Cerebral venous thrombosis (CVT) is still a significant diagnostic and therapeutic challenge, due to its high variability of clinical manifestations and its lack of a clear therapeutic consensus.
SourcesA search of the medical literature was made through PubMed using the conjoined terms of CVT and epidemiology (428 results), pathophysiology (504 results), aetiology (2714 results), diagnosis (2802 results), treatment (2173 results) and outcome (648 results). Original and review publications deemed to be useful for this review were selected. Classical and historical works on CVT were also included.
DevelopmentThe present paper reviews the fundamental aspects of the epidemiology, pathophysiology and aetiology of CVT. There is a comparison of the most common initial clinical manifestations along with a description of the most important neuroradiological studies needed to establish a diagnosis, all based on multiple published series. Moreover, in order to serve as an important tool in both clinical practice and continuing research, there is also an analysis of recent evidence on treatment and prognosis.
ConclusionsCVT represents approximately 0.5% of all stroke cases worldwide. Headache, focal deficits and seizures are the most frequent initial clinical manifestations, representing 89%, 50%, and 35% of appearances, respectively. Magnetic resonance imaging (MRI) in combination with magnetic resonance venography has proved to have the highest sensitivity and specificity in establishing a diagnosis. An equal alternative to MRI is computed tomography venography due to similar diagnostic results. Pharmacological treatment with heparin is widely accepted today. Recurrence and mortality rates of CVT are 2.8 per 100 cases and 10%, respectively, despite of anticoagulation treatment.
La trombosis venosa cerebral (TVC) representa hasta la fecha tanto un reto diagnóstico como terapéutico, debido principalmente a la alta variabilidad de presentación y a la falta de un consenso terapéutico claro.
FuentesSe realizó la búsqueda de literatura médica en PubMed con el término TVC y epidemiología (428 resultados), fisiopatología (504 resultados), etiología (2714 resultados), diagnóstico (2.802 resultados), tratamiento (2.173 resultados) y pronóstico (648 resultados). Se seleccionaron las publicaciones originales y de revisión consideradas como más útiles para la revisión. Se incluyeron también textos clásicos o históricos.
DesarrolloEn la presente revisión se destacan los aspectos epidemiológicos, fisiopatológicos y etiológicos fundamentales de la TVC. Se comparan las manifestaciones clínicas iniciales más frecuentes de acuerdo a diferentes series y se exponen las pruebas neurorradiológicas de elección actual para su diagnóstico. Asimismo, se analiza la evidencia disponible hasta el momento en lo que corresponde al tratamiento y pronóstico, con el propósito de brindar una herramienta sólida para la práctica clínica y la investigación.
ConclusionesLa TVC representa alrededor del 0,5% de todos los casos de enfermedad vascular cerebral a nivel mundial. La cefalea, los déficits focales y las crisis convulsivas constituyen las manifestaciones iniciales más comunes con el 89, 50 y 35% de frecuencia respectivamente. El diagnóstico neurorradiológico más sensible y específico es la imagen por resonancia magnética (IRM) combinada con venorresonancia. La venografía por tomografía computarizada constituye una buena alternativa debido a resultados equiparables a los de la IRM. El tratamiento con heparina es en la actualidad el más aceptado. Tiene una mortalidad del 10% y la recurrencia se sitúa en 2,8 por cada 100 casos a pesar de terapia anticoagulante.
The first cases of cerebral venous thrombosis (CVT) were reported by Ribes and Abercrombie at the beginning of the 19th century and they included the first case associated with puerperium.1,2 For many decades, CVT was mainly linked to septic processes.3,4 However, once the use of antibiotics became common, the infectious aetiology of CVT was reduced considerably and primary or aseptic CVT is the most common form of this disease today.
Cerebral venous thrombosis is characterised by the polymorphism of its neurological manifestations, difficulty in diagnosing it, the amount of different medical conditions that cause it and its variable prognosis. Despite the disease being rare on a global scale, CVT is of particular interest in poor countries due to its greater frequency. In these countries, it is mainly associated with puerperium. Although there is no clear explanation, it is possible that inappropriate perinatal care, dehydration, iron deficiency anaemia and infections associated to childbirth are factors that account for this greater frequency.5,6
The aim of this paper is to review the current most important aspects of the epidemiology, diagnosis, treatment and prognosis of cerebral venous thrombosis.
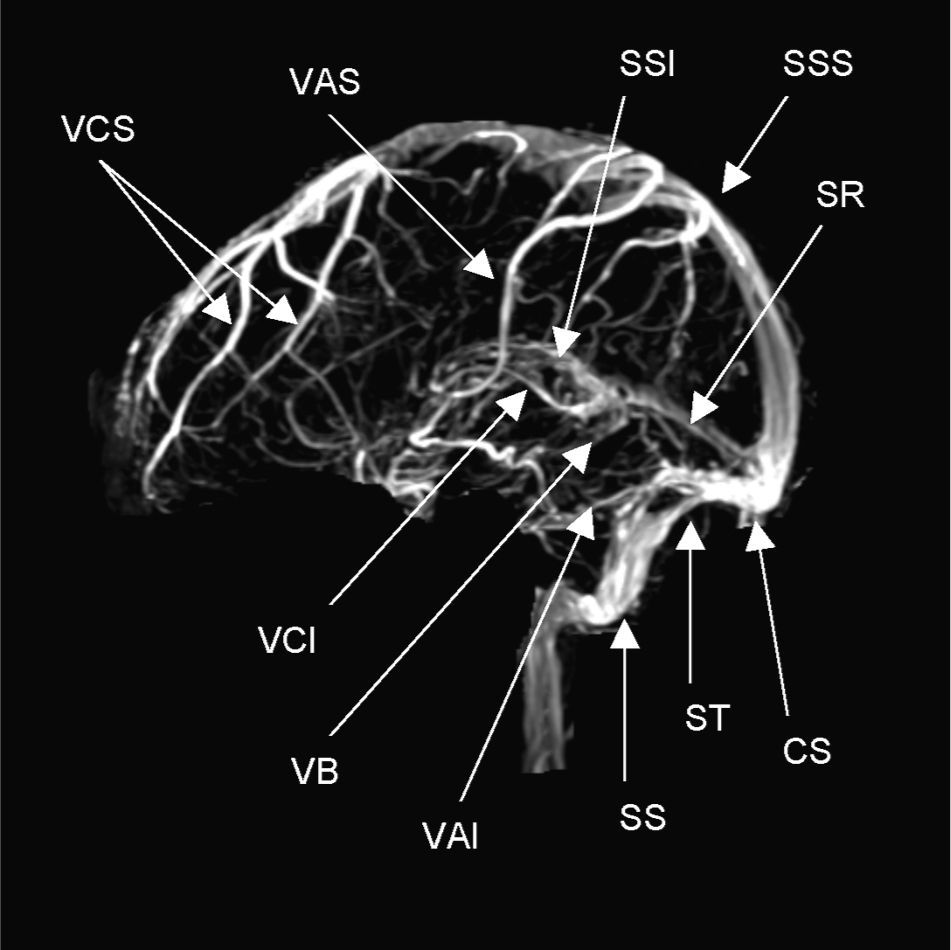
AnatomyThe venous drainage of the brain is carried out by a superficial venous system and another deep one that drains towards the major dural sinuses: superior sagittal sinus (SSS), inferior sagittal sinus (ISS), lateral sinus (LS), cavernous sinus and straight sinus (Fig. 1). The final drainage is accomplished through the internal jugular vein. The veins of the superficial venous system, which principally drain the SSS and the LS, have numerous anastomoses and are difficult to diagnose in cases of occlusion. The deep venous system drains blood from the deep white matter of the hemispheres and the basal ganglia in the vein of Galen. Both the superficial as well as the deep venous systems have many anastomoses. The superficial system allows the venous drainage to take alternative routes according to its different needs, as in the case of a thrombosis.4,5
Normal anatomy of the major venous sinuses on magnetic resonance imaging in the venous phase. CS: confluence of sinuses (torcular herophili); VAI: inferior anastomotic vein (v. of Labbé); VAS: superior anastomotic vein (v. of Trolard); VB: basal vein of Rosenthal; VCI: internal cerebral vein (v. of Galen); VCS: superficial cerebral veins; SR: straight sinus; SS: sigmoid sinus; SSI: inferior sagittal sinus; SSS: superior sagittal sinus; ST: transverse or lateral sinus [Note: acronyms in Spanish].
There are two groups of dural sinuses, posterior superior and anterior inferior. The posterior superior group is composed of the SSS, ISS, LS with its transverse and sigmoid portions, the straight sinus and the occipital sinus. The anterior inferior group includes the superior and inferior petrosal sinuses and the cavernous sinus. Dural venous sinuses play an important role in absorbing cerebrospinal fluid (CSF), as they contain arachnoid villi.
The superior sagittal sinus drains most of the cerebral cortex and corresponds anatomically to the edge of the falx cerebri. The lateral sinuses originate in the confluence of sinuses and extend to the jugular bulb, with transverse and sigmoid sections. The sigmoid portion is joined to the mastoid and is prone to thrombosis in patients with mastoiditis or otitis media. The lateral sinus drains blood from the cerebellum, brain stem and the back of the cerebral hemispheres.
The cavernous sinuses are located at the base of the skull, in a superolateral position to the sphenoid sinuses. The oculomotor (III), trochlear (IV) and ophthalmic and maxillary branches of the trigeminal (V) nerves run through its lateral walls. The abducent (VI) nerve and the internal carotid artery surrounded by its sympathetic plexus are found in its medial part. The cavernous sinuses drain the internal jugular veins through the petrosal sinuses.7
EpidemiologyIt is calculated that CVT incidence rates in autopsy series are about 3–4 cases per million adults and 7 cases per million children and infants, while in clinical series the incidence rate is 10 times greater. Currently, CVT is particularly common in women of 20–35 years old and is associated to pregnancy or puerperium and the use of oral contraceptives.8,9 It constitutes 0.5% of all cerebrovascular events on a global scale.10–13 The international study on cerebral venous thrombosis (ISCVT) has provided significant information related to the aetiology differences in different populations. This study included 624 patients from 21 countries. One significant difference that stands out is that in cases in Mexico, 58% were secondary to pregnancy or puerperium, as compared to 8% of cases in other countries.14,15 The aforementioned exemplifies the different CVT aetiologies in countries that have different socio-economic conditions. In 1998, Lanska and Kryscio carried out a census on cerebrovascular events, based on the National Discharge Survey, in a sample of 280,000 births, discovering that there were 32 CVT events during the study period (1979–1991): 7 occurred before childbirth (22.6%), 9 during puerperium (29%) and the remaining 16 (48.4%) at unspecified moments. When middle-aged women (from 25 to 34 years old) were compared to younger women (15–24 years old), the latter had a 3.7 times greater risk of suffering from CVT.16 In an additional study, these same authors found that there was a total number of 170 CVT cases in the puerperium in a sample of 1,408,015 births, which meant a frequency of 11.6 CVT cases per 100,000 births. After carrying out a multi-variant analysis, they found that the risk factors associated with CVT included pregnancy outcome by caesarean section with an odds ratio (OR) of 3.1 (CI: 2.2–4.2), hypertension (OR: 1.93; CI 1.2–3.0) and the presence of infections other than pneumonia or influenza (OR: 3.1; CI: 1.8–5.2).17 In contrast to these results, where hypertension during pregnancy was associated with CVT, in the series of 67 cases associated to pregnancy or puerperium reported by Cantú and Barinagarrementería, none of them were related to hypertension during pregnancy.11 More recently James et al.,18 in a study that used a database from the Health Care and Utilization Project of the Agency for Healthcare Research and Quality, assessed more than 9 million pregnant or post-partum patients. They found 2850 stroke cases, equivalent to 34 cases per 100,000 births. Cerebral venous thrombosis represented 2% of all cerebrovascular events. The greatest risk was for patients under 20 years old and for those between 35 and 39 years of age. This shows that there are differences between the reported incidence rate of CVT among populations, as well as the factors associated to a greater risk for developing it.
PathophysiologyThere are two possible scenarios: thrombosis in cerebral veins with local effects and thrombosis in the venous sinuses that increase intracranial pressure (ICP). The main mechanisms through which CVT causes deleterious effects on the brain are: cytotoxic or vasogenic cerebral oedema at the venous obstruction site and ICP increase secondary to the obstruction in the CSF drainage. Thrombosis in a cerebral vein causes the formation of a focal cerebral oedema area and later a venous infarct area, which is characterised pathologically by dilated veins, oedema, petechial haemorrhages and ischemic neuronal damage. Thrombosis in the venous sinuses causes an increase in blood pressure through a delay in venous emptying and a reduction in CSF absorption in the arachnoid villi. Occlusion in a venous sinus first causes an increase in retrograde venous pressure, venous congestion and blood drainage from the collaterals. When there is sufficient blood drainage through the collaterals, then only symptoms related to intracranial hypertension appear. If the latter is insufficient, then the venous congestion causes an ischemia that ends up as a venous infarction. Haemodynamics explain that this condition can have an acute, sub-acute or chronic course.
The SSS, LS and transverse sinuses, in decreasing order, are the most common location sites for CVT. In approximately two-thirds of thrombotic events, more than one cerebral vein is involved.19
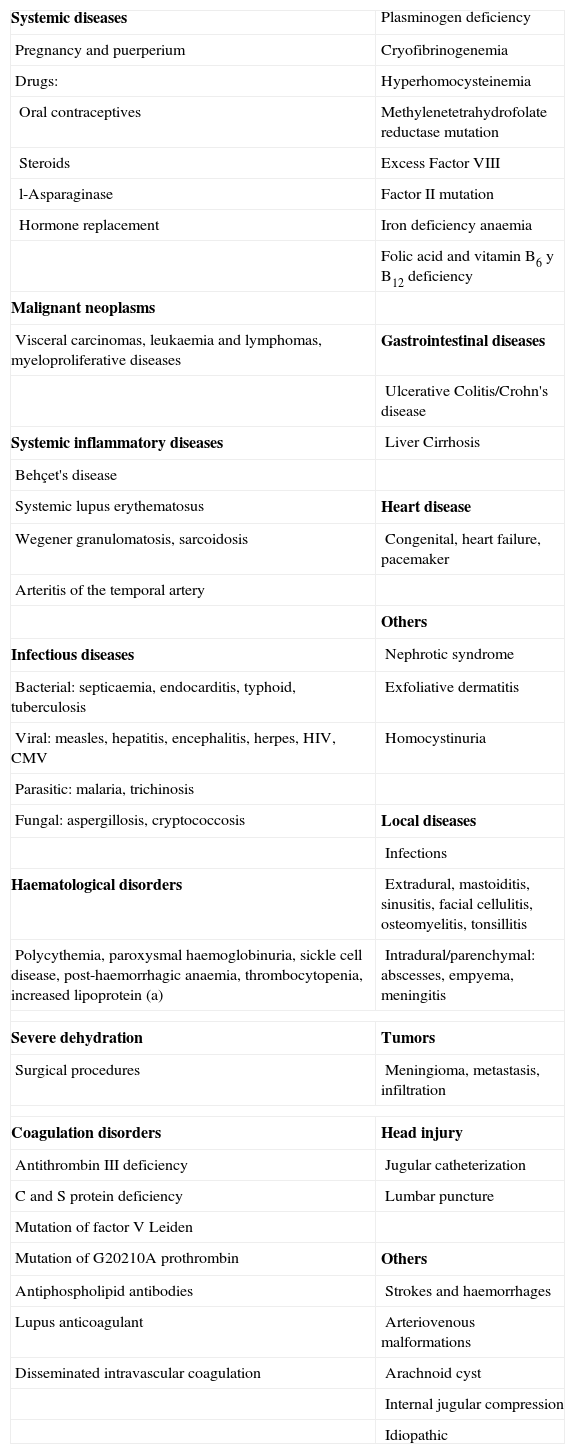
Aetiology and risk factorsCerebral venous thrombosis is a serious but potentially treatable disease that, unlike arterial strokes, affects young adults more frequently.8 Traditionally CVT is divided into 2 groups: septic and aseptic. Currently there are more aseptic cases. Table 1 summarises the most frequent causes. Although more than 100 causes that could cause a CVT episode have been described, the aetiology is not identified in 15–20% of cases.20 Post-partum CVT nearly always appears in the first 3 weeks after childbirth, and in nearly 15% of cases appears within the first 48h of the puerperium. The most probable pathophysiological mechanism for CVT in pregnancy and post-partum is the hypercoagulable state that exists during gravidity, in some cases exacerbated by dehydration and iron deficiency anaemia that arises from inappropriate perinatal care.21 A factor V Leiden mutation and antithrombin III deficiency are responsible for CVT in 15–20% of cases. Hyperhomocysteinemia has been identified as an independent risk factor in recent years, as it is found in up to 27–43% of patients diagnosed with this disease and in only 8–10% of controls.22,23
Causes and factors associated to cerebral venous thrombosis.
| Systemic diseases | Plasminogen deficiency |
| Pregnancy and puerperium | Cryofibrinogenemia |
| Drugs: | Hyperhomocysteinemia |
| Oral contraceptives | Methylenetetrahydrofolate reductase mutation |
| Steroids | Excess Factor VIII |
| l-Asparaginase | Factor II mutation |
| Hormone replacement | Iron deficiency anaemia |
| Folic acid and vitamin B6 y B12 deficiency | |
| Malignant neoplasms | |
| Visceral carcinomas, leukaemia and lymphomas, myeloproliferative diseases | Gastrointestinal diseases |
| Ulcerative Colitis/Crohn's disease | |
| Systemic inflammatory diseases | Liver Cirrhosis |
| Behçet's disease | |
| Systemic lupus erythematosus | Heart disease |
| Wegener granulomatosis, sarcoidosis | Congenital, heart failure, pacemaker |
| Arteritis of the temporal artery | |
| Others | |
| Infectious diseases | Nephrotic syndrome |
| Bacterial: septicaemia, endocarditis, typhoid, tuberculosis | Exfoliative dermatitis |
| Viral: measles, hepatitis, encephalitis, herpes, HIV, CMV | Homocystinuria |
| Parasitic: malaria, trichinosis | |
| Fungal: aspergillosis, cryptococcosis | Local diseases |
| Infections | |
| Haematological disorders | Extradural, mastoiditis, sinusitis, facial cellulitis, osteomyelitis, tonsillitis |
| Polycythemia, paroxysmal haemoglobinuria, sickle cell disease, post-haemorrhagic anaemia, thrombocytopenia, increased lipoprotein (a) | Intradural/parenchymal: abscesses, empyema, meningitis |
| Severe dehydration | Tumors |
| Surgical procedures | Meningioma, metastasis, infiltration |
| Coagulation disorders | Head injury |
| Antithrombin III deficiency | Jugular catheterization |
| C and S protein deficiency | Lumbar puncture |
| Mutation of factor V Leiden | |
| Mutation of G20210A prothrombin | Others |
| Antiphospholipid antibodies | Strokes and haemorrhages |
| Lupus anticoagulant | Arteriovenous malformations |
| Disseminated intravascular coagulation | Arachnoid cyst |
| Internal jugular compression | |
| Idiopathic | |
The way the symptoms appear varies greatly and is summarised in Table 2; it also depends on the venous sinus and the affected veins. It is a dynamic process that is characterised by progressive venous stenosis, collateral development and subsequent recanalization, which is why there are a great variety of manifestations that make diagnosis difficult. In 30% of CVT cases, it presents in an acute fashion and the symptoms develop in less than 48h. In up to 50% of cases, it presents in a sub-acute fashion and the symptoms develop between 48h and 30 days. The chronic form corresponds to 20% of cases and the symptoms develop over a period greater than 30 days and up to 6 months. Patients in whom CVT develops acutely usually present neurological targeting data, while isolated ICP rise is the most common form of presentation in those where the process develops chronically. An acute onset presents more frequently in an obstetric or infectious framework; a sub-acute or chronic onset is more frequently found in inflammatory diseases and coagulation disorders.20
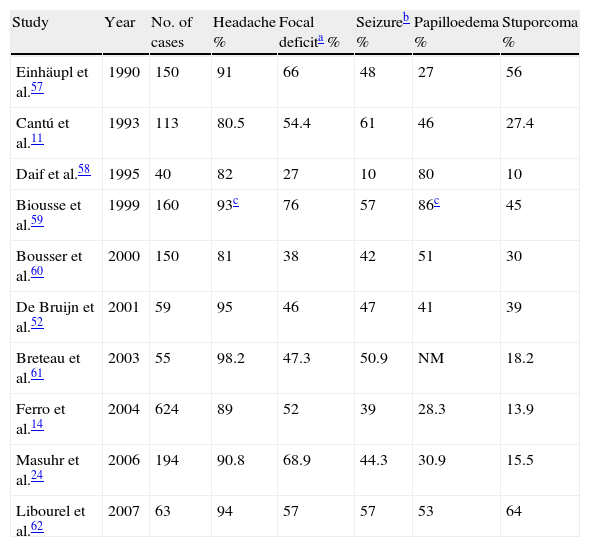
Comparison of the most frequent signs and symptoms at the onset of cerebral venous thrombosis according to different series.
| Study | Year | No. of cases | Headache % | Focal deficita % | Seizureb % | Papilloedema % | Stuporcoma % |
| Einhäupl et al.57 | 1990 | 150 | 91 | 66 | 48 | 27 | 56 |
| Cantú et al.11 | 1993 | 113 | 80.5 | 54.4 | 61 | 46 | 27.4 |
| Daif et al.58 | 1995 | 40 | 82 | 27 | 10 | 80 | 10 |
| Biousse et al.59 | 1999 | 160 | 93c | 76 | 57 | 86c | 45 |
| Bousser et al.60 | 2000 | 150 | 81 | 38 | 42 | 51 | 30 |
| De Bruijn et al.52 | 2001 | 59 | 95 | 46 | 47 | 41 | 39 |
| Breteau et al.61 | 2003 | 55 | 98.2 | 47.3 | 50.9 | NM | 18.2 |
| Ferro et al.14 | 2004 | 624 | 89 | 52 | 39 | 28.3 | 13.9 |
| Masuhr et al.24 | 2006 | 194 | 90.8 | 68.9 | 44.3 | 30.9 | 15.5 |
| Libourel et al.62 | 2007 | 63 | 94 | 57 | 57 | 53 | 64 |
A study carried out by Masuhr et al. on 194 patients with CVT found that headache was present in 90.8% of cases, some motor deficit in 50% and early seizures in 44% of patients. In patients with seizures, 54% presented Todd's paralysis.24 Another study published by Gosk-Bierska et al.25 with 154 patients, also described the clinical presentation of CVT. The most frequently found symptoms were: headache in 87%, seizures in 26.6%, nausea and vomiting in 24.7% and focal deficits in 23.3%. Based on the different series reviewed, headache is the symptom that presents in more than 80% of cases and represents the initial symptom in at least 75% of patients. Headache characteristics are very variable regarding location, intensity, onset and evolution and headache can also occur with no other neurological signs being present. When it appears suddenly, it can be confused with a subarachnoid haemorrhage. Papilloedema is identified in approximately a third of cases. Seizures, whether partial or generalised, including epilepsy, occur in 50% of cases and in 15% of patients and constitute the initial form of presentation. Focal sensory and motor deficits are very common and occasionally suggest the location site, especially when there is a paralysis of cranial nerves such as in IV paralysis. An ICP increase can present in an isolated fashion (that is, with no focalisation data) and can be confused with benign intracranial hypertension (ICH) or pseudotumor cerebri. The main difference between ICH that is secondary to CVT and pseudotumor cerebri is that the latter predominates in obese women.24,26
Based on Bousser,3 4 clinical patterns for CVT have been identified:
- 1.
Focal syndrome: presence of focal signs associated with headache, seizures or changes in mental state.
- 2.
Isolated ICH: with headache, nausea, vomiting and papilloedema.
- 3.
Diffuse subacute encephalopathy: with changes in mental state.
- 4.
Cavernous sinus syndrome: painful ophthalmoplegia, chemosis and proptosis.
Given the wide range of manifestations for CVT, it should be excluded by using available neuroimaging studies when there is clinical suspicion.
Computerised tomographyThe first study that should be carried out in the emergency department is a brain tomography (CT)–with or without contrast–which will allow us to eliminate many of the conditions that could simulate it. It also allows detection of parenchymal lesions, which could have been caused by the thrombosis itself or by the rupture of dilated veins. Venous infarcts can undergo haemorrhagic transformation and occasionally even frank intracranial haemorrhages can be presented, including subarachnoid ones. Sometimes a hyperdense area of the cerebral venous sinus thrombi is detected, but the CT is normal in up to 30% of cases. There are direct and indirect neuroradiological signs to diagnose CVT.27,28 The direct signs are characterised by seeing the thrombus in the affected vessel, while indirect signs are caused by brain parenchyma damage from ischemia as a consequence of the venous flow obstruction.
Direct signs of cerebral venous thrombosis- 1.
String sign. This is found in up to 25% of patients and is non-specific, as slow flow can also produce it. This is identified on a CT scan without contrast when there is cortical vein thrombosis, which is seen as an elongated hyperdense image relating to the brain parenchyma.
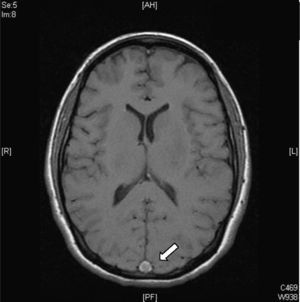
- 2.
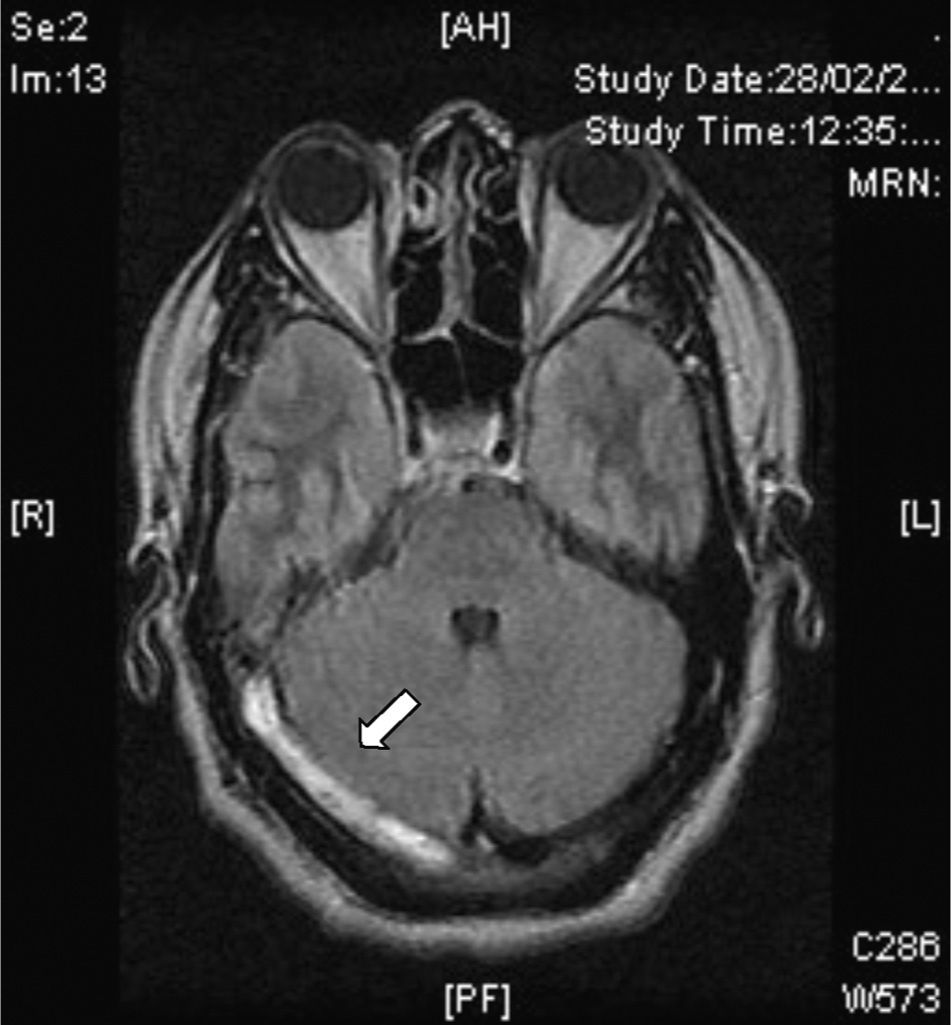
Dense triangle sign. This can be seen during the first 2 weeks in up to 60% of patients and corresponds to a fresh thrombus in the posterior part of the SSS (Fig. 2). It is non-specific and false positives occur in patients with a raised haematocrit or with dehydration.
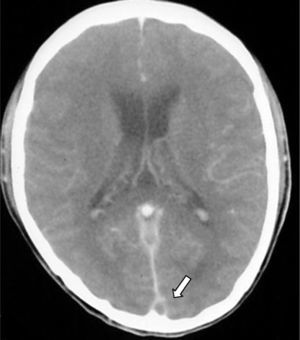
- 3.
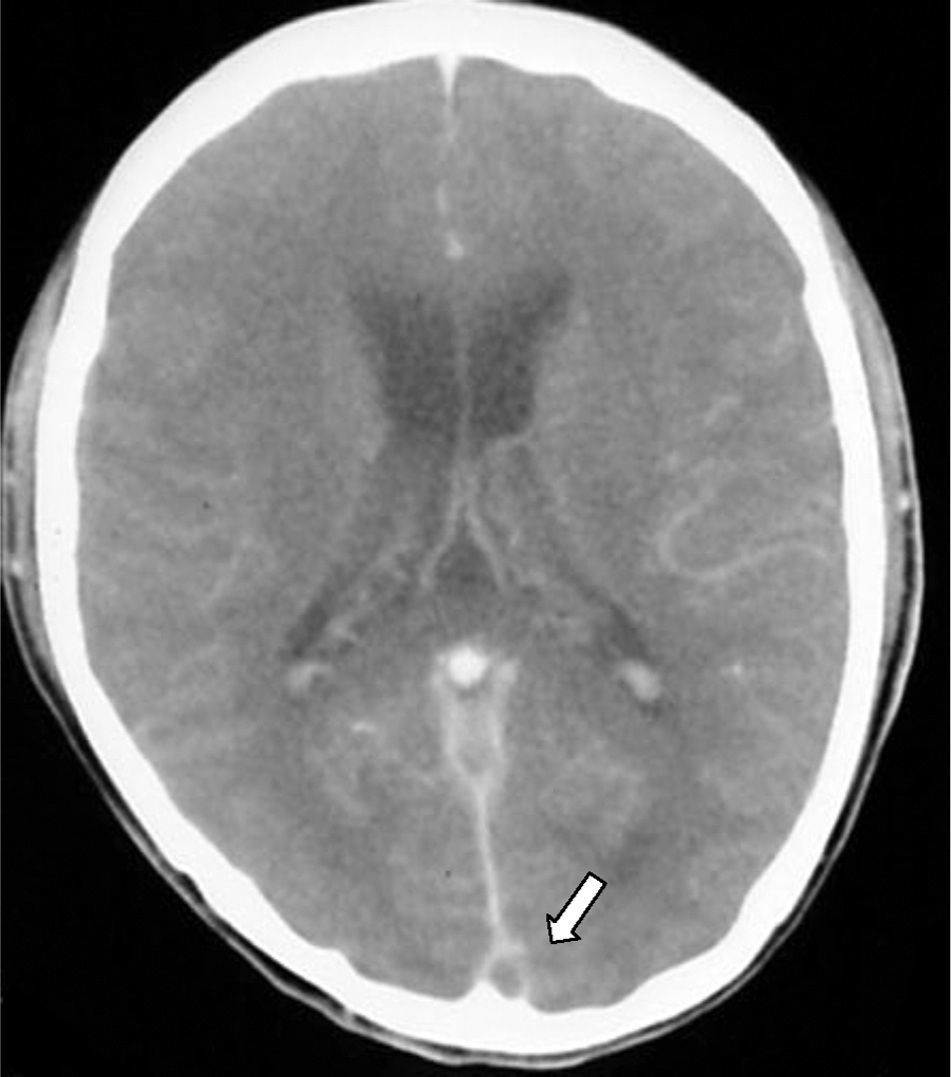
Empty delta (or empty triangle) sign. This is seen after the contrast medium is administered and it is formed due to an intraluminal filling defect surrounded by contrast in the posterior portion of the SSS. This presents in 30% of cases (Fig. 3).
- 1.
Erosion in the middle ear structures and changes in the mastoid region. It is frequent in septic lateral sinus thrombosis.
- 2.
Hydrocephalus and compression of the fourth ventricle. This can be seen in patients with transverse sinus thrombosis.
- 3.
Strokes secondary to CVT are present in up to 40% of cases, focal or diffuse oedema, brain furrow effacement and strengthening the falx or tentorium. Strokes secondary to CVT can be bleeding or non-bleeding and usually affect structures near the damaged site.
- 4.
Reduction in ventricle size secondary to cerebral oedema.
Nowadays, CT venography is considered a good alternative to diagnose CVT, as it is carried out quickly, is accessible and has results that are very similar to those of magnetic resonance imaging (MRI). Studies where CT venography is compared to MRI show a sensitivity and specificity of between 75% and 100% in response to the affected venous sinus.29,30 An examination using this technique should include the region that goes from the vertex to the first cervical vertebra so as to examine the origin of the jugular veins as well.28
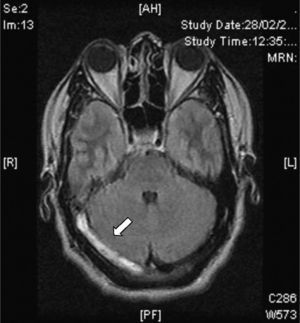
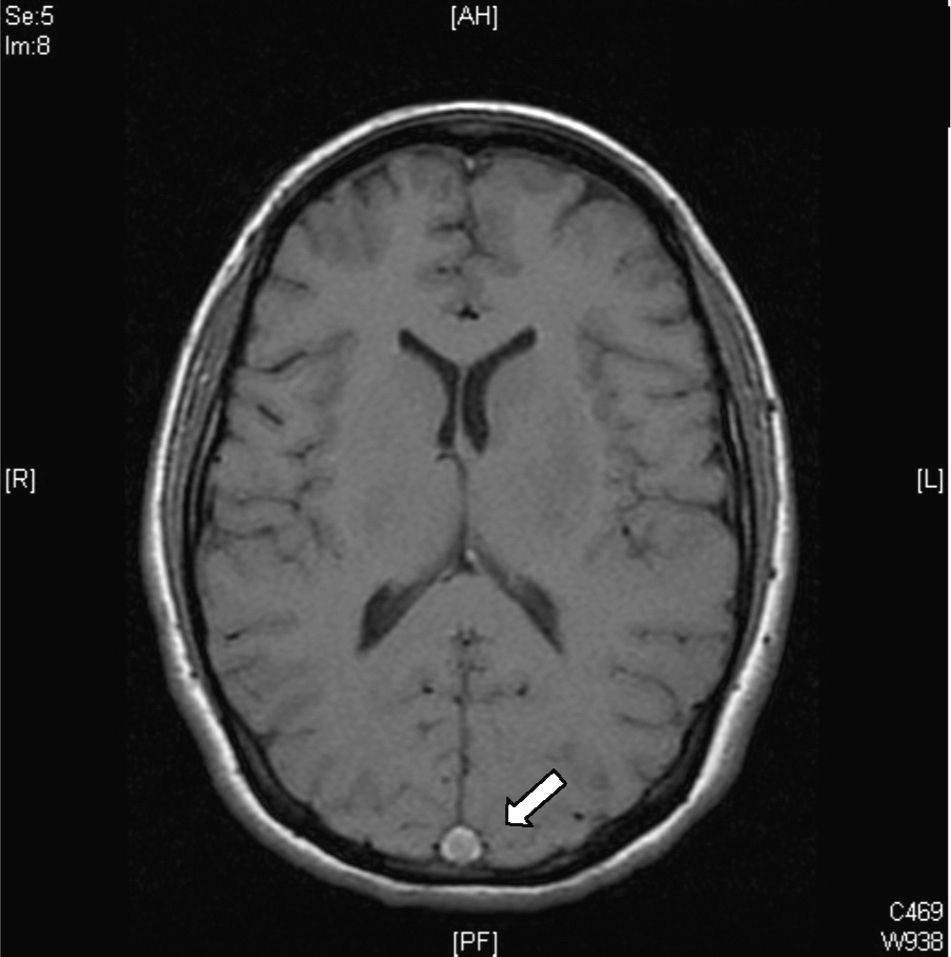
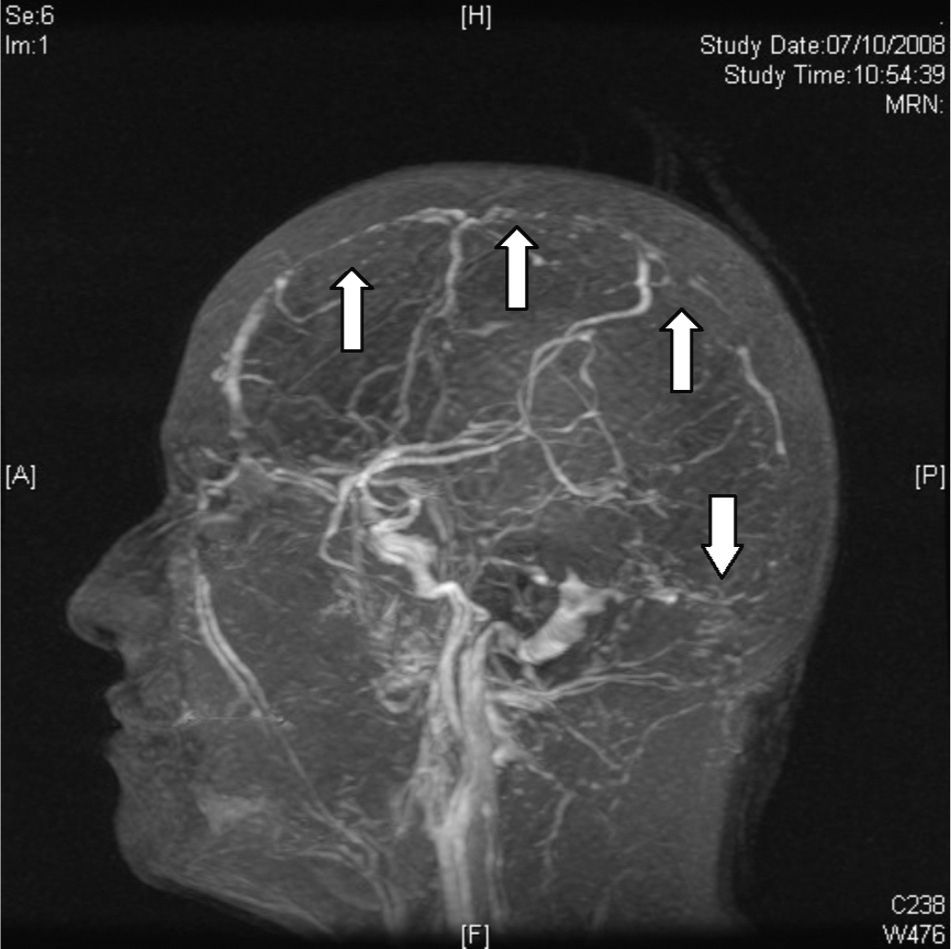
Magnetic resonanceCurrently, CVT diagnosis is confirmed with MRI combined with resonance venography. There is a greater sensitivity in MRI for detecting changes in brain parenchyma, formation of blood clots, petechial haemorrhages and blood flow.31 The study protocol includes T1 sequences (with and without contrast), T2 sequences, FLAIR sequences, diffusion and venography27 (Fig. 4). The clot can take on different aspects depending on how the CVT evolves, for example, during early or acute stages (<5 days), the occluded vessels appear isointense on T1-weighted spin–echo sequences and hypointense on T2. From day 5 to day 35, the thrombus oxyhaemoglobin gradually becomes methaemoglobin and we can observe hyper-intense images in T2 T1.32 During late or chronic stages, the presentation pattern in the MRI is more variable. The thrombosed venous sinus can recanalize itself or remain either partially or completely occluded, which can be interpreted as a recurrent CVT. Due to this, venous resonance (Fig. 5) and spiral CT scans are indicated for the early stages of less than 5 days and for later stages (>6 weeks), as these are periods where the MRI can show false negatives. At 6 months, the abnormalities in the imaging remain in approximately 2/3 of patients. False positives are due to slow venous blood flow without thrombosis. The venous resonance sequences include: TOF (time-of-flight) and phase contrast. Those of phase contrast enhancement are of quick acquisition and allow analysing not only the flow direction but its quantification in the obstructed vasculature.33
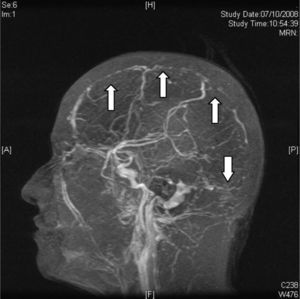
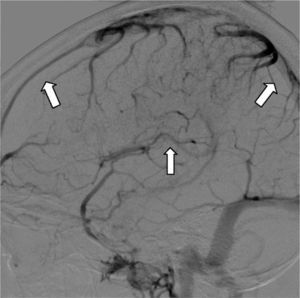
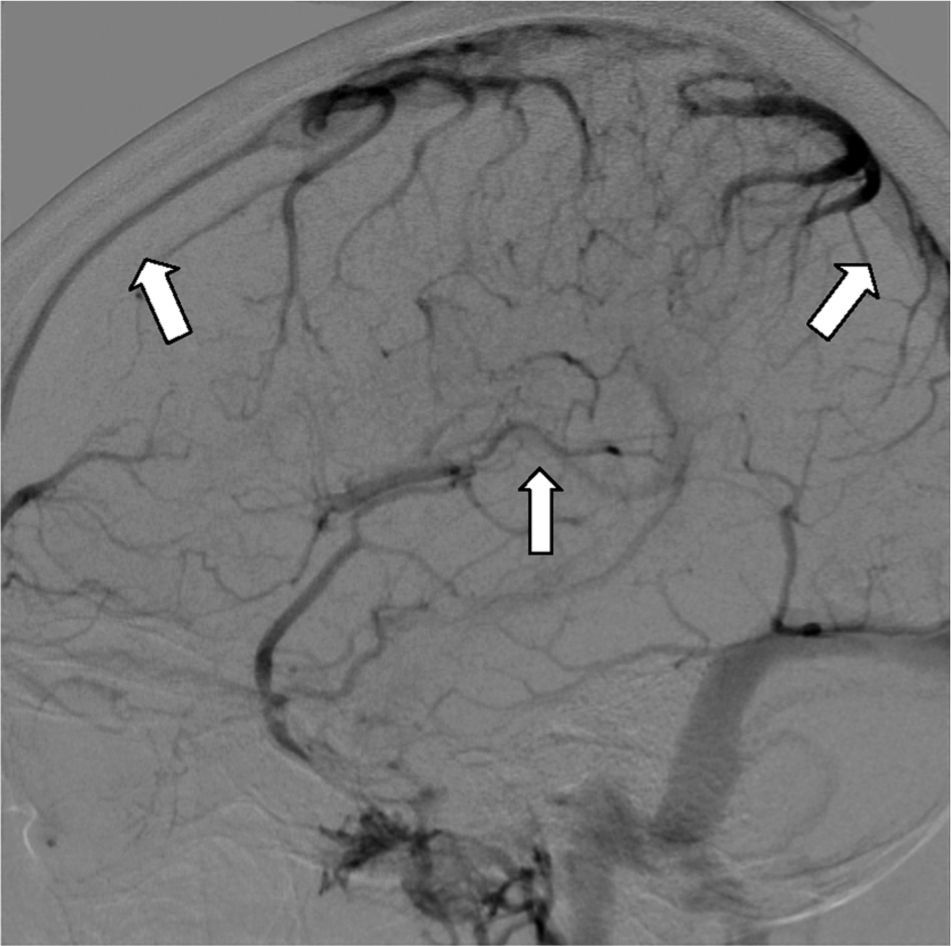
Digital subtraction angiography (DSA) is rarely used nowadays to diagnose CVT. It is useful in cortical vein thrombosis cases where non-invasive imaging studies are not conclusive. It is also useful to exclude the presence of a dural fistula or in cases where chemical or mechanical endovascular thrombolytic treatment is planned34 (Fig. 6).
As well as the studies mentioned, we should take into account that the presence of spontaneous CVT during pregnancy or puerperium makes the investigation of pro-thrombotic states necessary to determine the treatment to follow. In ISCVT, we found that CVT was secondary to a genetic or acquired thrombophilia in 34.1%, secondary to the use of oral contraceptives in 58.6% and to local or systemic infections in 12.3% of patients.35 In 2004, Kosinski et al. proposed the determination of d-dimer levels36 as a diagnostic marker for CVT, because of how useful it was in deep vein thrombosis. In this condition, the high values of d-dimer can be non-specific but very sensitive. However, in a systematic review by Haapaniemi and Tatlisumak in 2009, they warned of its limited use for patients with CVT.37
TreatmentGeneral measuresThe usual measures for handling ICH are recommended, such as keeping the headboard at an approximate inclination of 40°, proper oxygenation and airway protection if there is deterioration in consciousness or patient intubation if the airways are compromised. Seizures present in 35–45% of CVT cases and anti-convulsive treatment is not indicated to prevent them. Patients who initially present seizures, haemorrhage, targeting data or thrombosis in the cortical veins are candidates for anti-convulsive drugs.38,39
The increase in ICP is a complication that is sometimes seen and is related to a bad prognosis. In these cases, the risk of herniation is a serious threat that should be treated by protecting the airways, with hyperventilation and mannitol to reduce the ICP. The use of steroids to reduce vasogenic oedema has not been shown to be of benefit, and is associated with a worse prognosis in patients with parenchymal lesions.40
AnticoagulantsHeparin has become more and more widely used due to increasing evidence regarding its efficacy and safety. The use of heparin and oral anticoagulants (OA) is based on reversing the causal thrombotic process and on preventing other complications such as pulmonary embolism, which presents in up to 11% of patients where there is thrombosis in the jugular veins. Administration of anticoagulants is controversial as, in approximately 40% of CVT cases with a venous infarct, there is a haemorrhagic element that could be susceptible to increase with this treatment. Clinical trials to resolve this dilemma are difficult to carry out due to the rarity of this disease; there have only been 2 small random studies to date that comply with minimum methodological standards. The first of them is a study that included 20 patients in just 1 centre, comparing the use of unfractionated heparin (UFH) with a placebo. In the group of 10 patients who received UFH, a good prognosis was observed at 3 months, while 3 patients died in the placebo group.41 The second study randomised 60 patients with CVT for treatment with low molecular weight heparin (LMWH) or a placebo.42 In the group that received LMWH (nadroparin), 13% had a bad prognosis at 3 months, compared to 21% in the placebo group. In a meta-analysis of the two studies, heparin was associated with an absolute reduction in mortality in 13% (CI 95%: 27–1%; P=.08) and a reduction in absolute risk of death or dependence of 15%, without causing an increase in new haemorrhagic lesions.43 In this same analysis, the patients who did not receive anticoagulants presented a greater frequency of pulmonary embolism. Although these results were not significant statistically, they confirm the clinical improvement associated with heparin treatment by the majority of experts. The additional evidence of heparin use comes from the prospective ISCVT cohort, where 39% of cases had an intracerebral haemorrhage before treatment and 83% of all patients were treated with heparin without their prognosis worsening.
Although the aforementioned studies did not report any new haemorrhagic events, these findings cannot be generalised when we consider the reduced number of patients included. To date, there are no precise instructions on which type of heparin to use. In the ISCVT study, UFH was used in approximately 75% of cases. The main advantage of this heparin use is that it is easy to antagonise in situations such as the need for surgical intervention. Some centres now prefer the use of LMWH in therapeutic doses because it provides more steady anticoagulation and does not require dosage adjustment based on coagulation times. Unfractionated heparin is administered intravenously at an initial dose of 5000 units and then the infusion is maintained at 1000UI/h or a response dose to achieve an activated partial thromboplastin time of 60–80s.41
After the acute phase, we recommend the use of OA unless there is a clear contraindication. In cases of CVT associated with a transient risk factor such as infection, trauma or pregnancy, a treatment period of 3 months is enough. In other conditions, with a greater risk of recurrence, such as pro-thrombotic states, the duration of anticoagulation should be longer. Although there is no solid data, we normally recommend between 6 and 12 months, but treatment may occasionally be needed indefinitely. We suggest maintaining anticoagulation with an international normalised ratio of between 2.0 and 3.0.44
Endovascular treatmentThrombolytic agents applied locally with endovascular jugular or femoral access have been used since 1971. In the review of the two largest series where fibrinolytic agents were used, blood flow was restored in the majority of cases (71.4%).45,46 According to existing studies, local fibrinolytic treatment restores blood flow more quickly and efficiently than heparin, but it has the great disadvantage of increasing the risk of haemorrhage. Up to now, there have been no clear indications for the use of local or systemic thrombolytic agents due to the lack of conclusive studies supporting it. Such agents could be an alternative for CVT patients with a bad prognosis despite anticoagulant treatment.34
There are other options for endovascular treatment. One type is mechanical techniques by extracting the clot with waves, which reduce the required thrombolytic dosage and therefore decrease the risk of intracranial haemorrhage.47
Due to the fact that the current evidence is anecdotal and based on retrospective series, it is impossible to extract conclusions right now with respect to the benefit of endovascular therapy in CVT.
Decompressive hemicraniectomyA decompressive craniectomy should be considered in cases of severe ICH with little or no response to initial treatment. The technique is useful because it gives the brain parenchyma a window to mitigate excessive intracranial pressure.48 Coutinho et al.,49 in a small series of 3 cases, and later Théaudin et al.,50 in a series of 12 cases with CVT and with evolution listed as “malignant”, showed that decompressive surgery not only saved the life of patients but improved functional prognosis even in patients with bilateral pupil dilation. In the ISCVT study, decompressive craniectomy was used in only 9 patients (1.4% of cases), which reflects how little this measure is used in daily practice.
PrognosisThe advent of new imaging methods and the opportunity of having accurate diagnosis and early treatment has meant that CVT prognosis has been changed favourably over the last 30 years. Before the 1960s, it was considered a practically mortal illness; later, with the use of angiography, mortality was reported in 30–50% of cases.51 In the 1980s, the arrival of the CT scan and generalised treatment with anticoagulants contributed to series showing diverse mortality rates of between 4% and 33%.25 More recently and with MRI, series have reported mortality rates in the acute phase of 4.3% and of 3.4% in evolution after 30 days. In the ISCVT, the overall mortality at the end of follow-up was 8.3%, while in the systematic review of 19 articles it was estimated that the overall mortality in the acute phase was 5.6% and the mortality at the end of the follow-up (12–145 months) was 9.4%.14,25,37,52,53 In these studies, we found that early mortality was secondary to transtentorial herniation through multiple lesions, diffuse oedema or mass effect, while deaths that occurred later were secondary to sepsis, pulmonary embolism, sudden death and other deaths related to the base illness. Other studies have found that intracranial haemorrhage at the time of diagnosis is a factor for bad prognosis, as it increases the risk of death and residual disability. In the same way as with haemorrhage, seizures seem to be a factor for bad prognosis and they also present themselves more frequently when there is an intracranial haemorrhage (55% versus 29%; P<.0001).35 In an observational study, Masuhr et al.48 found that mortality was three times greater in patients who had seizures. Other factors for a bad prognosis were age over 37 years old, male gender, consciousness assessed with the Glasgow coma scale as less than 9, changes in mental functions, deep CVT, right intracranial haemorrhage, lesion of the posterior fossa, papilloedema, worsening of previous focal and de novo deficits, neuroinfection and malignant neoplasm. On the other hand, isolated ICH and young age were factors for a good prognosis.24 Headache, which was a symptom present at onset in the majority of patients with CVT, generally resolved itself after about a month with no side effects. However, in some cases (≥30%), headache, whether through tension or migraine, persisted after 6 months.52 Putaala et al.,54 in a retrospective study with 91 patients, found that evolution at 6 months with patients who did not have recanalization presented greater frequency of residual headache.
With regard to functional evolution, we found in ISCVT35 that only 5.1% of patients presented serious residual disability, while 70–85% of patients presented a complete recovery 2 months after follow-up. The overall recurrence rate, according to the different studies (including a systematic review), was 2.8/100 and the patients were taking anticoagulant treatment at the time of recurrence in 90% of cases. A different venous thrombosis site to that of the cerebral one was reported in 3.7% of cases.55 Patients with pro-thrombotic states or with a deep venous thrombosis associated to the lower limbs presented the greatest risk of suffering a recurrence of thrombosis. The recurrence rate ranged from 0% in the first year to up to 12% at 6.5 years.52,53
Finally, there are currently different risk factors that have been identified, not only cognitive but acquired, that contribute to the development of CVT. The diagnosis techniques we have at present allow us to identify individuals with a higher innate risk of presenting thrombotic diseases. We can use these diagnostic tools to set up preventative measures that will lead us to avoid risk factors in individuals with acquired morbid disposition. The development of new, safer and more efficient antithrombotic drugs will undoubtedly lead us to reduce the morbidity and mortality associated to cerebral thrombotic diseases even more. The evolution of diagnostic imaging methods and endovascular therapy will surely be very useful in improving the treatment and prognosis of CVT.
To conclude, CVT is a neurological illness with many clinical manifestations, whose diagnosis requires the clinician's skill. Its proper assessment includes, among other aims, confirming whether it is a CVT and defining its mechanism of pathogenesis, as well as establishing its proper treatment early on.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Guenther G, Arauz A. Trombosis venosa cerebral: aspectos actuales del diagnóstico y tratamiento. Neurología. 2011;26:488–9.





![Normal anatomy of the major venous sinuses on magnetic resonance imaging in the venous phase. CS: confluence of sinuses (torcular herophili); VAI: inferior anastomotic vein (v. of Labbé); VAS: superior anastomotic vein (v. of Trolard); VB: basal vein of Rosenthal; VCI: internal cerebral vein (v. of Galen); VCS: superficial cerebral veins; SR: straight sinus; SS: sigmoid sinus; SSI: inferior sagittal sinus; SSS: superior sagittal sinus; ST: transverse or lateral sinus [Note: acronyms in Spanish]. Normal anatomy of the major venous sinuses on magnetic resonance imaging in the venous phase. CS: confluence of sinuses (torcular herophili); VAI: inferior anastomotic vein (v. of Labbé); VAS: superior anastomotic vein (v. of Trolard); VB: basal vein of Rosenthal; VCI: internal cerebral vein (v. of Galen); VCS: superficial cerebral veins; SR: straight sinus; SS: sigmoid sinus; SSI: inferior sagittal sinus; SSS: superior sagittal sinus; ST: transverse or lateral sinus [Note: acronyms in Spanish].](https://static.elsevier.es/multimedia/21735808/0000002600000008/v1_201305151307/S2173580811000149/v1_201305151307/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)