Gait alterations are present in a high percentage of patients with multiple sclerosis (MS). They appear from early stages of the disease and can limit patients’ capacity to perform basic activities of daily living, affecting their quality of life. Visual biofeedback cycling training appears to be a useful tool in treating these impairments. This study aims to evaluate the short-term effect of visual biofeedback cycling training on gait in patients with MS.
Material and methodsA total of 61 patients with mild to moderate MS were randomly assigned to a control group and an intervention group. The intervention group received visual biofeedback cycling training (MOTOmed viva2 system) once per week for 3 months, and a home exercise programme. The control group only received the home exercise programme. Both groups were evaluated using the GAITRite® Walkway gait assessment system before the intervention, during the first month of the programme, and after the intervention.
ResultsIn the intervention group, the analysis revealed statistically significant differences between Functional Ambulation Profile (FAP) scores before and during the intervention (P=.014), and before and after the intervention (P=.002). A statistically significant improvement was observed in step length in the intervention group between pre- and post-intervention scores (P=.001) and between first-month and post-intervention scores (P=.004).
ConclusionsVisual biofeedback cycling training improved specific gait parameters in the short term and appears to be a therapeutic option for gait retraining in patients with MS.
Las alteraciones de la marcha están presentes en un alto porcentaje en los pacientes con esclerosis múltiple (EM); aparece desde estadios iniciales pudiendo limitar la realización de las actividades básicas de la vida diaria y afectando a su calidad de vida. El entrenamiento en bicicleta con retroalimentación visual se presenta como una herramienta útil en el tratamiento de estas alteraciones. El objetivo del presente estudio es valorar el efecto a corto plazo del entrenamiento en bicicleta con retroalimentación visual sobre las alteraciones de la marcha en pacientes con EM.
Material y métodosSesenta y un pacientes con EM con afectación leve-moderada, distribuidos aleatoriamente en un grupo control y un grupo experimental participaron en el estudio. El grupo experimental fue sometido a entrenamiento con bicicleta con biofeedback visual (sistema MOTOmed Viva2) un día en semana durante 3 meses y un programa domiciliario de ejercicios. El grupo control tuvo solo el programa domiciliario de ejercicios. Valoración mediante el sistema de análisis de marcha GAITRite® Walkway de ambos grupos, pre, al mes y postintervención.
ResultadosResultados estadísticamente significativos en el parámetro FAP del grupo experimental entre el pre y al mes (p=0,014) y el pre y postintervención (p=0,002). Mejoró significativamente la diferencia de la longitud de paso del grupo experimental entre el pre y post (p=0,001) y entre el mes y el postintervención (p=0,004).
ConclusionesEl tratamiento con la bicicleta mejoró a corto plazo determinados parámetros de la marcha, pudiéndose mostrar como opción terapéutica en la reeducación de la marcha en pacientes con EM.
Multiple sclerosis (MS) is a degenerative, inflammatory, autoimmune disease of the central nervous system (CNS). The most frequent symptoms are motor alterations (in 90%-95% of patients), followed by sensory alterations (77%) and cerebellar involvement (75%). Patients with MS may also present brainstem involvement, poor motor control, and cognitive and visual alterations. However, disease progression is unpredictable.1
MS is the most common non-traumatic cause of neurological disability among young and middle-aged adults,2 affecting 2.5 million Europeans.1
A large percentage of patients with MS (75%) present gait impairment,3 which limits their ability to perform activities of daily living (ADL) and has a negative impact on their quality of life.4
Two of the functional tests most frequently used by neurologists to assess these patients are the Timed 25-Foot Walk (T25FW) and the Expanded Disability Status Scale (EDSS); the latter is the most widely used tool for evaluating MS severity.5 The EDSS measures disability based on a quantitative neurological examination and is scored from 0 (no disability) to 10 (death due to MS).6 The scale comprises 8 subscales assessing the following functional systems: pyramidal (motor), sensory, cerebellar, brainstem, visual, mental, bowel and bladder, and other.
Patients scoring ≥4 display severe gait impairment: they more frequently need aid for walking, have greater postural instability, and show increased energy cost of walking.7
According to recent studies analysing spatio-temporal parameters of gait in MS, these patients show reduced walking speed, cadence, and stride length and increased step time.8
Pedalling and walking have similar kinematic patterns since both tasks are cyclical and require the legs (hips, knees, and ankles) to alternate in flexion and extension, with extensor muscles alternating with their anatomic antagonists synchronously and in coordination.9
Visual biofeedback cycling training has been shown to have positive effects on patients with neurological diseases associated with asymmetrical gait patterns, resulting in overuse of the less affected side of the body.10 In patients with stroke, this treatment has been found to improve step symmetry and leg loading; the visual feedback enables patients to adjust leg movement and transfer weight more evenly while walking. This results in increased walking speed.10 However, the usefulness of this technique in patients with MS is yet to be explored.
Another study found that biofeedback cycling training improves muscle control and activation, and reports no adverse events.11
Other authors suggest that this type of training has an impact on central pattern generators (CPG), which are responsible for lower limb flexion and extension during walking. This pattern may be regulated by external sensory stimuli, responding to visual feedback provided by the stationary bicycle's console display during training.12
Visual biofeedback cycling training seems to be beneficial in patients with marked step asymmetry and inefficient gait, as well as in those displaying overuse of the healthy limb, which increases asymmetry.13
We hypothesised that visual biofeedback cycling training over 3 months would improve not only pedalling but also other gait parameters in patients with MS.
The main objective of our study was to analyse the effectiveness of a neurophysiotherapy programme based on feedback cycling for improving gait in patients with MS. We evaluated the programme's impact on step asymmetry, functional ambulatory performance (FAP), and walking speed and cadence using the GAITRite® electronic walkway system (CIR Systems, Inc., USA).
Patients and methodsPatientsParticipants were recruited from the multiple sclerosis unit at Hospital Universitario Virgen Macarena (Seville, Spain). Participants had been admitted to the multiple sclerosis unit and met the inclusion and exclusion criteria listed below.
Inclusion criteria: (1) referral by the neurologist to our hospital's multiple sclerosis unit; (2) diagnosis of definite MS according to the McDonald criteria at least 2 years previously; (3) EDSS score ≤7 (established by a neurologist); (4) age between 20 and 70 years; (5) clinical stability during the 3 months previous to recruitment; (6) no cognitive impairment according to the Mini-Mental State Examination; (7) willingness to sign an informed consent form; and (8) EDSS score between 2 and 6.5. Exclusion criteria: (1) severe comorbidities that may involve a risk of death in patients receiving biofunctional neurophysiotherapy; (2) medical conditions or psychological disorders potentially limiting their ability to understand and/or answer questions or to complete questionnaires; (3) relapse occurring less than 3 months prior to treatment onset or during the intervention; and (4) visual defects.
Of an initial sample of 70 patients, a total of 61 were included in the study (25 men and 36 women).
The research protocol was reviewed and approved by our hospital's research ethics committee (C.P.-C.I. 1896). All participants gave written informed consent to be included in the study.
DesignWe conducted a double-blind, randomised clinical trial. Patients were randomly assigned either to the intervention group (n=30) or to the control group (n=31); the random allocation sequence was generated using MAS version 2.1 (Glaxo Wellcome). During the study period, none of the participants received other types of physiotherapy. Results were evaluated by a researcher blinded to treatment allocation.
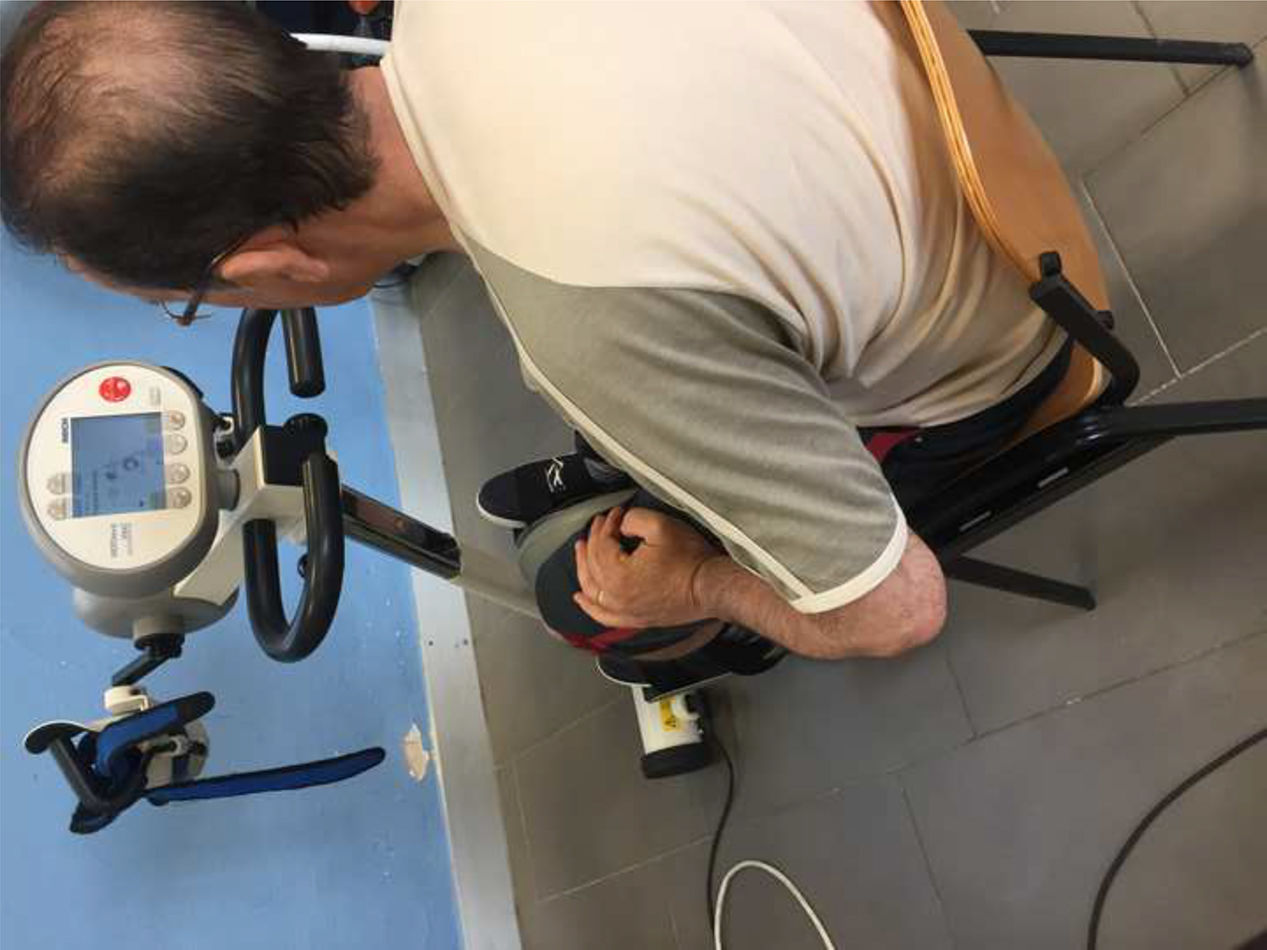
InterventionTreatment was administered at our unit's biofunctional neurophysiotherapy treatment room. The intervention group used a stationary bicycle with a coordination feedback programme to work on lower limb strength asymmetry using 75% of the maximum resistance. Patients in the intervention group completed one 30-minute session per week for 3 months using the MOTOmed Viva2 Movement Trainer® (RECK-Technik GmbH, Germany) (Fig. 1). They also received a personalised exercise programme to be completed at home. Control patients received the personalised exercise programme only.
VariablesStudy variables were evaluated at treatment onset, one month after treatment onset, and at the end of the 3-month intervention period. Assessments were performed by an independent researcher blinded to treatment allocation.
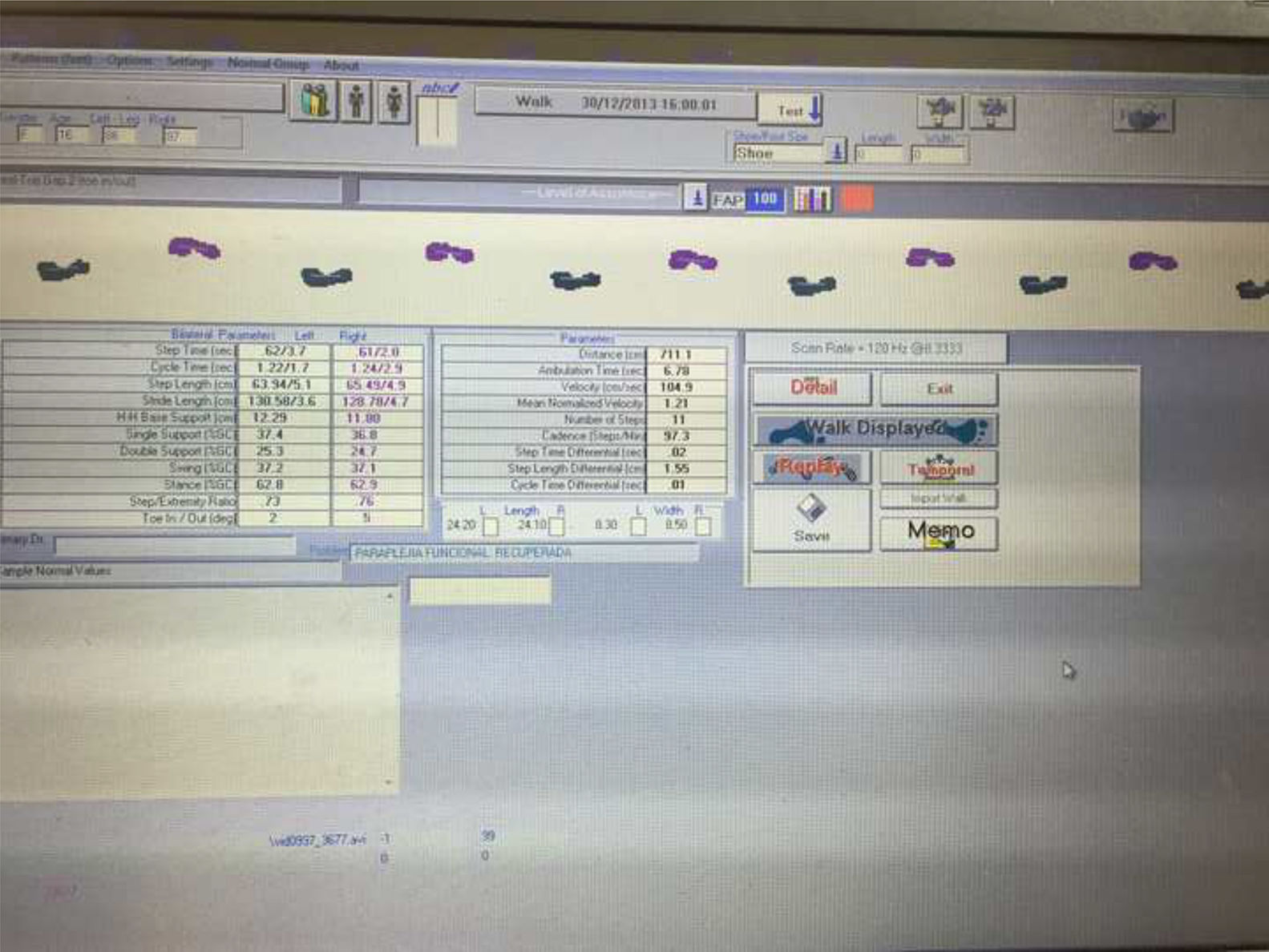
Gait alterations were analysed using the GAITRite® system (Fig. 2), a mat equipped with pressure sensors.14,16 Its results are equivalent to those of the T25FW test, but provide more precise, detailed information.15
GAITRite® was used to measure the following variables (Fig. 3):
- -
Stride length (cm): distance from the centre of the heel of one footprint to the centre of the heel of the previous footprint made by the opposite foot, measured on the line of progression.14
- -
Walking speed (cm/s): quotient of the distance divided by walking time.14
- -
Walking cadence: number of steps taken per minute.14
- -
FAP (%): percentage of gait function of a patient as compared to a group of healthy individuals, calculated on the basis of a series of spatio-temporal gait parameters.14
- -
Gait speed and stride length decrease with increasing EDSS scores.7
The Shapiro-Wilk test was used to test for normality.
Baseline data on quantitative variables were analysed with the independent-samples t test or the Mann-Whitney U test, depending on whether they were normally distributed.
Normally-distributed quantitative data were analysed using the paired-samples t test for intragroup comparisons (3 measurements) and the independent-samples t test for intergroup comparisons (difference between means).
Non-normally distributed quantitative variables were compared with the paired-samples Wilcoxon test for intragroup comparisons (3 measurements), or with the Mann-Whitney U test for independent samples for intergroup comparisons (difference between means).
Data were analysed with the SPSS statistical software, version 23. The significance threshold was established at P<.05.
ResultsWe initially recruited 70 individuals, 61 of whom were finally included in the study (9 patients were excluded due to relapses). The intervention group included 30 individuals and the control group 31.
The homogeneity of the sample was analysed in terms of age. The Shapiro-Wilk test (W=.395 for the intervention group and W=0.91 for the control group) confirmed the normality of age distribution in both groups.
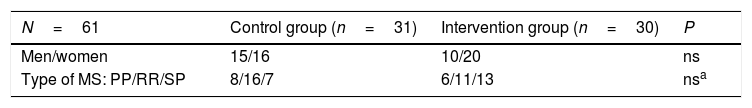
Regarding the sex distribution in both groups, the chi-square value (0.30) indicates that there were no statistically significant differences between groups, despite the intervention group having a larger percentage of women. The sample included 25 men and 36 women (Table 1).
Baseline characteristics of the sample.
| N=61 | Control group (n=31) | Intervention group (n=30) | P |
|---|---|---|---|
| Men/women | 15/16 | 10/20 | ns |
| Type of MS: PP/RR/SP | 8/16/7 | 6/11/13 | nsa |
The chi-square value (0.22) indicates that there were no statistically significant differences in terms of MS type.
According to the results of the Shapiro-Wilk test, EDSS scores were normally distributed in the intervention group only (W=0.84, P<.001 vs W=0.90, P<.05 in the control group). The Mann-Whitney U test (U=377.0) demonstrated the absence of statistically significant differences in EDSS scores between groups. The mean EDSS score was 4.3. Therefore, we may conclude that both groups are comparable in terms of age and sex.
Stage 0 corresponds to baseline status, stage 1 corresponds to status at one month, and stage 2 corresponds to the 3-month assessment.
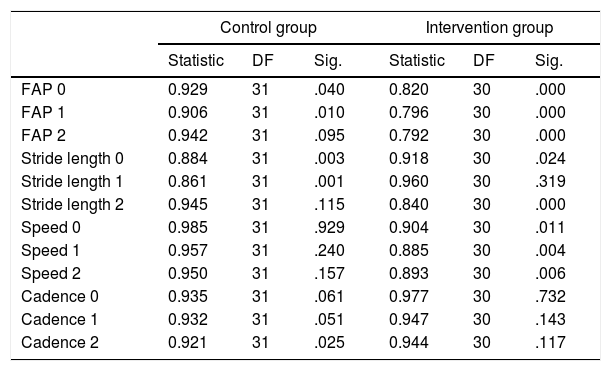
The Shapiro-Wilk test of normality is more appropriate for small datasets; data are considered to be normally distributed when the level of significance is >.05. Walking speed and cadence follow a normal distribution, whereas FAP and stride length do not. We therefore used one statistic or the other depending on data distribution (Table 2).
Results from the Shapiro-Wilk test for normality.
| Control group | Intervention group | |||||
|---|---|---|---|---|---|---|
| Statistic | DF | Sig. | Statistic | DF | Sig. | |
| FAP 0 | 0.929 | 31 | .040 | 0.820 | 30 | .000 |
| FAP 1 | 0.906 | 31 | .010 | 0.796 | 30 | .000 |
| FAP 2 | 0.942 | 31 | .095 | 0.792 | 30 | .000 |
| Stride length 0 | 0.884 | 31 | .003 | 0.918 | 30 | .024 |
| Stride length 1 | 0.861 | 31 | .001 | 0.960 | 30 | .319 |
| Stride length 2 | 0.945 | 31 | .115 | 0.840 | 30 | .000 |
| Speed 0 | 0.985 | 31 | .929 | 0.904 | 30 | .011 |
| Speed 1 | 0.957 | 31 | .240 | 0.885 | 30 | .004 |
| Speed 2 | 0.950 | 31 | .157 | 0.893 | 30 | .006 |
| Cadence 0 | 0.935 | 31 | .061 | 0.977 | 30 | .732 |
| Cadence 1 | 0.932 | 31 | .051 | 0.947 | 30 | .143 |
| Cadence 2 | 0.921 | 31 | .025 | 0.944 | 30 | .117 |
DF: degrees of freedom; FAP: functional ambulatory performance; Sig.: level of statistical significance.
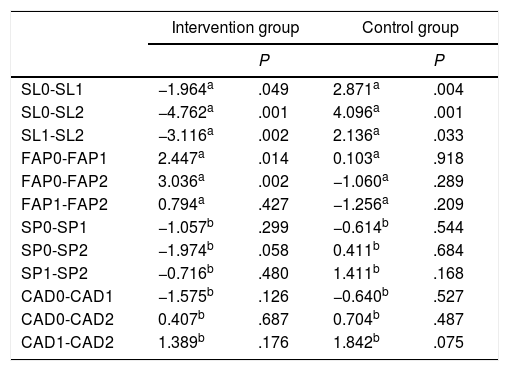
Firstly, we compared the variable FAP, which does not follow a normal distribution. The intervention group showed statistically significant changes between stages 0 and 1 (P<.014) and between stages 0 and 2 (P<.002); no such differences were observed in the control group. This reflects significant improvements in performance between the baseline assessment and subsequent assessments (Table 3).
Comparison of values for study variables between the intervention and the control groups.
| Intervention group | Control group | |||
|---|---|---|---|---|
| P | P | |||
| SL0-SL1 | −1.964a | .049 | 2.871a | .004 |
| SL0-SL2 | −4.762a | .001 | 4.096a | .001 |
| SL1-SL2 | −3.116a | .002 | 2.136a | .033 |
| FAP0-FAP1 | 2.447a | .014 | 0.103a | .918 |
| FAP0-FAP2 | 3.036a | .002 | −1.060a | .289 |
| FAP1-FAP2 | 0.794a | .427 | −1.256a | .209 |
| SP0-SP1 | −1.057b | .299 | −0.614b | .544 |
| SP0-SP2 | −1.974b | .058 | 0.411b | .684 |
| SP1-SP2 | −0.716b | .480 | 1.411b | .168 |
| CAD0-CAD1 | −1.575b | .126 | −0.640b | .527 |
| CAD0-CAD2 | 0.407b | .687 | 0.704b | .487 |
| CAD1-CAD2 | 1.389b | .176 | 1.842b | .075 |
CAD: walking cadence; FAP: functional ambulatory performance; SL: stride length; SP: walking speed.
The intervention group also displayed significant differences in stride length between stages 0 and 2 (P<.001) and between stages 1 and 2 (P<.002), whereas controls only displayed significant differences in stride length between stages 0 and 2 (P<.004) (Table 3).
The paired-data t test was used to analyse walking speed and cadence, as these variables were normally distributed. No significant differences in walking speed or cadence were observed between stages in any of the groups (Table 3).
DiscussionNo previous study addresses the effects of the MOTOmed Viva2 system on gait in patients with MS. The available evidence focuses on the effects of visual biofeedback cycling training on gait in patients with stroke.10,11 Previous studies have shown that visual feedback movement training provides intrinsic and extrinsic sensory information, improving learning and motor control.
Ours is the first study to be conducted in Spain that evaluates the effects of MOTOmed Viva2 on step asymmetry, FAP, walking speed, and walking cadence in patients with MS.17,18
We used gold-standard tools for MS, with good intra- and inter-rater reliability, to minimise the methodological limitations observed in previous studies. More specifically, we used the validated GAITRite® system to evaluate gait parameters. The GAITRite® mat was useful and effective for evaluating gait quality in patients with MS; this easy-to-use system provides fast, thorough analysis of the variability of spatio-temporal gait parameters in patients with MS.15
One of the most important considerations was whether the effects of the treatment on cycling symmetry may be transferred to functional gait.
The intervention group displayed significant improvements only in FAP and stride length. FAP showed improvements at one month and at 3 months after treatment onset. In the intervention group, stride length showed no significant changes one month after treatment onset but did improve significantly at 3 months, both compared to baseline stride length and to stride length one month after treatment onset. The most significant improvements were observed at the end of the 3-month intervention period. Patients showed significant improvements in gait symmetry. No patient in either group showed improvements in either walking speed or cadence. This suggests that the duration of the intervention was too short to induce changes in these gait parameters. Our results also suggest that 12 sessions are insufficient to fully determine the potential of cycling training for improving gait. Longer treatment duration may enable greater improvements in aspects from physical performance to locomotor function. Treatment duration should be adapted to each patient's circumstances. According to our results, this type of treatment seems to be most beneficial in patients with more marked asymmetry and who overuse the less affected leg.
Pedalling and walking involve similar muscle activation patterns since both tasks require the legs to alternate in flexion and extension, with agonist and antagonist muscles alternating synchronously.19,20 Previous research suggests that leg flexion and extension patterns generated by the CPG may be regulated by peripheral sensory input.21 A possible explanation for the positive effects of treatment is that visual feedback provided through the stationary bicycle's display during training may be beneficial for muscle control and muscle activation in the affected leg.
Some studies with stroke patients have used a frequency-domain near-infrared spectroscopy system to detect haemodynamic changes resulting from neuronal activity during pedalling. These studies have shown greater premotor cortex activation during active cycling with visual feedback than during active cycling without visual feedback.22,23 However, limited data are available on the impact of extrinsic feedback cycling training on brain activation, especially regarding its long-term effects. Future studies including patients with MS could use MRI to explore this question.
Our study has several limitations, including the lack of follow-up and the small size of our sample, which prevent us from generalising our results. We may conclude, however, that visual biofeedback cycling training may optimise lower limb function and improve gait parameters in patients with MS. This type of training may be used in MS rehabilitation therapy or performed by patients at home.
Our results show that visual biofeedback cycling training may be a viable, effective treatment in patients with MS. Future controlled studies including larger samples may confirm the benefits of this safe, inexpensive treatment for home rehabilitation.
Conflicts of interestThe authors have no conflicts of interest to declare.
We are grateful to the Hogar Virgen de los Reyes social centre for providing us with a space to set up our unit's biofunctional neurophysiotherapy treatment room, where the experiment was conducted.
Please cite this article as: Hochsprung A, Granja Domínguez A, Magni E, Escudero Uribe S, Moreno García A. Efectos del entrenamiento en bicicleta con retroalimentación visual sobre la marcha en pacientes con esclerosis múltiple. Neurología. 2020;35:89–95.
Presented in poster format as a pilot study at the 66th Annual Meeting of the Spanish Society of Neurology, 2014.