Cerebral venous thrombosis (CVT) is an uncommon and clinically heterogeneous cerebrovascular particularly in children, only a few published case series focused in the pediatric population.
Patients and methodsRetrospective single-center observational and analytical study of consecutive pediatric patients admitted in a level II Portuguese hospital with a confirmed diagnosis of CVT, from 2003 to 2021. Clinical presentation, neuroimaging findings, prothrombotic factors, treatment strategies, outcome and recanalization were documented.
ResultsTwelve children were included (58% female). Mean age was 7.3 years. The most frequent symptoms were vomiting, headache and behavioral alterations. Infection was the triggering factor in 50% of the cases. The diagnosis of CVT was made based on imaging evidence of thrombosis through magnetic imaging resonance (MRI) with venography and/or computed tomography (CT) with venography. In 67% of cases there were multiples sinuses involved; the transverse sinus was the most affected, followed by the sigmoid sinus. In 83% of cases anticoagulant therapy was initiated with low molecular weight heparin (LMWH) and associated prothrombotic factors were investigated, with no major prothrombotic factors identified. No deaths occurred, but 30% had long-term neurological sequelae. One patient recurred 18 years later.
ConclusionThe results of this study are consistent with data from other published studies. MRI is the preferred imaging method for diagnosis in children by avoiding ionizing radiation and allowing identification of subjacent causes. Anticoagulation with LMWH is recommended and important to reduce mortality and sequelae. Infectious diseases are the most common trigger for CVT and can also be the cause for high morbidity and poor outcomes.
La trombosis venosa cerebral (TVC) es una enfermedad cerebrovascular poco común y clínicamente heterogénea, especialmente en niños, con pocas series de casos publicadas centradas en la población pediátrica.
Pacientes y métodos Estudio observacional y analítico, retrospectivo, unicéntrico, de pacientes pediátricos consecutivos ingresados de 2003 a 2021 en un hospital portugués de nivel II con diagnóstico confirmado de TVC. Se documentó: presentación clínica, hallazgos de neuroimagen, factores protrombóticos, estrategias de tratamiento, evolución y recanalización.
ResultadosSe incluyeron 12 niños (58% mujeres). La edad media fue de 7,3 años. Los síntomas más frecuentes fueron vómitos, dolor de cabeza y alteraciones del comportamiento. La infección fue el factor desencadenante en el 50% de los casos. El diagnóstico de TVC se realizó con base en la evidencia de imágenes de trombosis mediante resonancia magnética con venografía y/o tomografía computarizada (TC) con venografía. En el 67% de los casos hubo múltiples senos afectados; el seno transverso fue el más afectado, seguido del seno sigmoideo. En el 83% de los casos se inició tratamiento anticoagulante con heparina de bajo peso molecular (HBPM) y se investigaron los factores protrombóticos asociados, sin identificar factores protrombóticos importantes. No se produjeron muertes, pero el 30% tuvieron secuelas neurológicas a largo plazo. Un paciente recidivó 18 años después.
ConclusiónLos resultados de este estudio son consistentes con los datos de otros estudios publicados. La resonancia magnética es el método de imágenes preferido para el diagnóstico en niños al evitar la radiación ionizante y permitir la identificación de las causas subyacentes. Se recomienda la anticoagulación con HBPM, que es importante para reducir la mortalidad y las secuelas. Las enfermedades infecciosas son el desencadenante más común de TVC y también pueden ser la causa de una alta morbilidad y de malos resultados.
Cerebral venous thrombosis (CVT) is a rare cause of stroke that affects mainly young adults and children, in contrast to arterial stroke and venous thromboembolism.1 CVT has an incidence of 0.4–0.7 cases per infant per year.2 The incidence has increased over time, probably due to increased clinical awareness as well as improvement in neuroimaging techniques that enable the diagnosis of less severe cases.1 Indeed, the diagnosis of CVT needs to be confirmed by neuroimaging, including computed tomography (CT) and/or magnetic resonance imaging (MRI) with venography and/or catheter angiography.
CVT represents a serious and life-threatening disease with significant morbidity and mortality, usually due to hemorrhagic or non-hemorrhagic venous infarction.3
Affected patients usually present with non-focal neurological signs and symptoms, including headache, seizures and vomiting. Other possible presentations include focal neurologic deficits (hemiparesis and aphasia), isolated intracranial hypertension, diffuse encephalopathy or cavernous sinus syndrome.1
CVT is a multicausal disease triggered by the interaction of several risk factors, such as inherited or acquired thrombophilia, pregnancy and the most common, infection.4
When assessing the thrombotic risk, it is important to assess genetic and environmental risk factors.5 Hereditary thrombophilia, with a prevalence of <1% in the general population, may be caused primarily by deficiencies of natural anticoagulants including protein C and protein S or by genetic mutation of prothrombin or factor V.6 Several conditions, such as antiphospholipid syndrome (APS), are associated with acquired thrombophilia.7 Anticoagulation is the primary treatment regardless of the cause. Although intracerebral thrombosis secondary to thrombophilia is not very rare, it may be susceptible to misdiagnosis.
As this is a rare disorder in pediatric patients, there are only a few published case series of cases of CVT focused in children and we aimed to provide a description of all our pediatric diagnosed cases of CVT, including their clinical presentation, laboratory and neuroimaging studies and outcomes.
Patients and methodsThis is a retrospective single-center observational and analytical study of all consecutive pediatric patients admitted to Centro Hospitalar Vila Nova de Gaia/Espinho, Portugal from 2003 to 2021 with the diagnosis of cerebral venous thrombosis. The diagnosis was made based on the clinical presentation and confirmed by neuroimaging (CT and/or MRI with venography (CTV/MRV)) at the time of the initial diagnosis. Demographic and clinical information, as well as imaging findings, predictor and prothrombotic factors, treatment strategies and outcome of CVT, were documented for each patient. No patients were excluded.
ResultsEpidemiologyTwelve patients were included. Fifty-eight per cent (7/12) were female. Mean age was 7.3 years-old (range: 1 day to 16 years-old), including two newborn patients (Fig. 1).
Clinical presentationThe most common clinical presentation in our cohort was vomiting (50%), headache (42%) and behavioral alterations, such as irritability or prostration (33%). Other described signs and symptoms were fever (33%), seizures (17%) and one case of right hemiparesis (Table 1).
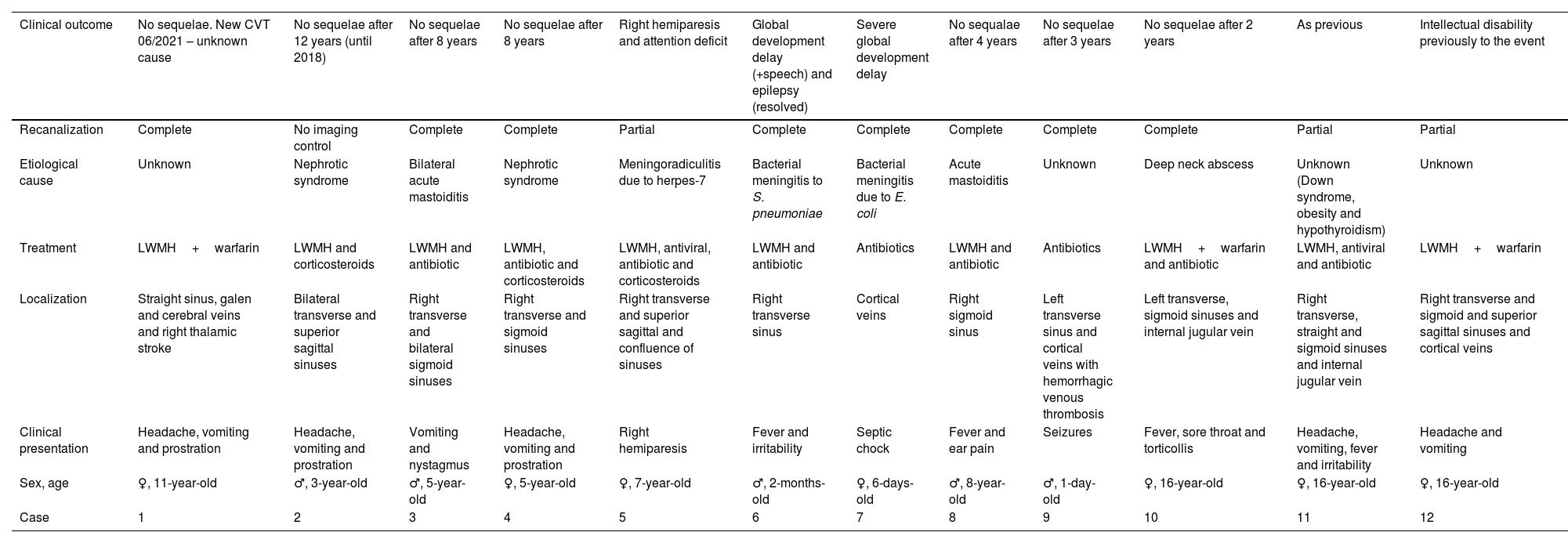
Complete demographic, clinical presentation, localization of affected sinuses, treatment in the acute phase, etiological cause, recanalization and clinical outcome at last time follow-up of all twelve patients engaged in this study. CVT – cerebral venous thrombosis; LWMH – low weight molecular heparin.
| Clinical outcome | No sequelae. New CVT 06/2021 – unknown cause | No sequelae after 12 years (until 2018) | No sequelae after 8 years | No sequelae after 8 years | Right hemiparesis and attention deficit | Global development delay (+speech) and epilepsy (resolved) | Severe global development delay | No sequalae after 4 years | No sequelae after 3 years | No sequelae after 2 years | As previous | Intellectual disability previously to the event |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Recanalization | Complete | No imaging control | Complete | Complete | Partial | Complete | Complete | Complete | Complete | Complete | Partial | Partial |
| Etiological cause | Unknown | Nephrotic syndrome | Bilateral acute mastoiditis | Nephrotic syndrome | Meningoradiculitis due to herpes-7 | Bacterial meningitis to S. pneumoniae | Bacterial meningitis due to E. coli | Acute mastoiditis | Unknown | Deep neck abscess | Unknown (Down syndrome, obesity and hypothyroidism) | Unknown |
| Treatment | LWMH+warfarin | LWMH and corticosteroids | LWMH and antibiotic | LWMH, antibiotic and corticosteroids | LWMH, antiviral, antibiotic and corticosteroids | LWMH and antibiotic | Antibiotics | LWMH and antibiotic | Antibiotics | LWMH+warfarin and antibiotic | LWMH, antiviral and antibiotic | LWMH+warfarin |
| Localization | Straight sinus, galen and cerebral veins and right thalamic stroke | Bilateral transverse and superior sagittal sinuses | Right transverse and bilateral sigmoid sinuses | Right transverse and sigmoid sinuses | Right transverse and superior sagittal and confluence of sinuses | Right transverse sinus | Cortical veins | Right sigmoid sinus | Left transverse sinus and cortical veins with hemorrhagic venous thrombosis | Left transverse, sigmoid sinuses and internal jugular vein | Right transverse, straight and sigmoid sinuses and internal jugular vein | Right transverse and sigmoid and superior sagittal sinuses and cortical veins |
| Clinical presentation | Headache, vomiting and prostration | Headache, vomiting and prostration | Vomiting and nystagmus | Headache, vomiting and prostration | Right hemiparesis | Fever and irritability | Septic chock | Fever and ear pain | Seizures | Fever, sore throat and torticollis | Headache, vomiting, fever and irritability | Headache and vomiting |
| Sex, age | ♀, 11-year-old | ♂, 3-year-old | ♂, 5-year-old | ♀, 5-year-old | ♀, 7-year-old | ♂, 2-months-old | ♀, 6-days-old | ♂, 8-year-old | ♂, 1-day-old | ♀, 16-year-old | ♀, 16-year-old | ♀, 16-year-old |
| Case | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
Neuroimaging studies were available for review by the authors in 11/12 patients. In one patient imaging findings were retrieved from the clinical chart.
CT/CTV and/or MRI/MRV were performed during the acute episode in 11/12 and 9/12 patients, respectively, with all of them showing CVT.
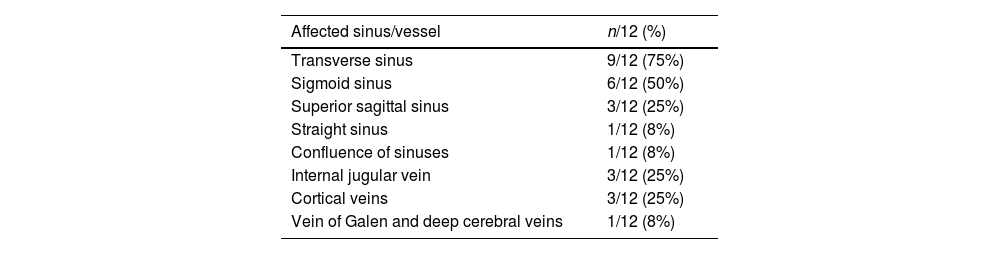
In 67% (8/12) of cases multiple sinuses were involved. Transverse (9/12) and sigmoid (6/12) sinuses were the most frequently affected ones, followed by the superior sagittal sinus (3/12) (Table 2). Three cases had superficial cerebral cortical veins thrombosis, that was isolated in one newborn patient. There was one case of deep venous system thrombosis. Only one patient (1/12, 8%) showed signs of venous hemorrhagic infarction as well as subarachnoid hemorrhage.
Distribution of affected sinus of included patients with CVT (n, %).
| Affected sinus/vessel | n/12 (%) |
|---|---|
| Transverse sinus | 9/12 (75%) |
| Sigmoid sinus | 6/12 (50%) |
| Superior sagittal sinus | 3/12 (25%) |
| Straight sinus | 1/12 (8%) |
| Confluence of sinuses | 1/12 (8%) |
| Internal jugular vein | 3/12 (25%) |
| Cortical veins | 3/12 (25%) |
| Vein of Galen and deep cerebral veins | 1/12 (8%) |
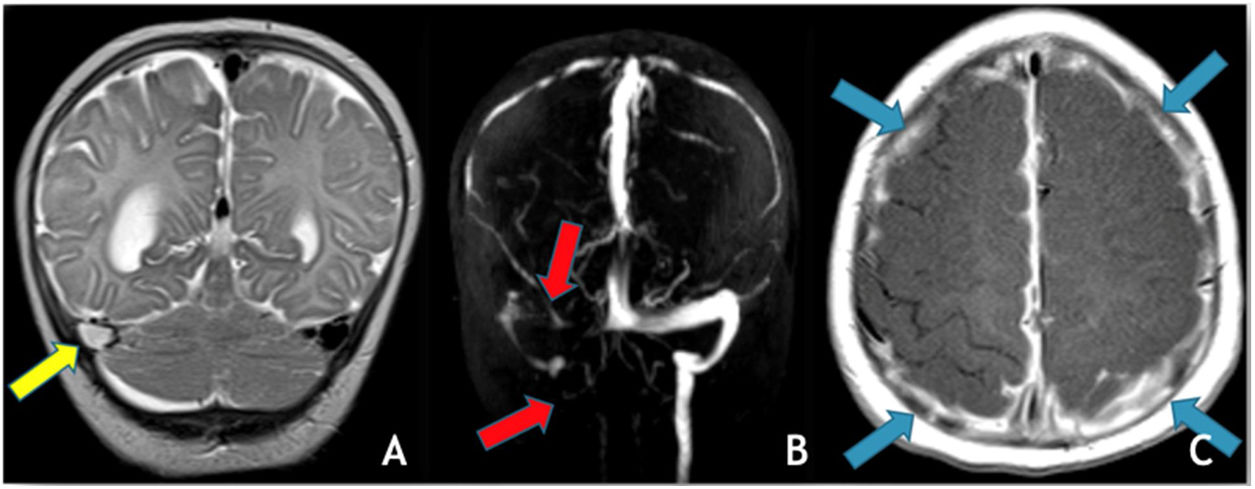
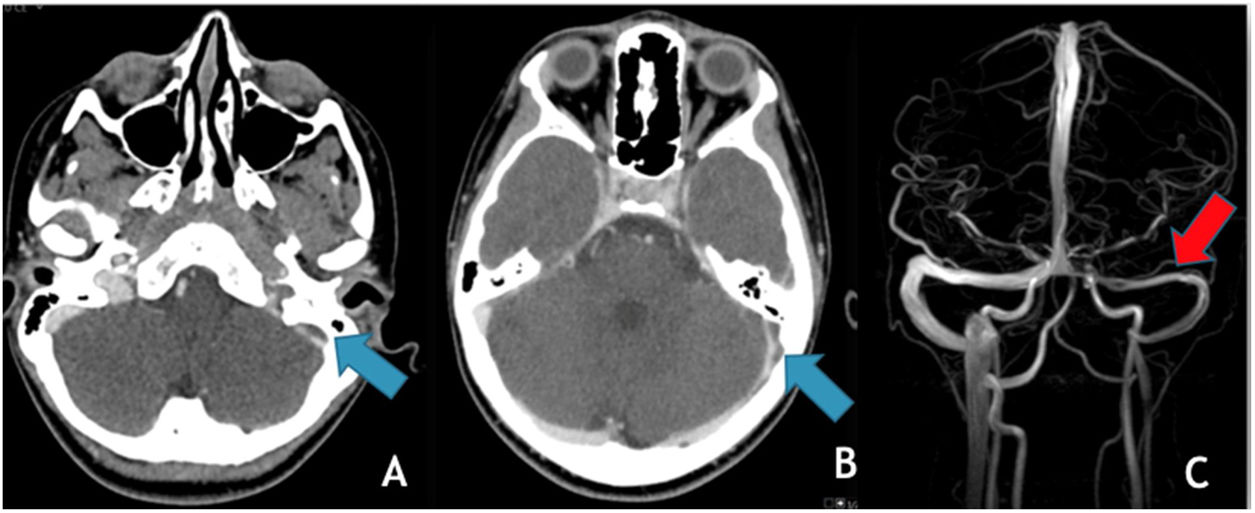
The most common trigger for cerebral venous thrombosis was infection (50%). There were two cases of acute mastoiditis, two cases of bacterial meningitis (Fig. 2), one case of deep neck abscess and one case of meningoradiculitis due to herpes-7. Nephrotic syndrome is a well-known prothrombic trigger and was found in two cases. The use of oral contraceptive was considered as a possible trigger in the adolescent females. One of them also has Down syndrome with obesity and hypothyroidism which may have also been contributing factors.
(A) Coronal T2 demonstrates absent flow void in the right transverse sinus (yellow arrow); (B) MRV (3D reconstruction), exhibits absent flow signal in the right transverse and sigmoid sinuses as well as ipsilateral jugular vein (red arrows); (C) Axial T1post-GAD showing diffuse pachymeningeal and leptomeningeal enhancement (blue arrows).
Initial CT/CTV allowed the diagnosis of two cases of mastoiditis (one with epicrania abscess), one case of deep neck abscess and two cases suspicious of cerebral empyema, both of them latter confirmed by MRI and lumbar puncture.
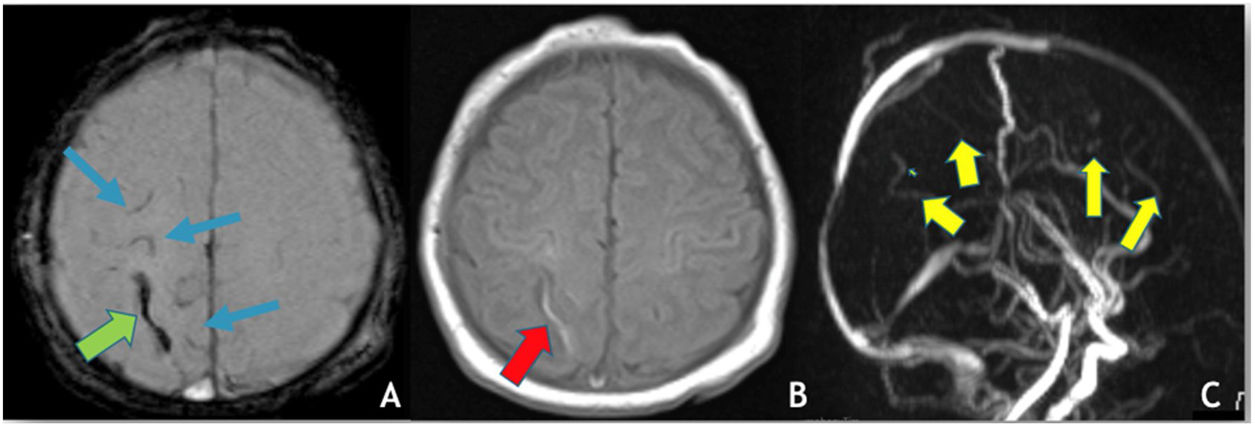
There were two additional cases where no etiological cause was found: one case of right thalamic venous stroke with thrombosis of the straight sinus, galen and cerebral veins and one newborn with left transverse sinus and cortical veins thrombosis (Fig. 3), with a perinatal history of a cesarean section birth with ventouse, for stationary labor.
(A) Axial depicts SWI, hypointensity (“blooming effect”) along a cortical vein located in the high convexity (green arrow) indicating the presence of thrombus and sub-arachnoid hemorrhage (blue arrows); (B) axial T1WI reveals spontaneous hyperintensity in a cortical vein located in the high convexity on the right – endoluminal thrombus; (C) MRV (3D reconstruction), poor visualization of the cortical veins draining into the superior sagittal sinus, which is itself patent.
Screening for genetic and acquired prothrombotic factors was made after the recommended anticoagulation period for each case.
Prothrombotic factors were investigated in 83% (10/12) of cases since two patients lost Immunohemotherapy follow-up at our hospital. Laboratory testing included evaluation of antithrombin, APS antibodies, lupus anticoagulant, serum homocysteine levels, functional protein C, functional protein S and fibrinogen. All patients had normal results. Genetic prothrombotic factors such as methylenetetrahydrofolate reductase (MTHFR), plasminogen activator inhibitor type 1 (PAI-1), prothrombin and factor V gene mutations were also investigated. No mutations were found in three patients. Five had heterozygous mutations in one or two genes and one was homozygous for the 4G/4G polymorphism of the PAI-1 gene. All had normal homocysteine levels, so no major genetic prothrombic factor was found in our series.
TreatmentExcept for the two newborns, all other 10 patients were promptly anticoagulated with low molecular weight heparin. Oral warfarin was started after the acute phase in three of them. The total anticoagulation therapy time varied from 3 to 18 months. Subsequently all patients were followed-up in an Immunohemotherapy consultation and the need for maintenance of anticoagulation was decided based on the imaging study and on the value of d-dimers (adjusted for age).
In addition, corticosteroids were administered in the acute phase to one third of patients (4/10), two of them with nephrotic syndrome, one with deep neck abscess and the other with acute mastoiditis. Finally, 75% (9/12) of patients were treated with antibiotics and two patients also initiated antiviral therapy. The newborn presenting with seizures was started on empiric antibiotics, treated with a single dose of phenytoin and admitted to the NICU. He was discharged ten days later, with infra-therapeutic levels of phenytoin and no other convulsive episodes. Antibiotics were suspended at 72 hours as all blood tests and blood and cerebrospinal fluid cultures were negative for infection.
Clinical outcomeClinical follow-up ranged from 21 months to 18 years (median 12 years). Only one case was lost to follow-up. No deaths were reported.
Three patients evolved with neurological sequelae at last clinical follow-up, one case of right hemiparesis and learning difficulties with attention deficit hyperactivity disorder, one with severe global development delay without speech and one case with epilepsy (suspended medication after three year without seizure and normal EEG) and global development delay, with predominant affected speech. These three patients had central nervous system infection (two cases of bacterial meningitis and one of meningoradiculitis) that most likely is the cause of the sequelae rather than the CVT. The female adolescent with Down syndrome was intellectually disabled and could not read before the event, and no aggravation happened. Similar to the other female adolescent (patient 12), that had previous follow-up in a Pediatrics Consultation for intellectual disability and attention deficit hyperactivity disorder, medicated previously with methylphenidate and no significant changes were found after the event.
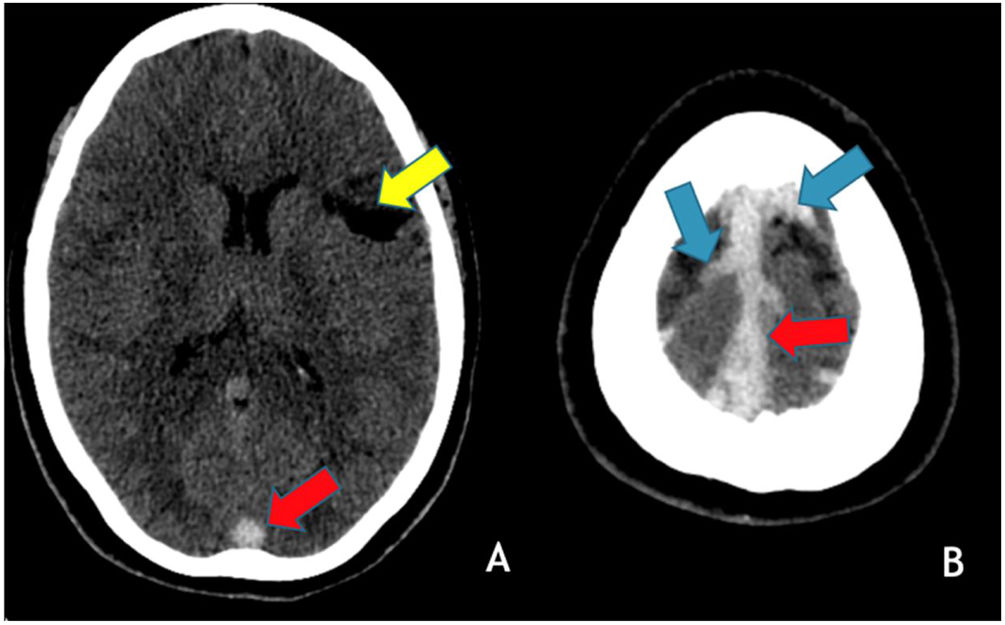
In June 2021, patient 1, at 29-years-old, went to the emergency room due to vomiting and headache and the head CT/CTV showed thrombosis of the right lateral and the superior sagittal sinuses (Fig. 4). She was admitted to the Neurology infirmary and discharged 5 days later. She remains without any neurological deficits or other sequelae and no cause for the two CVT, separated of 18 years, has been found.
Axial brain CT without contrast administration obtained 18 years after the primary event shows a left frontal cortico-subcortical vascular sequela (yellow arrow) as well as hyperdensity within the superior sagittal sinus (red arrows) and cortical veins in the high convexity (blue arrows) in keeping with recurrent cerebral venous thrombosis.
No other recurrences occurred.
RecanalizationOf the 12 included patients, 10 of them performed at least one follow-up neuroimaging with either MRI/MRV (n=7) and/or CT/CTV (n=3), with a median follow-up time of 7 months (range from 1 to 13 months).
Approximately 58% of the patients (7/11) experienced complete resolution of the thrombosis at the last neuroimaging study, while three cases exhibited only with partial recanalization (Fig. 5). None of the cases presented progression of the thrombus. The patient that recurred 18 years later (patient 1) showed a de novo CVT involving different sinuses when compared with the thrombus distribution of the first event. No dural arteriovenous fistulas were detected at longitudinal neuroimaging in any of the patients.
DiscussionHerein we present the clinical and imaging findings at presentations of our 12 pediatric patients (including two newborns) with CVT, as well as prothrombotic factors, treatment strategies, clinical outcomes and recanalization.
In children, CVT is relatively equally distributed between genders in all age groups.8 However, we found a higher proportion of females, against to previous articles2,9 eventually due to our small sample size. Some studies exclude newborns since the clinical presentation is different, but in this study we included two newborns.
Some reports describe seizures, decreased consciousness and headaches as the most common initial symptoms,10 with seizures being common in newborns.11 Focal and diffuse neurologic signs are likely to occur3 and other possible presenting symptoms may vary according to the cause/trigger of the CVT. As previously described, neurologic signs as vomiting and headaches were the most common presenting symptoms in our study.
There are three possible neuroimaging techniques to diagnose CVT: CT/CTV (computed tomography-venography), MRI/MRV (magnetic resonance imaging with venography) and catheter angiography.1 MRI/MRV is the imaging technique of choice for the diagnosis of CVT in children, allowing visualization of the thrombus within the vessel combined with absent flow on MRV as well as presence of associated venous infarction and/or subjacent causes, without the use of ionizing radiation.4 However, CT/CTV is frequently the initial diagnostic imaging method in the acute setting due to the easy access in the emergency room and the speed of acquisition, while diagnosis with catheter angiography is exceptional. Indeed, except for one newborn diagnosed at presentation by MRI/MRV, all our patients were initially investigated with CT/CTV, although majority of them also performed subsequently an MRI/MRV. Nevertheless, sedation techniques may be required for both techniques, especially MRI due to the longer acquisition time.
Multiple sinuses thrombosis was the most frequent scenario in our population, which is concordant with previous pediatric studies.8–10 Transverse and sigmoid sinuses thrombosis were the most frequently affected sinuses in our study, followed by the superior sagittal sinus, also as previously reported in children.11
The most common trigger for cerebral venous thrombosis in our cohort was central nervous system (CNS) or head and neck infection, as reported in most previous studies.2,3,11 Most patients with CNS or head and neck infections had multiple sinuses involvement, as well as the two cases of nephrotic syndrome. Oral contraceptive may have contributed for the CVT in two female adolescents, one with Down Syndrome, obesity and hypothyroidism. No trigger nor prothrombic factor was discovered in the first event of the patient with recurrent CVT. In the subsequent CVT episode, she was on oral contraceptives for more than three years against medical advice. In newborns, there is an increased risk of cerebral venous thrombosis that may be related with alternative mechanisms such as mechanical compression.12 The patient presenting with seizures in the neonatal period had no other risk factor and the cesarean section delivery with ventouse probably contributed to the CVT, in a multifactorial process.
It remains unclear whether all individuals with thrombosis should be screened for thrombophilia. Screening is generally not recommended for the general population, but there is consensus for some specific indications that include: thrombosis during perinatal period, following the use of oral contraceptives or estrogen replacement therapy and venous thromboembolism in uncommon sites such as the brain.13 Therefore, there is a formal recommendation for screening prothrombotic factors in all the patients included in this study. At our center, the screening for thrombophilia is mainly performed after the recommended anticoagulation period. All patients, except the two lost to follow-up, were investigated for prothrombotic factors. No major genetic prothrombotic factor was found. Nonetheless it is always important to exclude these since there may be an indication for lifetime anticoagulation.
The American Society of Hematology (ASH) recommends and suggests the use of anticoagulants in pediatric CVT with and without hemorrhagic venous infarction, respectively.14 Except for the two newborn patients, all cases were anticoagulated with low molecular weight heparin (LMWH). Three patients were latter switched to warfarin. ASH recommendations do not prefer LMWH to warfarin or the opposite and determine the decision depends on patients values and preferences, underlying conditions and comorbidities and health resources.14 Most patients were treated for a period of 3–6 months. One of them was treated for almost 18 months but he missed several appointments and continued anticoagulated longer than recommended by the specialist. Prothrombotic factors were investigated in 83% (10/12) of cases. A thrombophilia panel was performed including Protein C, Protein S and antithrombin activity, Activated protein C resistance, and the genetic mutations of the factor V (Leiden), prothrombin, Methylenetetrahydrofolate reductase and Plasminogen activator inhibitor 1 (PAI-1) gene. Five had heterozygous mutations in one or two genes and one was homozygous for the 4G/4G polymorphism of the PAI-1 gene. All had normal homocysteine levels, so no major genetic prothrombotic factor was found in our series.
Pediatric antiphospholipid syndrome was also excluded discarding the presence of lupus anticoagulant in plasma, anticardiolipin antibodies of IgG or IgM isoforms and anti-beta-2 glycoprotein I (anti-β2GPI) antibodies of IgG or IgM isoforms.
As the most common trigger of CVT in our cohort was CNS or neck/head infection, 75% of patients was treated with antibiotics. The used antibiotic varied depending on the primary infection focus. Two patients were treated with antivirals because of the presenting symptoms: one presented with right hemiparesis and the other was a 16-year-old female with seizures at day 2 of hospital admission. Two cases of severe infection (deep neck abscess and acute mastoiditis) and the two cases of nephrotic syndrome were treated with corticosteroids. Our two cases with nephrotic syndrome had hypoalbuminemia upon presentation, and low albumin levels has been considered the major risk factor associated with thromboembolic events in this syndrome.15
No deaths occurred in our study during the follow-up time and most patients evolved without any major sequelae. Only one case of recurrence after 18 years of the primal event, both without any neurological sequelae. Our data is similar to other pediatric studies, with overall favorable prognosis being described in this age group,1 and mainly dependent on the etiological factor for thrombosis11 and the occurrence (or not) of venous infarction. Indeed, cerebral thromboembolism is the most common neurological complication of nephrotic syndrome with favorable outcome in approximately 90% of patients.15 Accordingly, our two patients with CVT associated with nephrotic syndrome are now adults without any neurological sequelae. Ritchey et al. described a worse outcome for patients with cortical vein thrombosis without involvement of the dural sinuses,16 we have found similar results. Indeed, our patient with isolated cortical vein occlusion evolved with severe global development delay, most likely due to the bacterial meningitis. The other two patients with neurological sequelae had severe central nervous system infections, one other case of bacterial meningitis with empyema and a case of meningoradiculitis due to herpes-7 and this girl maintained right hemiparesis and learning difficulties. The injury related with CNS infection is more likely the cause of the long-term neurological sequelae than the CVT itself.
There are currently no guidelines requiring a systematic venographic follow-up evaluation in children for assessment of recanalization nor establishing the ideal imaging method to confirm recanalization.17 Indeed, long term recanalization is so far not considered a significant outcome factor of CVT11 as persistent intracranial venous occlusion does not appear to mean worse outcome in both children and adults.8 Therefore, imagiologic resolution of CVT is not always documented and depends on clinical judgement at an individual basis. However, clinical symptomatic control should be performed.
Although all our cases performed at least one neuroimaging study after the initial event, in most cases they were acquired in the emergency room in the setting of other acute, unrelated clinical reasons. Nevertheless, recanalization could be assessed in 10/12 cases, and considered complete in 8 of them. Concerning the two cases with only partial recanalization, it should be noted that their last imaging study was performed less than a year after the initial CVT event, and therefore subsequent evolution towards complete recanalization cannot be fully excluded.18
Finally, a recent study demonstrated a very low yield of routine follow-up neuroimaging aiming to detect new dural arteriovenous fistulae 6 months after CVT19 and accordingly we have neither detected development of this type of brain vascular malformations in any of our patients during imaging follow-up.
ConclusionThe results of this cohort were overall similar to those currently found in current literature. The clinical presentation of CVT in pediatric patients is not specific, most often with headache and seizures, therefore a high level of suspicion should be present. MRI/MRV is the preferred imaging method for diagnosis in children, despite not always being available in an emergency setting. Multiple sinuses involvement was the most common pattern, with the transverse and sigmoid sinuses being the most affected vessels. Anticoagulation should be started at diagnosis and the presence of hereditary or acquired thrombophilia evaluated after the minimum recommended time of anticoagulation. Anticoagulation treatment with LMWH is recommended and important to reduce the mortality rate, as seen in our case series. CVT is frequently associated with CNS and/or head and neck infections in children, and the former can also lead to parenchymal injury and lead to high morbidity and poor outcomes as demonstrated in a few of our cases. Recanalization does not appear to be a significant factor for prognosis and predictor of neurological outcome in both children and adults and there are currently no studies or guidelines concerning the expected time of recanalization and if follow-up neuroimaging control MRI/MRV should be routinely performed and at what time point.
Conflict of interestThe authors declare that there is no conflict of interest.