants, ensuring that if 20% are lost to follow-up, the intervention will still be adequately powered at 80% to detect a meaningful change. A dropout rate of 20% is a conservative estimation, given previous dropout rates of ∼10% in similar intervention designs for survivors (Bennett et al., 2007; Short, James, Girgis, Mcelduff, & Plotnikoff, 2012).
InstrumentsThe primary outcome will be minutes of MVPA and sedentary behaviour ascertained from the Actigraph GT9X (Actigraph, LLC, Pensacola, Florida, USA). Participants will wear the accelerometer on their right hip for all waking hours across 7 consecutive days. Individual days of wear time must exceed 10hours to be considered valid for analysis. Non-wear periods will be defined as intervals of at least 60 consecutive minutes of zero counts will be excluded from analyses. Activity counts will be categorised as: sedentary (<100cpm), light-intensity (100-1951cpm), moderate-intensity (1952-5724cpm) and vigorous-intensity (>5725cpm), using data recorded in 60-s epochs (Lynch et al., 2016).
Sedentary behaviour will be defined by accelerometer activity counts of <100cpm, for 20 consecutive minutes or more, which corresponds to clinical changes in cardio-metabolic biomarkers (Lynch et al., 2016). The accelerometer log completed by participants will assist in differentiating sedentary time from non-wear time.
The International Physical Activity Questionnaire, Short-Form (Craig et al., 2003) will assess self-reported physical activity at T1, T2, and T3. This questionnaire is scored based on the amount and intensity of accumulated minutes of exercise in the previous week, with activity being converted into MET minutes as a function of intensity. This tool is reliable (Cronbach's alpha of .80) and had demonstrated adequate validity across 12 countries (Craig et al., 2003; Mama et al., 2015).
Quality of life will be measured using the Medical Outcomes Study Short-Form survey (Ware, Kosinski & Keller, 1996). This instrument is considered reliable across both mental and physical components (Cronbach's alpha of .87 and .84, respectively), and valid when compared to the 36-item version (Dritsaki, Petrou, Williams, & Lamb, 2017; Ware et al., 1996).
Physical activity attitudes will be measured using previously published, validated items from the HAPA inventory, with Cronbach's alpha scores for the subscales below ranging from .73 to .87 (Parschau et al., 2014). Some items have been amended, based on the specific barriers identified by survivors (Bennett et al., 2007; Hardcastle, Glassey et al., 2017; Maxwell-Smith et al., 2017; Short et al., 2012), and physical activity guidelines for survivors (Rock et al., 2012). The following constructs will be assessed:
Outcome expectations. Twelve items will assess outcome expectations. Five items are derived from the validated exercise pros subscale (Plotnikoff, Blanchard, Hotz & Rhodes, 2001) and 7-items are tailored based on formative research with cancer survivors (Bennett et al., 2007; Hardcastle, Maxwell-Smith et al., 2017; Short et al., 2012). The items measure the extent to which participants agree or disagree (1=disagree very strongly to 6=agree very strongly) that regular physical activity over the next 12-weeks will help to: reduce tension or stress; feel more confident about my own health; sleep better; have a positive outlook; control my weight; regain lost strength; prevent cancer recurrence; increase fatigue; increase joint pain; weaken my immune system; feel better about my body, and increase my longevity. For example, ‘Doing regular physical activity over the next 12-weeks will help me to reduce tension or stress’.
Action self-efficacy. Four items will assess action self-efficacy, based on previous research with breast cancer survivors (Rogers et al., 2005). Items assess participants’ confidence to complete 150-minutes of physical activity per week, with the item stems: ‘I believe I have the ability to…’; ‘I am confident I can do…’; ‘If I wanted to I could…’ and ‘For me to do…’, For example, ‘I am confident I can do 150-minutes of moderate-intensity physical activity per week for the next 12-weeks’. Possible responses range from 1=extremely difficult, disagree very strongly, extremely unconfident to 6=extremely easy, agree very strongly, extremely confident.
Maintenance self-efficacy. Thirteen items will assess maintenance self-efficacy, with based on formative research (Hardcastle, Maxwell-Smith et al., 2017; Short et al., 2012). Items measure confidence to participate in regular physical activity over the next 12-weeks when: I lack discipline; exercise is not a priority; the weather is bad; I am feeling tired; I lack time; I do not enjoy exercising; I do not have someone to encourage me to exercise; I am in a bad mood or feeling depressed; I have to exercise alone; I can’t notice any improvements in physical fitness; I feel stiff or sore; I feel unwell, and I can’t notice any improvements in my body. Responses are scored on a six-point Likert scale from 1=disagree very strongly to 6=agree very strongly.
Action planning. Four items will assess action planning for the next 3-weeks, based on an amended scale (Rhodes, Blanchard, Matheson, & Coble, 2006). Participants will be asked to respond on a scale of 1=disagree very strongly to 6=agree very strongly about whether they have made plan concerning what, when, where, and how they will engage in regular physical activity.
Risk perception. Four items will measure risk perception, based on a previous scale (Graham, Prapavessis, & Cameron, 2006). Items are scored on a six-point Likert scale from 1=disagree very strongly, extremely unlikely, very much lower, to 6=agree very strongly, extremely likely, very much higher. Items measure ‘perceived risk…’, ‘vulnerability…’, ‘likelihood…’ and ‘chance…of developing health problems related to an inactive lifestyle, compared to the average person’.
Intention. Two items will measure intention to engage in moderate-intensity physical activity for at least 150-minutes per week in the next 12-weeks, based on previously established measures (Ajzen, Brown & Carvajal, 2004). Items are ‘I intend…’ and ‘I will try…to participate in moderate-intensity physical activity for at least 150-minutes per week in the next 12-weeks’. Items will be scored on a six-point Likert scale from 1=disagree very strongly to 6=agree very strongly.
Several demographic characteristics and comorbidities have been identified as covariates in cancer survivors, based on similar research (Loprinzi & Lee, 2014). Therefore, we will obtain this information at T1 including marital status, household income and educational attainment. Cardiovascular risk will be measured at each assessment using the QRISK2, which has been validated in the UK and is used internationally (Collins & Altman, 2010). The QRISK2 data will be entered into the online algorithm (www.qrisk.org), where scores will be calculated.
Blood pressure will be measured using an Omron IC-10 Upper Arm Blood Pressure Monitor (HEM 7070-E), which has been validated for use by the British Hypertension Society (British Hypertension Society, 2017). BMI will be calculated by measuring height and weight.
Intervention acceptability will be assessed at T3, where participants will be invited to provide feedback concerning the effective and ineffective components and the practicality of the intervention via interviews. Fitbit™ use will be monitored weekly using the Fitbit™ software, to assist with the assessment of intervention adherence.
ProcedureParticipants will contact a member of the trial team to express their interest in participating in the study. Those who express interest will undergo phone screening to assess eligibility, before organising their baseline assessment appointment.
Assessments will be conducted in a clinic room at St. John of God Subiaco Hospital, Perth, Australia. The baseline assessments will be performed by a member of the trial team, prior to randomisation. Subsequent assessments will be performed by a trial co-ordinator who is blinded to group allocation and not involved in the administration of the intervention. Group sessions will be held in a meeting room at St. John of God Subiaco Hospital and led by team investigators. Text messages and phone calls will act as reminders for participants to attend group sessions. Attendance at group sessions will be monitored as a measure of intervention adherence.
The baseline assessment will begin with participants reading the information sheet before providing consent to participate. Participants will be required to complete a demographic, physical activity, quality of life, exercise attitudes, and cardiovascular risk questionnaire. Height (at T1), weight, and blood pressure will then be recorded by the assessor. At the end of their assessment, participants will be provided with an Actigraph GT9X accelerometer to record their activity for the subsequent week, an accelerometer log for recording accelerometer wear, and a booklet. The assessor will inform the participant of the accelerometer wear instructions and provide a prepaid postage satchel for accelerometer return. The assessment procedure will be repeated at T2 and T3. The intervention will cease prior to the T2 assessment and the treatment group will be required to return their Fitbit™ at T3.
The intervention includes three components: (1) a Fitbit Alta™; (2) two group sessions; (3) one telephone-delivered feedback and support session. All participants will receive a printed booklet on physical activity guidelines, home-based strength exercises, benefits of regular physical activity, physical activity logs, confidence building, barrier solving, coping planning, action-planning and goal-setting activities.
Intervention group- I.
WAT tracker: Participants are provided with a Fitbit Alta™ activity tracker, which they will sign-out at their first group sessions, and be encouraged to wear for the duration of the trial. This is a slim, wrist-worn device that displays steps, distance, active minutes (MVPA) and caloric expenditure. The Fitbit™ was chosen because previous work demonstrates its usefulness and acceptance amongst cancer survivors (Nguyen et al., 2017) and older adults (>70; McMahon et al., 2016). The Fitbit Alta™ also alerts users to sedentary behaviour and progress towards activity goals. Data from the device can be uploaded to the Fitbit™ application via Bluetooth. At the first group session participants will be assisted to install the smartphone/tablet/computer application, and to pair their device with the application. A member of the trial team will send friend requests to each participant so that engagement with the application and activity can be monitored weekly.
- II.
Group sessions: Sessions lasting for approximately 2-hours will be delivered at weeks 1 and 4, with approximately 10-12 participants in each session. Group sessions will correspond with components of the HAPA-model.
- a.
Session one will focus on introducing participants to the Fitbit Alta™ and giving instructions on how to use the device as a self-monitoring tool. The first part of the session will be largely didactic covering risks of inactivity (corresponding to the ‘risk perceptions’ construct in HAPA), the benefits of physical activity (targeting positive ‘outcome expectancies’ of the HAPA), detailed physical activity guidelines (steps and MVPA) and enhancing confidence and importance to participate in physical activity at the recommended level (targeting ‘action self-efficacy’ and ‘intention’). In keeping with this approach, personalised physical activity feedback will be provided to each participant based on their T1 accelerometer data. During this session, participants will be encouraged to complete action-planning (corresponding to HAPA ‘action-planning’ as a strategy to aid the translation of intentions to behaviour), and goal-setting exercises from the intervention booklet for the following 3-weeks. Behaviour change specialist SH and CMS will assist participants with action-planning, goal-setting and self-monitoring activities.
- b.
Session two (week 4) will attend to support needs, problem solving and coping planning. The session will use the intervention booklet to prompt physical activity planning and coping planning (corresponding to HAPA ‘coping planning’) for the following four weeks, as well as targeting maintenance self-efficacy. Specifically, participants will be asked to consider situations or obstacles to implementing their physical activity plans, and form ‘if-then plans’. The final part of the session will involve demonstrations of strength-training exercises that could be performed at home, using household items. Participants will be given the opportunity to practice strength-based exercises during the session to check technique and foster perceptions of confidence. This session will also allow for trouble-shooting of problems that participants encounter regarding Fitbit™ use.
- a.
- III.
Telephone-delivered feedback and support session: A trial team member will telephone each participant during week 8 of the trial for approximately 20-minutes. The purpose of the call will be to discuss progress to date, with a focus on self-regulation, maintenance self-efficacy and coping planning based on the principles of the HAPA and the relational techniques of motivational interviewing (Hardcastle, Fortier, Blake, & Hagger, 2017). Coping planning to overcome barriers will also be discussed.
Control group. Participants in the control group will receive the intervention booklet containing physical activity guidelines and motivational tools. However, the control group will not receive group sessions, a Fitbit Alta™ or a telephone-delivered support session. Participants will receive feedback on their physical activity levels and be offered the opportunity to trial the Fitbit Alta™ for 6-weeks following trial completion (after T3).
Statistical analysisPrimary and secondary outcome variables will be analysed via a series of Generalised Linear Mixed Models (GLMMs) employing appropriate distributions and link functions for each outcome measure. All GLMMs will include the fixed effects Group (Intervention v Control), Time (T1, T2 and T3) and the 2-way interaction. A random effect for participant will be included to account for the correlation within people inherent in a longitudinal design.
Cancer type, gender, age, socio-economic status, BMI, and blood pressure will be included as covariates within the model. Compared to the traditional statistical procedures for analysing behavioural change, GLMM is less sensitive to participant attrition because it does not rely on participants providing data at every assessment point; the GLMM maximum likelihood procedure is a full information estimation procedure that uses all data present at each assessment time-point. Missing data will be investigated for patterns in terms of observed study variables. Multiple imputation will be considered if data are arguably missing at random and less than 20% of the data are missing. We will impute 25 data sets based on all relevant observed variables, including the interaction term and outcome measure of interest for each specific analysis. Sensitivity analyses will be conducted to consider the effect of potential missing not at random mechanisms on parameter estimates from imputed datasets (Sterne et al., 2009). Qualitative data from post-trial interviews will be analysed using inductive thematic analysis to identify common themes concerning active ingredients, barriers to behaviour change and acceptability (Braun & Clarke, 2006).
DiscussionThe trail will examine the effectiveness and acceptability of an intervention that combines WAT (The Fitbit Alta) with self-regulation techniques (action-planning, goal-setting, and coping planning) to increase physical activity and reduce sedentary behaviour in colorectal and gynecologic cancer survivors. This protocol describes the first intervention to employ the Fitbit Alta™ to promote physical activity in adult survivors, contributing to the growing research on the effectiveness of home-based, brief interventions to promote physical activity.
There is growing evidence to suggest that physical activity reduces risk of CVD and cancer recurrence (Hamer & Warner, 2017). However, few survivors are meeting the minimum physical activity guidelines (Rock et al., 2012). Physical activity interventions that meet the preferences and support needs of cancer survivors, are feasible, and can be integrated into routine practice are needed (Hardcastle & Cohen, 2017).
Previous research supports the exercise preferences of cancer survivors for home-based, unsupervised, self-paced, low-moderate intensity physical activity that involves primarily walking (Hardcastle & Cohen, 2017; Hardcastle et al., 2018; Maxwell-Smith et al., 2017), and the desire for monitoring and accountability (Bennett et al., 2007; Hardcastle, Maxwell-Smith et al., 2017). Since self-monitoring (Hardcastle et al., 2015) has been identified as effective strategies for increasing physical activity, WAT may serve as a valuable tool for measuring activity in a practical and motivational way. Further, home-based interventions offer advantages because they mitigate access and transport issues, and are less expensive than supervised, facility-based programs that require participants to attend classes or maintain a health club membership (Hardcastle, Glassey et al., 2017). Examination of intervention acceptability will indicate whether such programs can be implemented for improving physical activity of cancer survivors as part of follow-up care.
Between 12-weeks (T2) and 24-weeks (T3), intervention participants will keep the activity tracker but receive no formal support. Therefore, changes in physical activity between T2 and T3 in the intervention group will provide some insight concerning whether ongoing behavioural support is necessary in combination with WAT to sustain increases in MVPA and reductions in sedentary behaviour. Interventions that are able to demonstrate sustained increases in physical activity are needed.
ConclusionThe trial is pragmatic and primarily concerned with evaluating whether a low-intensity intervention package (WAT combined with limited behavioural support) is effective for increasing MVPA and reducing sedentary behaviour in survivors compared to usual care. If found to be effective, the low-cost intervention could be integrated into clinical practice and delivered by oncology clinicians/nurses, allied professionals or charitable organisations.
FundingThis work is sponsored by The Tonkinson Colorectal Cancer Research Fund (#57838). We also acknowledge the contribution from the St. John of God Gynecologic Oncology Research Group, Western Australia.