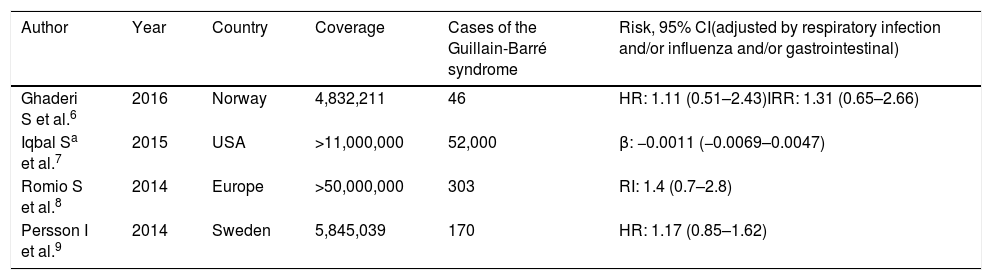
array:24 [ "pii" => "S2387020619302347" "issn" => "23870206" "doi" => "10.1016/j.medcle.2018.09.016" "estado" => "S300" "fechaPublicacion" => "2019-07-19" "aid" => "4690" "copyright" => "Elsevier España, S.L.U.. All rights reserved" "copyrightAnyo" => "2018" "documento" => "simple-article" "crossmark" => 1 "subdocumento" => "cor" "cita" => "Med Clin. 2019;153:90-1" "abierto" => array:3 [ "ES" => false "ES2" => false "LATM" => false ] "gratuito" => false "lecturas" => array:1 [ "total" => 0 ] "Traduccion" => array:1 [ "es" => array:19 [ "pii" => "S0025775318306638" "issn" => "00257753" "doi" => "10.1016/j.medcli.2018.09.022" "estado" => "S300" "fechaPublicacion" => "2019-07-19" "aid" => "4690" "copyright" => "Elsevier España, S.L.U." "documento" => "simple-article" "crossmark" => 1 "subdocumento" => "cor" "cita" => "Med Clin. 2019;153:90-1" "abierto" => array:3 [ "ES" => false "ES2" => false "LATM" => false ] "gratuito" => false "lecturas" => array:2 [ "total" => 8 "formatos" => array:2 [ "HTML" => 6 "PDF" => 2 ] ] "es" => array:10 [ "idiomaDefecto" => true "cabecera" => "<span class="elsevierStyleTextfn">Carta al Editor</span>" "titulo" => "Vacunación del paciente tratado con fármacos inmunodepresores, inmunomoduladores o biológicos" "tienePdf" => "es" "tieneTextoCompleto" => "es" "paginas" => array:1 [ 0 => array:2 [ "paginaInicial" => "90" "paginaFinal" => "91" ] ] "titulosAlternativos" => array:1 [ "en" => array:1 [ "titulo" => "Vaccination of patients under immunosuppressive, immunomodulatory or biologics drugs" ] ] "contieneTextoCompleto" => array:1 [ "es" => true ] "contienePdf" => array:1 [ "es" => true ] "autores" => array:1 [ 0 => array:2 [ "autoresLista" => "Juan Rodríguez-García, Rafael Fernández-Santos, José Antonio García-Erce" "autores" => array:3 [ 0 => array:2 [ "nombre" => "Juan" "apellidos" => "Rodríguez-García" ] 1 => array:2 [ "nombre" => "Rafael" "apellidos" => "Fernández-Santos" ] 2 => array:2 [ "nombre" => "José Antonio" "apellidos" => "García-Erce" ] ] ] ] ] "idiomaDefecto" => "es" "Traduccion" => array:1 [ "en" => array:9 [ "pii" => "S2387020619302347" "doi" => "10.1016/j.medcle.2018.09.016" "estado" => "S300" "subdocumento" => "" "abierto" => array:3 [ "ES" => false "ES2" => false "LATM" => false ] "gratuito" => false "lecturas" => array:1 [ "total" => 0 ] "idiomaDefecto" => "en" "EPUB" => "https://multimedia.elsevier.es/PublicationsMultimediaV1/item/epub/S2387020619302347?idApp=UINPBA00004N" ] ] "EPUB" => "https://multimedia.elsevier.es/PublicationsMultimediaV1/item/epub/S0025775318306638?idApp=UINPBA00004N" "url" => "/00257753/0000015300000002/v2_202005080620/S0025775318306638/v2_202005080620/es/main.assets" ] ] "itemSiguiente" => array:19 [ "pii" => "S2387020619302359" "issn" => "23870206" "doi" => "10.1016/j.medcle.2018.09.017" "estado" => "S300" "fechaPublicacion" => "2019-07-19" "aid" => "4679" "copyright" => "Elsevier España, S.L.U." "documento" => "simple-article" "crossmark" => 1 "subdocumento" => "cor" "cita" => "Med Clin. 2019;153:e7-e8" "abierto" => array:3 [ "ES" => false "ES2" => false "LATM" => false ] "gratuito" => false "lecturas" => array:1 [ "total" => 0 ] "en" => array:11 [ "idiomaDefecto" => true "cabecera" => "<span class="elsevierStyleTextfn">Letter to the Editor</span>" "titulo" => "Pleural extramedullary hematopoiesis due to severe pernicious anemia" "tienePdf" => "en" "tieneTextoCompleto" => "en" "paginas" => array:1 [ 0 => array:2 [ "paginaInicial" => "e7" "paginaFinal" => "e8" ] ] "titulosAlternativos" => array:1 [ "es" => array:1 [ "titulo" => "Hematopoyesis extramedular pleural secundaria a anemia perniciosa grave" ] ] "contieneTextoCompleto" => array:1 [ "en" => true ] "contienePdf" => array:1 [ "en" => true ] "resumenGrafico" => array:2 [ "original" => 0 "multimedia" => array:7 [ "identificador" => "fig0005" "etiqueta" => "Fig. 1" "tipo" => "MULTIMEDIAFIGURA" "mostrarFloat" => true "mostrarDisplay" => false "figura" => array:1 [ 0 => array:4 [ "imagen" => "gr1.jpeg" "Alto" => 1355 "Ancho" => 905 "Tamanyo" => 169846 ] ] "descripcion" => array:1 [ "en" => "<p id="spar0005" class="elsevierStyleSimplePara elsevierViewall">Cytology of pleural effusion showing plasma cells (red arrow) and multisegmented neutrophils (yellow arrow) in the upper panel; erythroblasts (black arrow) and myeloblasts (white arrow) in the lower panel.</p>" ] ] ] "autores" => array:1 [ 0 => array:2 [ "autoresLista" => "Sergio A. Castillo-Torres, Alexandro Atilano-Díaz, David Gómez-Almaguer" "autores" => array:3 [ 0 => array:2 [ "nombre" => "Sergio A." "apellidos" => "Castillo-Torres" ] 1 => array:2 [ "nombre" => "Alexandro" "apellidos" => "Atilano-Díaz" ] 2 => array:2 [ "nombre" => "David" "apellidos" => "Gómez-Almaguer" ] ] ] ] ] "idiomaDefecto" => "en" "Traduccion" => array:1 [ "es" => array:9 [ "pii" => "S0025775318306493" "doi" => "10.1016/j.medcli.2018.09.020" "estado" => "S300" "subdocumento" => "" "abierto" => array:3 [ "ES" => false "ES2" => false "LATM" => false ] "gratuito" => false "lecturas" => array:1 [ "total" => 0 ] "idiomaDefecto" => "es" "EPUB" => "https://multimedia.elsevier.es/PublicationsMultimediaV1/item/epub/S0025775318306493?idApp=UINPBA00004N" ] ] "EPUB" => "https://multimedia.elsevier.es/PublicationsMultimediaV1/item/epub/S2387020619302359?idApp=UINPBA00004N" "url" => "/23870206/0000015300000002/v2_202004301535/S2387020619302359/v2_202004301535/en/main.assets" ] "itemAnterior" => array:19 [ "pii" => "S2387020619302335" "issn" => "23870206" "doi" => "10.1016/j.medcle.2018.05.059" "estado" => "S300" "fechaPublicacion" => "2019-07-19" "aid" => "4534" "copyright" => "Elsevier España, S.L.U." "documento" => "simple-article" "crossmark" => 1 "subdocumento" => "crp" "cita" => "Med Clin. 2019;153:88-9" "abierto" => array:3 [ "ES" => false "ES2" => false "LATM" => false ] "gratuito" => false "lecturas" => array:1 [ "total" => 0 ] "en" => array:11 [ "idiomaDefecto" => true "cabecera" => "<span class="elsevierStyleTextfn">Scientific letter</span>" "titulo" => "Herpes zoster multiplex: Report on one case" "tienePdf" => "en" "tieneTextoCompleto" => "en" "paginas" => array:1 [ 0 => array:2 [ "paginaInicial" => "88" "paginaFinal" => "89" ] ] "titulosAlternativos" => array:1 [ "es" => array:1 [ "titulo" => "Herpes zóster multiplex: descripción de un caso" ] ] "contieneTextoCompleto" => array:1 [ "en" => true ] "contienePdf" => array:1 [ "en" => true ] "resumenGrafico" => array:2 [ "original" => 0 "multimedia" => array:7 [ "identificador" => "fig0005" "etiqueta" => "Fig. 1" "tipo" => "MULTIMEDIAFIGURA" "mostrarFloat" => true "mostrarDisplay" => false "figura" => array:1 [ 0 => array:4 [ "imagen" => "gr1.jpeg" "Alto" => 515 "Ancho" => 989 "Tamanyo" => 67989 ] ] "descripcion" => array:1 [ "en" => "<p id="spar0005" class="elsevierStyleSimplePara elsevierViewall">(a) Vesicular lesions with herpetiform distribution and erythematous fundus, located on the instep, extending towards the dorsum of the first toe of the right foot; (b) erythematous plaques with some bundles of fragile vesicular lesions, which in some cases have ruptured, leaving small erosions located in the right costal area.</p>" ] ] ] "autores" => array:1 [ 0 => array:2 [ "autoresLista" => "Ana Varela-Veiga, Benigno Monteagudo, Óscar Suárez-Amor" "autores" => array:3 [ 0 => array:2 [ "nombre" => "Ana" "apellidos" => "Varela-Veiga" ] 1 => array:2 [ "nombre" => "Benigno" "apellidos" => "Monteagudo" ] 2 => array:2 [ "nombre" => "Óscar" "apellidos" => "Suárez-Amor" ] ] ] ] ] "idiomaDefecto" => "en" "Traduccion" => array:1 [ "es" => array:9 [ "pii" => "S0025775318303063" "doi" => "10.1016/j.medcli.2018.05.007" "estado" => "S300" "subdocumento" => "" "abierto" => array:3 [ "ES" => false "ES2" => false "LATM" => false ] "gratuito" => false "lecturas" => array:1 [ "total" => 0 ] "idiomaDefecto" => "es" "EPUB" => "https://multimedia.elsevier.es/PublicationsMultimediaV1/item/epub/S0025775318303063?idApp=UINPBA00004N" ] ] "EPUB" => "https://multimedia.elsevier.es/PublicationsMultimediaV1/item/epub/S2387020619302335?idApp=UINPBA00004N" "url" => "/23870206/0000015300000002/v2_202004301535/S2387020619302335/v2_202004301535/en/main.assets" ] "en" => array:15 [ "idiomaDefecto" => true "cabecera" => "<span class="elsevierStyleTextfn">Letter to the Editor</span>" "titulo" => "Vaccination of patients under immunosuppressive, immunomodulatory or biologic drugs" "tieneTextoCompleto" => true "saludo" => "To the Editor," "paginas" => array:1 [ 0 => array:2 [ "paginaInicial" => "90" "paginaFinal" => "91" ] ] "autores" => array:1 [ 0 => array:4 [ "autoresLista" => "Juan Rodríguez-García, Rafael Fernández-Santos, José Antonio García-Erce" "autores" => array:3 [ 0 => array:4 [ "nombre" => "Juan" "apellidos" => "Rodríguez-García" "email" => array:2 [ 0 => "juan.rodriguezgarcia@ssib.es" 1 => "juanrodriguez.74@hotmail.es" ] "referencia" => array:2 [ 0 => array:2 [ "etiqueta" => "<span class="elsevierStyleSup">a</span>" "identificador" => "aff0005" ] 1 => array:2 [ "etiqueta" => "*" "identificador" => "cor0005" ] ] ] 1 => array:3 [ "nombre" => "Rafael" "apellidos" => "Fernández-Santos" "referencia" => array:1 [ 0 => array:2 [ "etiqueta" => "<span class="elsevierStyleSup">b</span>" "identificador" => "aff0010" ] ] ] 2 => array:3 [ "nombre" => "José Antonio" "apellidos" => "García-Erce" "referencia" => array:1 [ 0 => array:2 [ "etiqueta" => "<span class="elsevierStyleSup">c</span>" "identificador" => "aff0015" ] ] ] ] "afiliaciones" => array:3 [ 0 => array:3 [ "entidad" => "Unidad de Vacunación del Paciente Inmunodeprimido, Servicio de Medicina Preventiva, Hospital Universitario Son Espases, Mallorca, Balearic Islands, Spain" "etiqueta" => "a" "identificador" => "aff0005" ] 1 => array:3 [ "entidad" => "Unidad de Vacunación del Paciente Inmunodeprimido, Servicio de Medicina Preventiva, Hospital Obispo Polanco, Teruel, Spain" "etiqueta" => "b" "identificador" => "aff0010" ] 2 => array:3 [ "entidad" => "Banco de Sangre y Tejidos de Navarra, Pamplona, Navarra, Spain" "etiqueta" => "c" "identificador" => "aff0015" ] ] "correspondencia" => array:1 [ 0 => array:3 [ "identificador" => "cor0005" "etiqueta" => "⁎" "correspondencia" => "Corresponding author." ] ] ] ] "titulosAlternativos" => array:1 [ "es" => array:1 [ "titulo" => "Vacunación del paciente tratado con fármacos inmunodepresores, inmunomoduladores o biológicos" ] ] "textoCompleto" => "<span class="elsevierStyleSections"><p id="par0005" class="elsevierStylePara elsevierViewall">We have read with interest the review carried out by Peremiquel-Trillas et al., <a class="elsevierStyleCrossRef" href="#bib0050"><span class="elsevierStyleSup">1</span></a> and we would like to make some clarifications:</p><p id="par0010" class="elsevierStylePara elsevierViewall">The authors state that there is a relationship between the 2009 H1N1 monovalent influenza vaccine (Pandemrix®) and Guillain-Barré syndrome (GBS) quantifying its effect in an additional case per million doses. It is true that some population studies, such as that referenced by the authors and a meta-analysis with important methodological limitations, suggest this relationship. However, in none of the 2 cases was this association adjusted or stratified by suffering from influenza or other respiratory infection in that season. In fact, the study in which the authors base their claim indicated that 5 of the 9 cases of GBS in patients vaccinated with Pandemrix® had suffered a respiratory infection compared to one of 8 of those vaccinated with the seasonal vaccine. The meta-analysis of Martín Arias et al., <a class="elsevierStyleCrossRef" href="#bib0055"><span class="elsevierStyleSup">2</span></a> in addition to not excluding studies that do not adjust for suffering from respiratory or influenza infection on the development of GBS to eliminate its effect, it does not assess the quality of the studies. There are also population studies subsequent to those included in this meta-analysis with large samples conducted by the National Health Services of different countries, which do take this factor into account in the analysis. All of them conclude that it is the suffering from respiratory or influenza infection that coincides with the time of administration of Pandemrix® and not the vaccine itself, the factor associated with the development of GBS, finding no association between having received Pandemrix® and the development of GBS when adjusting or stratifying by respiratory infection or influenza, in addition to other factors. We have summarized the review of these studies in <a class="elsevierStyleCrossRef" href="#tbl0005">Table 1</a>.</p><elsevierMultimedia ident="tbl0005"></elsevierMultimedia><p id="par0015" class="elsevierStylePara elsevierViewall">On the other hand, the authors state that a reduction in the response to the pneumococcal vaccine is observed in patients on treatment with methotrexate, methotrexate and anti-TNF, azathioprine and 6-mercaptopurine. The study on which this claim is based, as it often happens when referring to the loss of immunogenicity of the pneumococcal vaccine in the patient with an impaired immune system, used the old pneumococcal polysaccharide vaccine. The conjugate vaccine, approved since 2011 for adults in our country, is more immunogenic and generates immune memory by stimulating a response “<span class="elsevierStyleItalic">T dependent</span>”, therefore, the loss of immunity is lower.<a class="elsevierStyleCrossRef" href="#bib0060"><span class="elsevierStyleSup">3</span></a> It would be desirable to base this claim on efficacy studies of the currently recommended pneumococcal conjugate vaccine regimens for these patients.</p><p id="par0020" class="elsevierStylePara elsevierViewall">It is also stated that patients under treatment with eculizumab have an increased risk of invasive infection by capsulated bacteria, such as meningococcus of any serogroup, type b <span class="elsevierStyleItalic">H. influenzae</span> and pneumococcus, recommending vaccination against meningococcus with vaccines available in each territory and also against type b <span class="elsevierStyleItalic">H. influenzae</span> and pneumococcus. It should be noted that patients with terminal complement deficiencies are especially at high risk of acquiring invasive <span class="elsevierStyleItalic">Neisseria meningitidis</span> infections, with the wrongly called “rare” serogroups being especially common. For this reason, coverage of serogroups W-135 and Y contained in the quadrivalent conjugate vaccine is particularly important and is also becoming more common in Spain.<a class="elsevierStyleCrossRef" href="#bib0065"><span class="elsevierStyleSup">4</span></a> So much so that the manufacturer requires the certificate of having received this vaccine (and not others) regardless of the vaccines available in each territory before the start of the administration of the first dose of eculizumab. Therefore, in addition to the vaccine against serogroup B it must be ensured that these patients always receive the quadrivalent conjugate vaccine. If there is a need to reinforce the protection conferred by the quadrivalent against the serogroup <span class="elsevierStyleSmallCaps">C</span>, the monovalent vaccine should be added to this serogroup at a later stage.<a class="elsevierStyleCrossRef" href="#bib0065"><span class="elsevierStyleSup">4</span></a></p><p id="par0025" class="elsevierStylePara elsevierViewall">Finally, in patients receiving immunosuppressive, immunomodulatory or biologic drugs, the administration of other vaccines, such as the antimeningococcal and those against <span class="elsevierStyleItalic">H. influenzae</span> type b should also be considered, since, to the state of functional hyposplenism commonly associated with the underlying disease, we need to add the one caused by some of these drugs, such as steroids or others that affect the different phases of maturation and trophism of the B lymphocyte such as rituximab, belimumab, ocrelizumab and others.<a class="elsevierStyleCrossRef" href="#bib0070"><span class="elsevierStyleSup">5</span></a></p><span id="sec0005" class="elsevierStyleSection elsevierViewall"><span class="elsevierStyleSectionTitle" id="sect0005">Conflict of interests</span><p id="par0030" class="elsevierStylePara elsevierViewall">The first author has received fees as a lecturer and grants for scientific activities by GSK, Pfizer and Pasteur. There are no other conflicts of interest on the part of the authors.</p></span></span>" "textoCompletoSecciones" => array:1 [ "secciones" => array:2 [ 0 => array:2 [ "identificador" => "sec0005" "titulo" => "Conflict of interests" ] 1 => array:1 [ "titulo" => "References" ] ] ] "pdfFichero" => "main.pdf" "tienePdf" => true "NotaPie" => array:1 [ 0 => array:2 [ "etiqueta" => "☆" "nota" => "<p class="elsevierStyleNotepara" id="npar0015">Please cite this article as: Rodríguez-García J, Fernández-Santos R, García-Erce JA. Vacunación del paciente con fármacos inmunodepresores, inmunomoduladores o biológicos. Med Clin. 2019;153:90–91.</p>" ] ] "multimedia" => array:1 [ 0 => array:8 [ "identificador" => "tbl0005" "etiqueta" => "Table 1" "tipo" => "MULTIMEDIATABLA" "mostrarFloat" => true "mostrarDisplay" => false "detalles" => array:1 [ 0 => array:3 [ "identificador" => "at1" "detalle" => "Table " "rol" => "short" ] ] "tabla" => array:3 [ "leyenda" => "<p id="spar0010" class="elsevierStyleSimplePara elsevierViewall">β: linear regression coefficient; HR: hazard ratio; 95% CI: 95% confidence interval; RI: relative incidence; IRR: incidence rate ratio.</p>" "tablatextoimagen" => array:1 [ 0 => array:2 [ "tabla" => array:1 [ 0 => """ <table border="0" frame="\n \t\t\t\t\tvoid\n \t\t\t\t" class=""><thead title="thead"><tr title="table-row"><th class="td" title="\n \t\t\t\t\ttable-head\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t" scope="col" style="border-bottom: 2px solid black">Author \t\t\t\t\t\t\n \t\t\t\t\t\t</th><th class="td" title="\n \t\t\t\t\ttable-head\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t" scope="col" style="border-bottom: 2px solid black">Year \t\t\t\t\t\t\n \t\t\t\t\t\t</th><th class="td" title="\n \t\t\t\t\ttable-head\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t" scope="col" style="border-bottom: 2px solid black">Country \t\t\t\t\t\t\n \t\t\t\t\t\t</th><th class="td" title="\n \t\t\t\t\ttable-head\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t" scope="col" style="border-bottom: 2px solid black">Coverage \t\t\t\t\t\t\n \t\t\t\t\t\t</th><th class="td" title="\n \t\t\t\t\ttable-head\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t" scope="col" style="border-bottom: 2px solid black">Cases of the Guillain-Barré syndrome \t\t\t\t\t\t\n \t\t\t\t\t\t</th><th class="td" title="\n \t\t\t\t\ttable-head\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t" scope="col" style="border-bottom: 2px solid black">Risk, 95% CI(adjusted by respiratory infection and/or influenza and/or gastrointestinal) \t\t\t\t\t\t\n \t\t\t\t\t\t</th></tr></thead><tbody title="tbody"><tr title="table-row"><td class="td-with-role" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t ; entry_with_role_rowhead " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">Ghaderi S et al.<a class="elsevierStyleCrossRef" href="#bib0075"><span class="elsevierStyleSup">6</span></a> \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="char" valign="\n \t\t\t\t\ttop\n \t\t\t\t">2016 \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">Norway \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">4,832,211 \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">46 \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">HR: 1.11 (0.51–2.43)IRR: 1.31 (0.65–2.66) \t\t\t\t\t\t\n \t\t\t\t</td></tr><tr title="table-row"><td class="td-with-role" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t ; entry_with_role_rowhead " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">Iqbal S<a class="elsevierStyleCrossRef" href="#tblfn0005"><span class="elsevierStyleSup">a</span></a> et al.<a class="elsevierStyleCrossRef" href="#bib0080"><span class="elsevierStyleSup">7</span></a> \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="char" valign="\n \t\t\t\t\ttop\n \t\t\t\t">2015 \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">USA \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">>11,000,000 \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">52,000 \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">β: −0.0011 (−0.0069–0.0047) \t\t\t\t\t\t\n \t\t\t\t</td></tr><tr title="table-row"><td class="td-with-role" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t ; entry_with_role_rowhead " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">Romio S et al.<a class="elsevierStyleCrossRef" href="#bib0085"><span class="elsevierStyleSup">8</span></a> \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="char" valign="\n \t\t\t\t\ttop\n \t\t\t\t">2014 \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">Europe \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">>50,000,000 \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">303 \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">RI: 1.4 (0.7–2.8) \t\t\t\t\t\t\n \t\t\t\t</td></tr><tr title="table-row"><td class="td-with-role" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t ; entry_with_role_rowhead " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">Persson I et al.<a class="elsevierStyleCrossRef" href="#bib0090"><span class="elsevierStyleSup">9</span></a> \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="char" valign="\n \t\t\t\t\ttop\n \t\t\t\t">2014 \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">Sweden \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">5,845,039 \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">170 \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">HR: 1.17 (0.85–1.62) \t\t\t\t\t\t\n \t\t\t\t</td></tr></tbody></table> """ ] "imagenFichero" => array:1 [ 0 => "xTab2281243.png" ] ] ] "notaPie" => array:1 [ 0 => array:3 [ "identificador" => "tblfn0005" "etiqueta" => "a" "nota" => "<p class="elsevierStyleNotepara" id="npar0005">Hospitalizations 2005–2009.</p> <p class="elsevierStyleNotepara" id="npar0010"><span class="elsevierStyleItalic">Source</span>: table based on the review of the originals by: Ghaderi S et al., Iqbal S et al., Romio S et al. and Persson I et al.</p>" ] ] ] "descripcion" => array:1 [ "en" => "<p id="spar0005" class="elsevierStyleSimplePara elsevierViewall">Güillain-Barré and influenza vaccination.</p>" ] ] ] "bibliografia" => array:2 [ "titulo" => "References" "seccion" => array:1 [ 0 => array:2 [ "identificador" => "bibs0015" "bibliografiaReferencia" => array:9 [ 0 => array:3 [ "identificador" => "bib0050" "etiqueta" => "1" "referencia" => array:1 [ 0 => array:2 [ "contribucion" => array:1 [ 0 => array:2 [ "titulo" => "Vaccines that should be administered to patients receiving treatment with immunosuppressive, immunomodulatory and/or biological drugs [Article in English, Spanish]" "autores" => array:1 [ 0 => array:2 [ "etal" => false "autores" => array:4 [ 0 => "P. Peremiquel-Trillas" 1 => "L.M. Leguízamo" 2 => "C. Asensio Ostos" 3 => "X. Martínez-Gómez" ] ] ] ] ] "host" => array:1 [ 0 => array:2 [ "doi" => "10.1016/j.medcli.2018.05.008" "Revista" => array:2 [ "tituloSerie" => "Med Clin (Barc)" "fecha" => "2018" ] ] ] ] ] ] 1 => array:3 [ "identificador" => "bib0055" "etiqueta" => "2" "referencia" => array:1 [ 0 => array:2 [ "contribucion" => array:1 [ 0 => array:2 [ "titulo" => "Guillain-Barré syndrome and influenza vaccines: a meta-analysis" "autores" => array:1 [ 0 => array:2 [ "etal" => false "autores" => array:5 [ 0 => "L.H. Martín Arias" 1 => "R. Sanz" 2 => "M. Sáinz" 3 => "C. Treceño" 4 => "A. Carvajal" ] ] ] ] ] "host" => array:1 [ 0 => array:2 [ "doi" => "10.1016/j.vaccine.2015.05.013" "Revista" => array:6 [ "tituloSerie" => "Vaccine" "fecha" => "2015" "volumen" => "33" "paginaInicial" => "3773" "paginaFinal" => "3778" "link" => array:1 [ 0 => array:2 [ "url" => "https://www.ncbi.nlm.nih.gov/pubmed/25999283" "web" => "Medline" ] ] ] ] ] ] ] ] 2 => array:3 [ "identificador" => "bib0060" "etiqueta" => "3" "referencia" => array:1 [ 0 => array:2 [ "contribucion" => array:1 [ 0 => array:2 [ "titulo" => "Pneumococcal conjugate and plain polysaccharide vaccines have divergent effects on antigen-specific B cells" "autores" => array:1 [ 0 => array:2 [ "etal" => true "autores" => array:6 [ 0 => "E.A. Clutterbuck" 1 => "R. Lazarus" 2 => "L.M. Yu" 3 => "J. Bowman" 4 => "E.A. Bateman" 5 => "L. Diggle" ] ] ] ] ] "host" => array:1 [ 0 => array:2 [ "doi" => "10.1093/infdis/jis212" "Revista" => array:6 [ "tituloSerie" => "J Infect Dis" "fecha" => "2012" "volumen" => "205" "paginaInicial" => "1408" "paginaFinal" => "1416" "link" => array:1 [ 0 => array:2 [ "url" => "https://www.ncbi.nlm.nih.gov/pubmed/22457293" "web" => "Medline" ] ] ] ] ] ] ] ] 3 => array:3 [ "identificador" => "bib0065" "etiqueta" => "4" "referencia" => array:1 [ 0 => array:2 [ "contribucion" => array:1 [ 0 => array:2 [ "titulo" => "Vaccination of patients with paroxysmal nocturnal hemoglobinuria under eculizumab treatment" "autores" => array:1 [ 0 => array:2 [ "etal" => false "autores" => array:3 [ 0 => "J. Rodríguez-García" 1 => "R. Fernández-Santos" 2 => "J.A. García-Erce" ] ] ] ] ] "host" => array:1 [ 0 => array:1 [ "Revista" => array:5 [ "tituloSerie" => "Med Clin (Barc)" "fecha" => "2012" "volumen" => "138" "paginaInicial" => "640" "paginaFinal" => "641" ] ] ] ] ] ] 4 => array:3 [ "identificador" => "bib0070" "etiqueta" => "5" "referencia" => array:1 [ 0 => array:2 [ "contribucion" => array:1 [ 0 => array:2 [ "titulo" => "Post-splenectomy and hyposplenic states" "autores" => array:1 [ 0 => array:2 [ "etal" => false "autores" => array:3 [ 0 => "A. Di Sabatino" 1 => "R. Carsetti" 2 => "G.R. Corazza" ] ] ] ] ] "host" => array:1 [ 0 => array:2 [ "doi" => "10.1016/S0140-6736(10)61493-6" "Revista" => array:6 [ "tituloSerie" => "Lancet" "fecha" => "2011" "volumen" => "378" "paginaInicial" => "86" "paginaFinal" => "97" "link" => array:1 [ 0 => array:2 [ "url" => "https://www.ncbi.nlm.nih.gov/pubmed/21474172" "web" => "Medline" ] ] ] ] ] ] ] ] 5 => array:3 [ "identificador" => "bib0075" "etiqueta" => "6" "referencia" => array:1 [ 0 => array:2 [ "contribucion" => array:1 [ 0 => array:2 [ "titulo" => "Risk of Guillain-Barré syndrome after exposure to pandemic influenza A(H1N1)pdm09 vaccination or infection: a Norwegian population-based cohort study" "autores" => array:1 [ 0 => array:2 [ "etal" => false "autores" => array:6 [ 0 => "S. Ghaderi" 1 => "N. Gunnes" 2 => "I.J. Bakken" 3 => "P. Magnus" 4 => "L. Trogstad" 5 => "S.E. Håberg" ] ] ] ] ] "host" => array:1 [ 0 => array:2 [ "doi" => "10.1007/s10654-015-0047-0" "Revista" => array:6 [ "tituloSerie" => "Eur J Epidemiol" "fecha" => "2016" "volumen" => "31" "paginaInicial" => "67" "paginaFinal" => "72" "link" => array:1 [ 0 => array:2 [ "url" => "https://www.ncbi.nlm.nih.gov/pubmed/26008750" "web" => "Medline" ] ] ] ] ] ] ] ] 6 => array:3 [ "identificador" => "bib0080" "etiqueta" => "7" "referencia" => array:1 [ 0 => array:2 [ "contribucion" => array:1 [ 0 => array:2 [ "titulo" => "Relationship between Guillain-Barré syndrome, influenza-related hospitalizations, and influenza vaccine coverage" "autores" => array:1 [ 0 => array:2 [ "etal" => false "autores" => array:4 [ 0 => "S. Iqbal" 1 => "R. Li" 2 => "P. Gargiullo" 3 => "C. Vellozzi" ] ] ] ] ] "host" => array:1 [ 0 => array:2 [ "doi" => "10.1016/j.vaccine.2015.02.064" "Revista" => array:6 [ "tituloSerie" => "Vaccine" "fecha" => "2015" "volumen" => "33" "paginaInicial" => "2045" "paginaFinal" => "2049" "link" => array:1 [ 0 => array:2 [ "url" => "https://www.ncbi.nlm.nih.gov/pubmed/25749247" "web" => "Medline" ] ] ] ] ] ] ] ] 7 => array:3 [ "identificador" => "bib0085" "etiqueta" => "8" "referencia" => array:1 [ 0 => array:2 [ "contribucion" => array:1 [ 0 => array:2 [ "titulo" => "Guillain-Barré syndrome and adjuvanted pandemic influenza A (H1N1) 2009 vaccines: a multinational self-controlled case series in Europe" "autores" => array:1 [ 0 => array:2 [ "etal" => true "autores" => array:6 [ 0 => "S. Romio" 1 => "D. Weibel" 2 => "J.P. Dieleman" 3 => "H.K. Olberg" 4 => "C.S. de Vries" 5 => "C. Sammon" ] ] ] ] ] "host" => array:1 [ 0 => array:1 [ "Revista" => array:4 [ "tituloSerie" => "PLoS One" "fecha" => "2014" "volumen" => "3" "paginaInicial" => "e82222" ] ] ] ] ] ] 8 => array:3 [ "identificador" => "bib0090" "etiqueta" => "9" "referencia" => array:1 [ 0 => array:2 [ "contribucion" => array:1 [ 0 => array:2 [ "titulo" => "Risks of neurological and immune-related diseases, including narcolepsy, after vaccination with Pandemrix: a population- and registry-based cohort study with over 2 years of follow-up" "autores" => array:1 [ 0 => array:2 [ "etal" => false "autores" => array:6 [ 0 => "I. Persson" 1 => "F. Granath" 2 => "J. Askling" 3 => "J.F. Ludvigsson" 4 => "T. Olsson" 5 => "N. Feltelius" ] ] ] ] ] "host" => array:1 [ 0 => array:2 [ "doi" => "10.1111/joim.12150" "Revista" => array:6 [ "tituloSerie" => "J Intern Med" "fecha" => "2014" "volumen" => "275" "paginaInicial" => "172" "paginaFinal" => "190" "link" => array:1 [ 0 => array:2 [ "url" => "https://www.ncbi.nlm.nih.gov/pubmed/24134219" "web" => "Medline" ] ] ] ] ] ] ] ] ] ] ] ] ] "idiomaDefecto" => "en" "url" => "/23870206/0000015300000002/v2_202004301535/S2387020619302347/v2_202004301535/en/main.assets" "Apartado" => array:4 [ "identificador" => "43309" "tipo" => "SECCION" "en" => array:2 [ "titulo" => "Letters to the Editor" "idiomaDefecto" => true ] "idiomaDefecto" => "en" ] "PDF" => "https://static.elsevier.es/multimedia/23870206/0000015300000002/v2_202004301535/S2387020619302347/v2_202004301535/en/main.pdf?idApp=UINPBA00004N&text.app=https://www.elsevier.es/" "EPUB" => "https://multimedia.elsevier.es/PublicationsMultimediaV1/item/epub/S2387020619302347?idApp=UINPBA00004N" ]
Journal Information
Vol. 153. Issue 2.
Pages 90-91 (July 2019)
Share
Download PDF
More article options
Vol. 153. Issue 2.
Pages 90-91 (July 2019)
Letter to the Editor
Vaccination of patients under immunosuppressive, immunomodulatory or biologic drugs
Vacunación del paciente tratado con fármacos inmunodepresores, inmunomoduladores o biológicos
Visits
3
a Unidad de Vacunación del Paciente Inmunodeprimido, Servicio de Medicina Preventiva, Hospital Universitario Son Espases, Mallorca, Balearic Islands, Spain
b Unidad de Vacunación del Paciente Inmunodeprimido, Servicio de Medicina Preventiva, Hospital Obispo Polanco, Teruel, Spain
c Banco de Sangre y Tejidos de Navarra, Pamplona, Navarra, Spain
This item has received
Article information
These are the options to access the full texts of the publication Medicina Clínica (English Edition)
Subscriber
Subscribe
Purchase
Contact
Phone for subscriptions and reporting of errors
From Monday to Friday from 9 a.m. to 6 p.m. (GMT + 1) except for the months of July and August which will be from 9 a.m. to 3 p.m.
Calls from Spain
932 415 960
Calls from outside Spain
+34 932 415 960
E-mail