Advanced interatrial block (IAB) is an independent risk factor for ischaemic stroke. This study aimed to analyse whether advanced IAB predicts recurrence of embolic stroke of undetermined source (ESUS).
Methods104 patients with a confirmed diagnosis of ESUS were followed up for a median period of 15 months (interquartile range, 10-48). We recorded data on clinical variables, P-wave characteristics, and presence of IAB on the electrocardiogram (ECG). ECG findings were interpreted by a blinded, centralised rater at (XXXX2). ESUS recurrence was the primary outcome variable.
ResultsMedian age was 47 years (range, 19-85); 50% of patients were women. IAB was detected in 36 patients (34.6%); IAB was partial in 29 cases (27.9%) and advanced in 7 (6.7%). Sixteen patients (15.4%) presented stroke recurrence; of these, 5 had partial and 4 had advanced IAB (P = .01; odds ratio [OR] = 9.44; 95% confidence interval [CI], 1.88-47.46; relative risk [RR] = 4.62; 95% CI, 2.01-10.61). Median P-wave duration was longer in patients with stroke recurrence (P = .009). The multivariate logistic regression analysis identified the following independent risk factors for stroke recurrence: advanced IAB (P < .001; OR = 10.86; 95% CI, 3.07-38.46), male sex (P = .028; OR = 4.6; 95% CI, 1.18-17.96), and age older than 50 years (P = .039; OR = 3.84; 95% CI, 1.06-13.88). In the Cox proportional hazards model, the risk variables identified were age older than 50 years (P = .002; hazard ratio, 7.04; 95% CI, 2.06-23.8) and P-wave duration (per ms) (P = .007; hazard ratio, 1.02; 95% CI, 1.01-1.04).
ConclusionsAdvanced IAB and age older than 50 years predict ESUS recurrence.
El bloqueo interatrial avanzado (BIA-a) es considerado un factor de riesgo independiente para infarto cerebral (IC). Nuestro objetivo fue analizar si el BIA-a predice recurrencia de IC en pacientes con infarto cerebral embólico de origen no determinado (ESUS).
MétodosCiento cuatro pacientes con diagnóstico confirmado de ESUS fueron seguidos durante una mediana de 15 meses (RIQ 10-48). Los datos clínicos, las características de la onda P y presencia de BIA en electrocardiograma realizado durante el evento índice, fueron registrados. La interpretación de los electrocardiogramas se realizó de forma centralizada y ciega en (XXXX2). La recurrencia de ESUS fue el desenlace primario.
ResultadosLa mediana de edad de los casos fue de 47 años (rango 19-85); 50% fueron mujeres. Se encontró BIA en 36 casos (34,6%); parcial (BIA-p) en 29 (27,9%) y BIA-a en 7 (6,7%). Dieciséis pacientes (15,4%) presentaron IC recurrente; de los cuales 5 tenían BIA-p y 4 BIA-a (p = 0,01;OR 9,44: IC 95% 1,88-47,46). La mediana de duración de la onda P fue mayor en pacientes con recurrencia (p = 0,009). En el análisis multivariado de regresión logística, los factores de riesgo independientes para recurrencia de IC fueron: el BIA-a (p < 0,001; OR 10,86: IC 95% 3,07-38,46), género masculino (p = 0,028; OR 4,6: IC 95% 1,18-17,96) y la edad mayor a 50 años (p = 0,039; OR 3,84: IC 95% 1,06-13,88); en riesgos proporcionales de Cox fueron: edad mayor a 50 años (p = 0,002; HR 7,04: IC 95% 2,06-23,8) y duración de la onda P (por ms) p = 0,007 (HR 1,02: IC 95% 1,01-1,04).
ConclusionesEl BIA-a y edad mayor a 50 años predicen recurrencia de ESUS.
Interatrial block (IAB), easily identifiable in surface electrocardiography (ECG), is a strong predictor of atrial tachyarrhythmia and has been associated with increased risk of atrial fibrillation (AF) and stroke.1–10
Patients with stroke and a high clinical risk of recurrence may benefit from pharmacological treatment, for example with antiarrhythmic and anticoagulant drugs, and IAB may help in guiding treatment.11–16
The term embolic stroke of unknown source (ESUS) was recently introduced by an international working group on cryptogenic stroke with a view to identifying patients with clinical and radiological characteristics suggesting embolic stroke, whose embolic source remains unknown despite proper assessment.17
Patients with ESUS constitute a heterogeneous group who are very likely to present an obscure embolic source and have a high risk of stroke recurrence.17–19 Recent studies support the role of the left atrium in stroke risk and prevention, suggesting greater relevance of atrial dysfunction in stroke risk.20–24
This study aimed to analyse whether IAB predicts higher risk of stroke recurrence in patients with ESUS.
MethodsWe analysed data from 104 patients with confirmed diagnosis of ESUS, attended at the Instituto Nacional de Neurología y Neurocirugía in Mexico City, between January 2000 and April 2018. We used data prospectively included in our centre’s stroke registry, following a standardised protocol. The registry records demographic data, vascular risk factors, stroke subtype, treatment, laboratory and imaging findings, and functional status at discharge and during progression.
We used the diagnostic criteria proposed by the Cryptogenic Stroke/ESUS International Working Group, which defines ESUS as a non-lacunar stroke in the absence of intra- or extracranial atherosclerosis (stenosis > 50% in the artery irrigating the infarcted area), with no cardioembolic sources detected by transthoracic echocardiogram and Holter cardiac monitoring for at least 24 hours, and no other identified causes of stroke.17 We calculated the score on the CHA2DS2VASc scale for all patients.
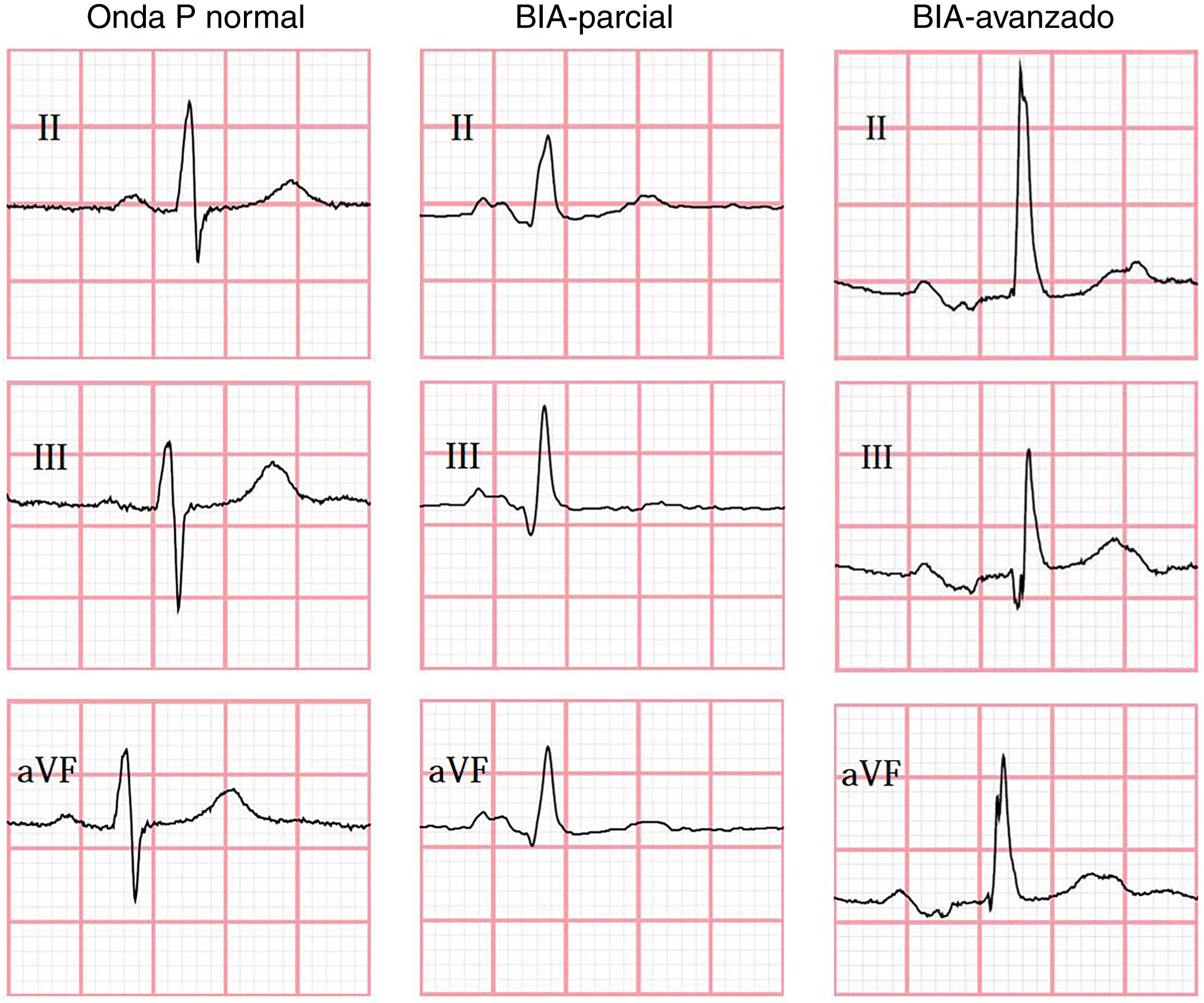
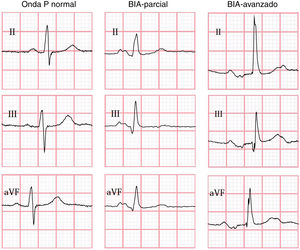
IAB was diagnosed according to the consensus criteria published by Bayés de Luna et al.1 in 2012, which define partial IAB (pIAB) as a P-wave duration > 120 ms, and advanced IAB (aIAB) as a P-wave with duration > 120 ms and biphasic morphology, with a negative terminal phase in leads II, III, and aVF.1
In all cases, the ECG performed during the index event was assessed and interpreted by blinded raters at Queen’s University (Canada), using the semi-automatic Cardio Calipers 3.3 software. We recorded data on heart rate, P-wave duration, PR interval, and P-wave voltage in lead I. Patients were subdivided into 3 groups, according to the ECG findings (Fig. 1): no IAB, pIAB, and aIAB. We excluded patients with non-sinus rhythm, multiple atrial or ventricular complexes, and/or second- or third-degree atrioventricular block.
The primary outcome was stroke recurrence in patients with ESUS, defined as a new stroke with a new neurological symptom or exacerbation of the previous neurological symptoms lasting at least 24 hours and confirmed by brain imaging studies.
Follow-up included neurological assessment at admission to our centre, at discharge, and at 3 and 6 months after hospital discharge, as well as at the end of follow-up.
Functional status was assessed using the modified Rankin Scale (mRS), with scores of 0-2 indicating good functional prognosis and scores of 3-5 suggesting poor prognosis.
The study was approved by our hospital’s ethics committee; due to the study characteristics, informed consent was not required.
Statistical analysisStatistical analysis was performed using the IBM SPSS software for Mac (version 22). Categorical variables are expressed as frequencies and percentages. Continuous variables are expressed as means and standard deviation (SD) or medians and interquartile ranges, in accordance with the results of the Kolmogorov-Smirnov test for normality. We used the chi-square test or the Fisher exact test to compare categorical variables. We compared the means of continuous variables with the t test or the Mann-Whitney U test and used ANOVA or the Kruskal-Wallis test to analyse variance, in accordance with data distribution. We conducted a multivariate analysis using binary logistic regression and a Cox proportional hazards model.
ResultsOur centre’s database included a total of 1673 patients with stroke; 104 (6.2%) met criteria for ESUS, and were included in our study. The median age of the study population was 47 years (range: 19-85); 57 patients (54%) were younger than 50 years and 52 (50%) were women. The most frequent risk factors were arterial hypertension, in 46 cases (44%), diabetes mellitus in 19 (18%), smoking in 34 (33%), dyslipidaemia in 35 (34%), and drug use in 6 cases (5.8%).
IAB was detected in 36 patients (34.6%): 29 with pIAB (27.9%) and 7 with aIAB (6.7%). Table 1 shows patients’ demographic and clinical characteristics according to presence of IAB. Presence of pIAB and aIAB was more frequently observed in patients older than 50 years.
Clinical and demographic characteristics of patients with ESUS.
| No IAB, n = 68 | Partial IAB, n = 29 | Advanced IAB, n = 7 | Fisher exact test P | |
|---|---|---|---|---|
| Age in years, median (Q1-Q3) | 47 (31.5-66) | 53 (34-68) | 66 (49-73) | .03a |
| Men, n (%) | 30 (44) | 19 (65.5) | 3 (43) | .16 |
| Arterial hypertension, n (%) | 27 (40) | 15 (52) | 4 (57) | .44 |
| Diabetes mellitus type II, n (%) | 9 (13) | 8 (27.6) | 2 (28.6) | .14 |
| Smoking, n (%) | 20 (29.4) | 12 (41) | 2 (28.6) | .96 |
| Dyslipidaemia, n (%) | 27 (31) | 7 (24) | 1 (14) | .71 |
| Ischaemic heart disease, n (%) | 1 (1.5) | 0 (0) | 1 (14.3) | .21 |
| History of drug dependence, n (%) | 6 (8.8) | 0 (0) | 0 (0) | .25 |
| CHA2DS2-VASc score ≥ 3, n (%) | 52 (76.5) | 23 (79) | 6 (86) | 1.0 |
| Stroke recurrence, n (%) | 7 (10) | 5 (17) | 4 (57) | .01 |
ESUS: embolic stroke of unknown source; IAB: interatrial block; Q1: first quartile; Q3: third quartile.
At the end of follow-up (median of 15 months, Q1-Q3: 10-48), functional progression was good (mRS of 0-2) in 82% of patients (n = 85), and poor (mRS of 3-5) in 18% (n = 19). No deaths were recorded in our cohort.
Stroke recurrence was reported in 16 patients (15.4%), with 14 in the territory of the middle cerebral artery and 2 in the posterior vascular territory; 4 of these patients were younger than 50 years, and 12 were older than 50 (P = .008; OR: 4.76 [95% CI, 1.42-15.98]; RR: 3.78 [95% CI, 1.31-10.96]); 7 of these patients did not present IAB, 5 presented pIAB (P = .76), and 4 presented aIAB (P = .010; OR: 9.44 [95% IC, 1.88-47.46]; RR: 4.62 [95% CI, 2.01-10.61]). The incidence of stroke recurrence in our series amounted to 0.046 (4.6 cases of stroke recurrence per 100 person-years of follow-up among patients with ESUS).
P-wave duration was greater in patients with stroke recurrence, with a median of 123 ms vs 113 ms in patients without recurrence (P = .009), whereas no significant differences were observed in the PR interval or P-wave voltage in lead I (Table 2).
Electrocardiographic characteristics of patients with or without stroke recurrence.
| Recurrence, n = 16 | Without recurrence, n = 88 | P | |
|---|---|---|---|
| Age in years, n (median, Q1-Q3) | 16 (62, 49-72) | 88 (44.5, 31-63) | .023 |
| Heart rate, n (mean ± SD) | 16 (70.6 ± 14) | 88 (72.6 ± 15) | .63a |
| P-wave duration (ms), n (median, Q1-Q3) | 16 (123, 114-134) | 88 (113, 102.6-120.7) | .009 |
| Duration of PR interval (ms), n (median, Q1-Q3) | 16 (162.5, 150-179.5) | 88 (157, 144-171) | .14 |
| P-wave voltage (mV) in lead I, n (median, Q1-Q3) | 15 (0.10, 0.08-0.12) | 86 (0.09, 0.07-0.12) | .35 |
| CHA2DS2-VASc score≥ 3, n | 12 | 69 | .75b |
Non-parametric Kolmogorov-Smirnov test, Mann–Whitney U test (bilateral significance), Kruskal-Wallis test with equality of variance.
Q1: first quartile; Q3: third quartile; SD: standard deviation.
A multivariate analysis using binary logistic regression (Table 3) identified the following independent risk factors for stroke recurrence: aIAB (P < .001; OR: 10.86 [95% CI, 3.07-38.46]), male sex (P = .028; OR: 4.60 [95% CI, 1.18-17.96]), and age older than 50 years (P = .039; OR: 3.84 [95% CI, 1.06-13.88]). The independent risk factors identified in the Cox proportional hazards model (Table 3) were age older than 50 years (P = .002; HR: 7.04 [95% CI, 2.06-23.8]) and P-wave duration (per ms) (P = .007; HR: 1.02 [95% CI, 1.01-1.04]).
Multivariate analysis of stroke using a logistic regression model and a Cox proportional hazards model.
| Risk factora | P | OR (95% CI) |
|---|---|---|
| aIAB | < .001 | 10.86 (3.07-38.46) |
| Male sex | .028 | 4.60 (1.18-17.96) |
| Age > 50 years | .039 | 3.84 (1.06-13.88) |
| Risk factorb | P | HR (95% CI) |
|---|---|---|
| Age > 50 years | .002 | 7.04 (2.06-23.8) |
| P-wave duration (per millisecond) | .007 | 1.02 (1.01-1.04) |
aIAB: advanced interatrial block; CI: confidence interval; ESUS: embolic stroke of unknown source; HR: hazard ratio; OR: odds ratio.
Patients with stroke of unknown origin must be properly assessed to rule out haematological disorders, given the high level of suspicion.25
Presence of aIAB is associated with increased risk of stroke.2,8,12,13 This association seems to be limited to non-lacunar strokes, and is independent of the documented presence of AF. In our study, aIAB was associated with a higher stroke recurrence rate in patients with ESUS.
Our findings support the idea that atrial dysfunction (atrial disease) should be considered an independent risk factor for ischaemic stroke, and also suggest that left atrial thromboembolism may occur regardless of the documented presence of AF. Although patients in our study may have presented subclinical AF, our most relevant finding is that the risk of recurrence exists when IAB is observed with surface ECG alone; this technique is simple, accessible, and highly reproducible.
O’Neal et al.8 examined the association between aIAB and stroke incidence in their study “The atherosclerosis risk in communities study”.8 Similarly to our findings, they identified aIAB as an independent risk factor for stroke.8 However, our study includes a carefully selected population of patients with stroke, in whom we had ruled out AF (clinically and by Holter monitoring) and carotid atherosclerotic disease, although clinical and imaging characteristics strongly suggested an emboligenic mechanism.
The incidence of stroke in the United States is estimated at approximately 700 000 new cases per year; of these, one-third correspond to cryptogenic strokes, for which the most effective treatment for secondary prevention has not yet been determined.26,27 Some ongoing studies are assessing whether treatment with direct oral anticoagulants (DOAC) decreases the risk of recurrence.28–30 To date, findings from 2 studies in patients with ESUS and treated with DOACs have been published: the NAVIGATE ESUS trial, which compared the effectiveness of 15 mg rivaroxaban against 100 mg aspirin, and the RE-SPECT ESUS trial, which compared the effectiveness of 2 doses of dabigatran (100 mg and 150 mg twice daily) against aspirin. Neither trial demonstrated the efficacy of DOAC in preventing stroke recurrence in this group of patients; in the case of rivaroxaban, the treatment was associated with increased risk of haemorrhage.28,31 However, an important consideration is that both treatment groups in the NAVIGATE ESUS trial showed a high rate of recurrence, which confirms the need for a better patient selection and therapeutic options. It is yet to be determined whether selecting patients using the same method as in our study and randomisation including only the group with aIAB may have segregated a population with higher risk.
The ARCADIA randomised trial is currently underway at the time of writing, and includes patients with ESUS and atrial cardiopathy, who were randomised to receive apixaban or aspirin.32 Its results may clarify whether the high risk of stroke recurrence in patients with ESUS and atrial dysfunction markers is reduced with other alternatives for anticoagulation.
Our findings suggest that the detection of aIAB in patients with ESUS enables us to identify patients at high risk of recurrence, who may benefit from particular pharmacological therapies.
LimitationsWe should mention some limitations of the study. Its design, a retrospective analysis of a series of prospectively included patients, may have introduced bias. To strengthen our findings, we should underscore that ECG recordings were assessed by raters blinded to the final outcomes. Furthermore, our study was performed at a single centre. Finally, our study sample is limited, with a mean age younger than that reported in other series, which prevents us from drawing firm conclusions; a prospective study with a larger number of patients would be highly desirable, as well as a study comparing stroke recurrence in patients with aIAB and with or without arterial hypertension, as outcomes may be different.33 Future trials should answer other existing questions, including whether antiarrhythmic and/or anticoagulant drugs may be used in this subgroup of patients to prevent atrial fibrillation and stroke recurrence.
ConclusionsAdvanced interatrial block and age older than 50 years predict recurrence of embolic stroke of unknown source.
Conflicts of interestThe authors have no conflicts of interest to declare.
Dr Karina Carrillo Loza would like to thank the Mexican National Council of Science and Technology (CONACYT) for supporting this project, as part of the Masters and Doctoral programme in medical, dental, and healthcare sciences at Universidad Autónoma de México.
Please cite this article as: Carrillo-Loza K, Baranchuk A, Serrano F, Hasseb S, Espinosa Lira F, Soriano E, et al. El bloqueo interatrial avanzado predice recurrencia de infarto cerebral embólico de origen no determinado. Neurología. 2022;37:647–652.