Acute pancreatitis (AP) is an inflammatory disease with multiple etiologies, and the emergence of complications. Between 0.1%–5% of cases are attributed to drugs. The absence of specific characteristics complicates the diagnosis and treatment of drug-induced AP. Reviewing patients admitted with the diagnosis of drug-induced AP can provide information and improve its management.
Patients and methodsThis is a descriptive, observational, and retrospective study. All patients admitted to the Hospital Universitari de Bellvitge (HUB) between June 2007 and March 2023 with suspected drug-induced AP were included. The data were obtained from the HUB pharmacovigilance program database.
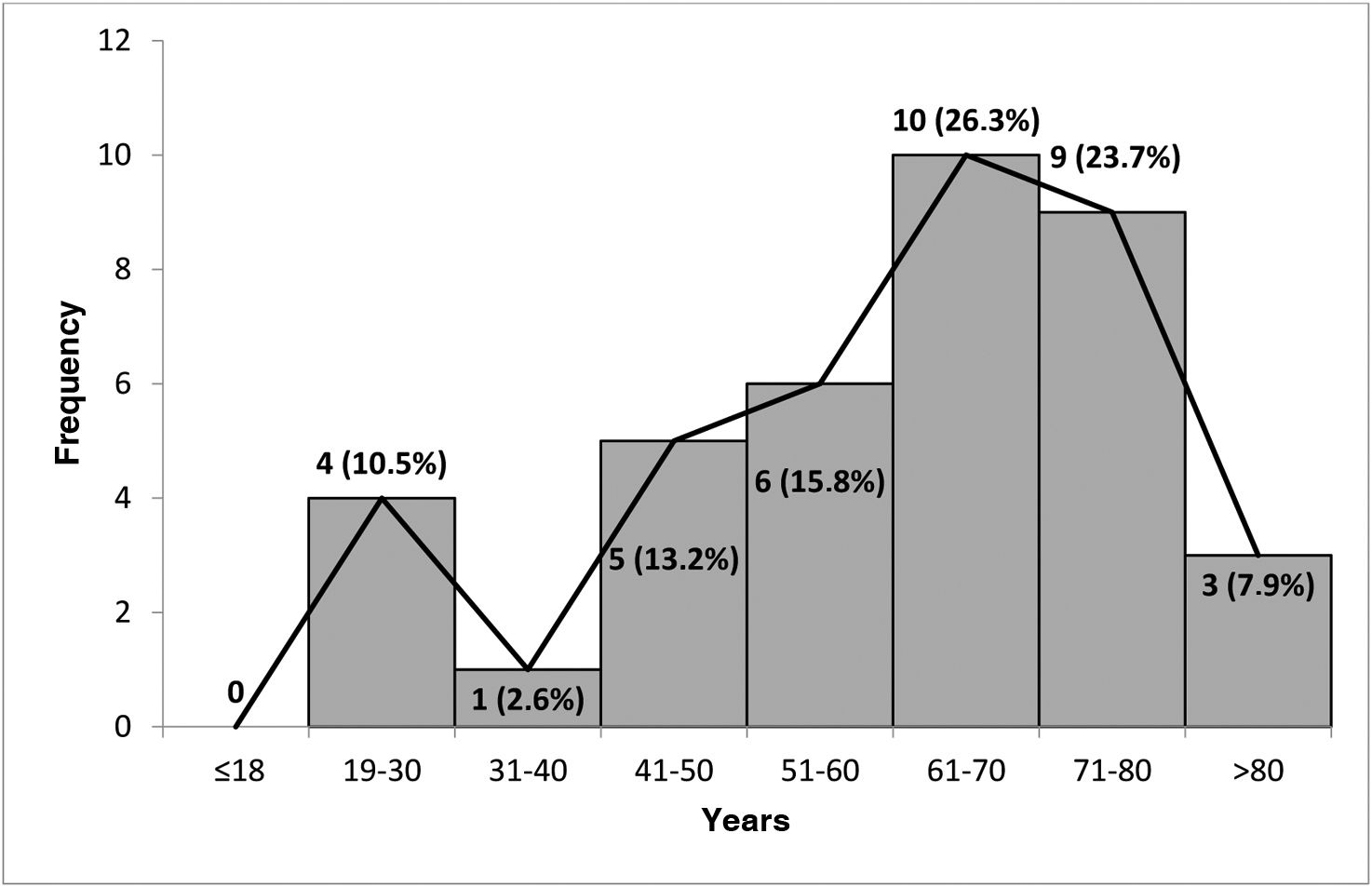
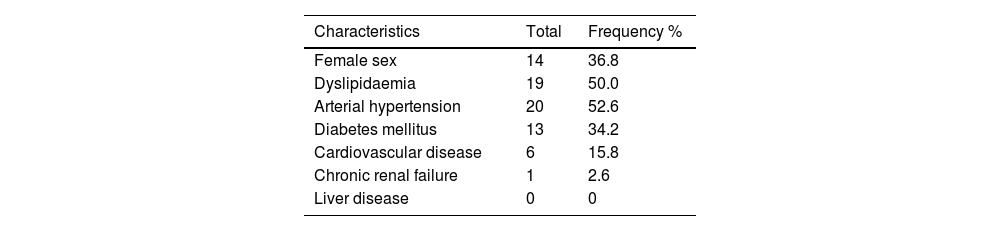
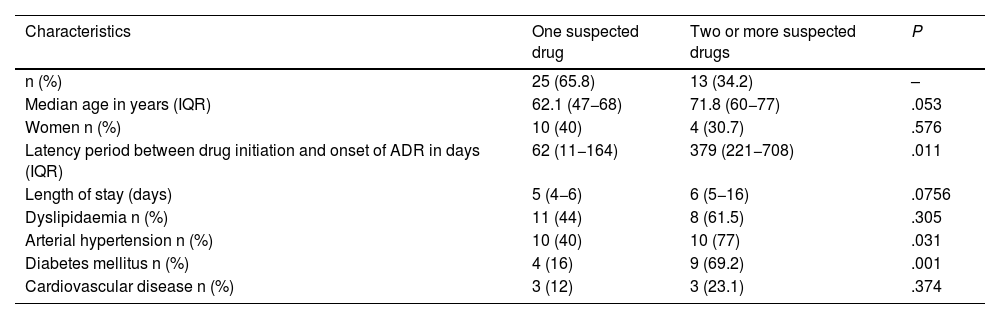
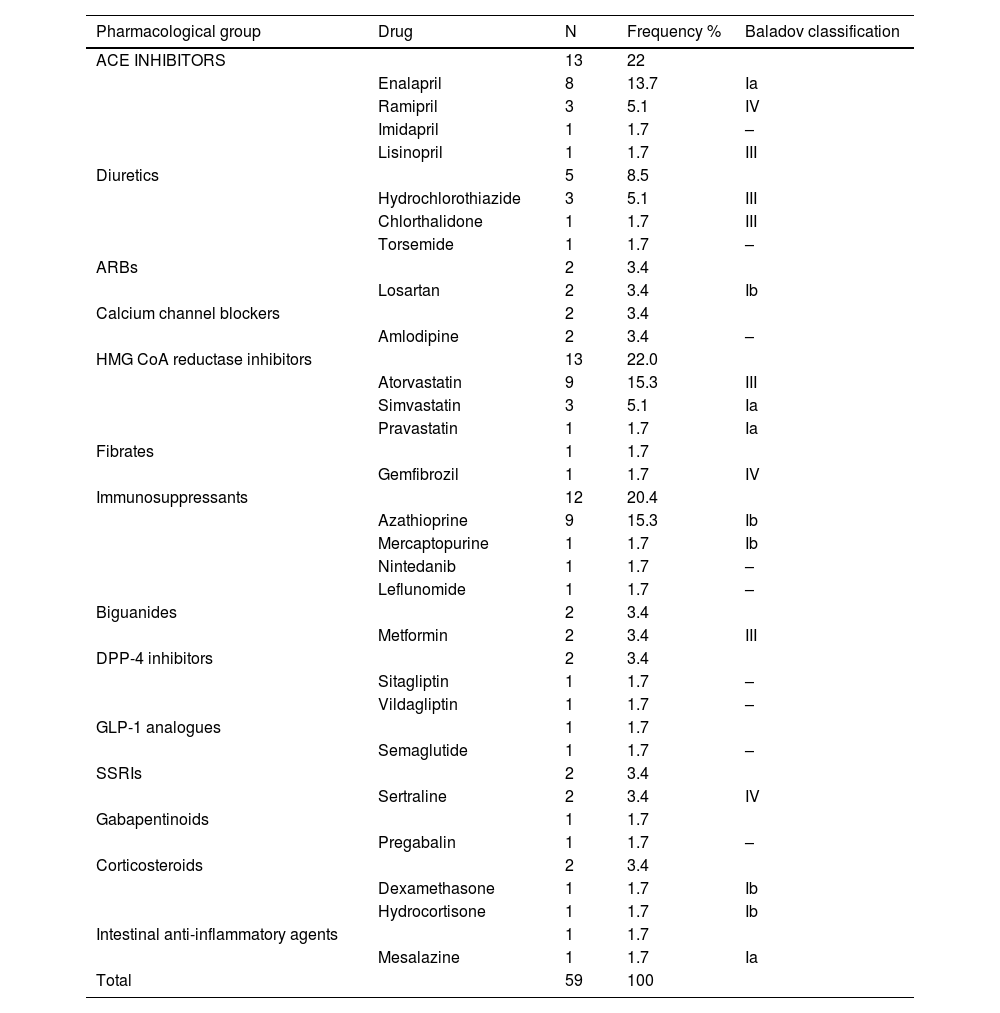
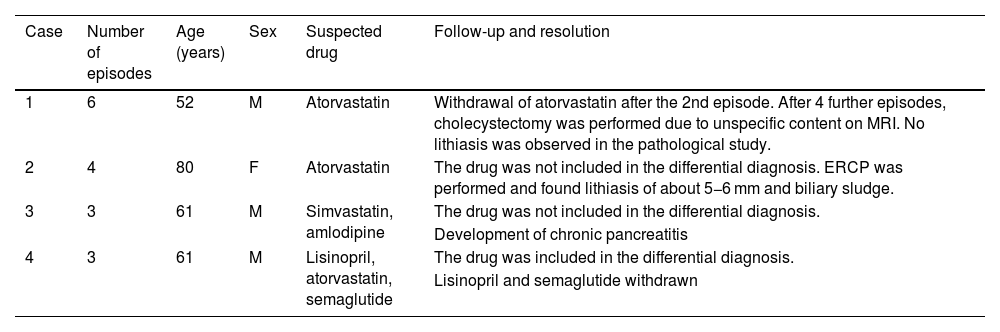
ResultsThirty-eight patients with suspected drug-induced AP were identified, representing 0.62% of all adverse drug reactions (ADRs) (n = 6085). Of these, 65.8% (n = 25) had a single suspected drug. The median latency period for the onset of ADRs was 160.5 days (IQR 18–582 days), and the median hospital stay was 5 days (IQR 3–7 days). Fifty-nine suspected drugs were identified, involving 26 active principles. Azathioprine and atorvastatin were the most frequent, with 9 cases each (15.2%), followed by enalapril with 8 cases (13.6%). Drug etiology was assessed in 23 cases (60.5%), and the suspected drug was discontinued in all cases. There was 1 fatal case documented (2.63%).
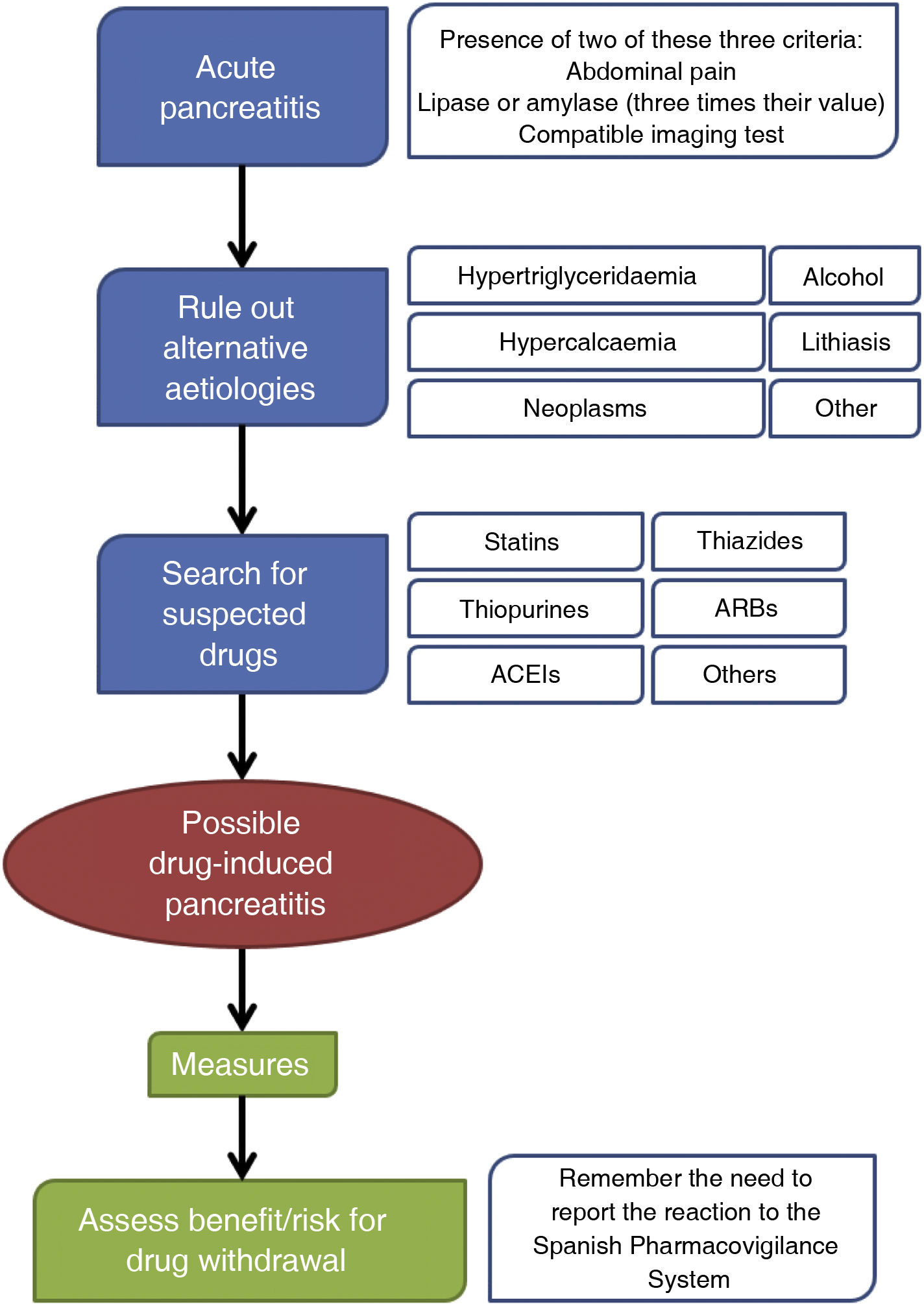
ConclusionThis study can contribute to better understanding of drug-induced pancreatitis episodes. We propose the creation of a diagnostic algorithm that includes the assessment of the drug as a possible cause.
La pancreatitis aguda (PA) es una enfermedad inflamatoria con múltiples etiologías y complicaciones. Entre un 0,1%–5% son secundarias a fármacos. La ausencia de características propias complica el diagnóstico y tratamiento de las PA fármaco-inducidas. La revisión de los pacientes ingresados con el diagnóstico de PA atribuida a fármacos puede aportar información y mejorar su abordaje.
Pacientes y métodosEstudio descriptivo, observacional y retrospectivo. Se incluyeron todos los pacientes ingresados en el Hospital Universitari de Bellvitge (HUB) entre junio de 2007 y marzo de 2023 con la sospecha de PA atribuida a fármaco. Los datos se obtuvieron a partir de la base de datos del programa de farmacovigilancia del HUB.
ResultadosSe identificaron 38 pacientes con sospecha de PA inducida por fármacos, un 0,62% del total de reacciones adversas medicamentosas (RAM) (n = 6085). Un 65,8% (n = 25) presentaban un solo fármaco sospechoso. La mediana de la latencia de aparición de la RAM fue de 160,5 días (IQR 18–582 días) y la duración del ingreso de 5 días (IQR 3–7 días). Se identificaron 26 principios activos como sospechosos. Azatioprina y atorvastatina en 9 casos (15,2%) y enalapril en 8 casos (13,6%) fueron los más frecuentes. La etiología farmacológica fue valorada en 23 casos (60,5%) y se retiró el fármaco sospechoso en todos. Se documentó 1 caso mortal (2,63%).
ConclusiónEste estudio puede contribuir a mejorar el conocimiento de los episodios de pancreatitis fármaco-inducidas. Proponemos la creación de un algoritmo diagnóstico en el que se incluye la valoración del fármaco como posible causa.