Alzheimer disease (AD) is a neurodegenerative disease characterised by progressive dementia associated with global cognitive dysfunction.
MethodsWe conducted a systematic review and meta-analysis of clinical trials evaluating omega-3 supplementation in patients with AD.
ObjectiveTo determine if there is scientific evidence of the effectiveness of omega-3 supplementation in improving cognitive function in patients with AD.
Search strategyWe included only randomised controlled trials (RCTs) from the following databases: Medline, Cochrane Central, Cinahl, and LILACS. An electronic search was also conducted using Google Scholar.
Study selectionSix articles met the eligibility criteria. The risk of bias was assessed following the Cochrane method.
ConclusionThere is no consistent evidence to support the effectiveness of omega-3 supplementation in improving cognitive function in AD patients in the short and medium term.
La enfermedad de Alzheimer (EA) es una patología neurodegenerativa caracterizada por demencia de carácter progresivo asociada a una perdida global de funciones cognitivas.
MétodosSe realizó una síntesis de evidencia a través de una revisión sistemática con metaanálisis de ensayos clínicos que hayan evaluado la suplementación de omega-3 en pacientes con EA.
ObjetivoDeterminar si existe evidencia científica que avale la efectividad de la suplementación omega-3 en la mejoría de la función cognitiva de pacientes con EA.
Estrategia de búsquedaLa estrategia de búsqueda incluyó solo ensayos clínicos aleatorizados (ECA). Las bases de datos usadas fueron: Medline, Cochrane Central, Cinahl y Lilacs. Además, se realizó una búsqueda electrónica en Google Scholar.
Selección de estudiosSe obtuvieron 6 artículos que cumplían con nuestros criterios de elegibilidad. Se evaluó el riesgo de sesgo según el método Cochrane.
ConclusiónNo hay evidencia consistente que avale la suplementación de omega-3 versus placebo en la mejoría de la función cognitiva de pacientes con EA a corto y mediano plazo.
Alzheimer disease (AD) is a neurodegenerative disease of multifactorial origin characterised by progressive dementia associated with global loss of cognitive function.1 The prevalence of the condition has increased greatly, particularly in countries that have seen an increase in life expectancy. Prevalence is below 1% in the population under 60 years of age, increasing to 40% among those older than 85.2 An individual's likelihood of developing AD depends on genetic and environmental factors that interact in the context of ageing3; the most relevant non-modifiable risk factors are age, sex, and apolipoprotein E polymorphism.2,4,5 The main modifiable risk and protective factors for AD are socioeconomic factors such as level of schooling; lifestyle factors such as alcohol and tobacco consumption and level of physical activity; and dietary factors such as the consumption of caffeine, antioxidants, and fatty acids.2,6,7
Several studies report a relationship between diet and cognitive impairment associated with AD.8–11 One of the dietary factors with the greatest effect on AD incidence is low consumption of oily fish, which has been linked to increased risk of developing the disease.12,13
Omega-3 fatty acids are a family of essential polyunsaturated fatty acids; while they are needed for normal development, the body is unable to synthesise them.14 Derivatives of docosahexaenoic acid (DHA), a type of omega-3 acid, are mainly found in seafoods such as seaweed, molluscs, crustaceans, and deep-water oily fish.15 Dietary omega-3 precursor is mainly metabolised to DHA or eicosapentaenoic acid (EPA) in the liver and, to a lesser extent, in the endothelium of the brain and in astrocytes, where the products are exported to neurons.16 DHA is incorporated into phospholipids in neuronal membranes, affording them structural and physical/chemical properties essential to synaptic functioning.17 High concentrations of DHA increase the fluidity of plasma membranes, facilitating the transport of neurotransmitters.18 Due to these properties, DHA is now considered a relevant factor in the prevention and treatment of neurodegenerative diseases.19 Experimental studies have found that DHA protects neurons against oxidative stress, especially in preserving the organisation of cytoskeletal microtubules.20 Several studies report that increasing DHA supplementation promotes neuronal maturation and development, specifically in the hippocampus, thereby increasing synaptic function.21–23
Despite these experimental results, systematic reviews only address the results of studies into the effect of omega-3 on the risk of developing AD.12,24–26 Mazereeuw et al.24 conclude that omega-3 supplementation improves cognitive function in patients with cognitive impairment but not dementia, although this effect is not observed in patients with AD. According to Wu et al.,25 increased consumption of fish is associated with reduced risk of developing AD, although no similar association was observed between supplementation with omega-3 fatty acids and risk of AD or dementia. Zhang et al.12 report an association between DHA consumption and reduced risk of dementia and AD, but did not find a linear dose/response relationship. Burckhardt et al.26 found no convincing evidence of the efficacy of omega-3 supplementation for the treatment of mild to moderate AD. However, a meta-analysis of animal models demonstrated that long-term supplementation with omega-3 does improve cognitive function.1
Given the evidence summarised above, we consider it timely to address the clinical issue of whether omega-3 supplementation is more effective than placebo for improving cognitive function in patients with AD.
ObjectivesThis study aims to analyse the available scientific evidence on the effectiveness of omega-3 supplementation in improving cognitive function in patients with AD.
Material and methodsThis study was conducted in accordance with the international PRISMA guidelines for systematic reviews and meta-analyses.27 It should be noted that the study protocol was not registered before the review was conducted.
Eligibility criteriaThe following characteristics were established for inclusion of studies in the review: (1) randomised clinical trials (RCTs); (2) including samples of patients diagnosed with AD, independently of race or sex; (3) comparing oral supplementation with omega-3 or derivatives against placebo; and (4) published in English or Spanish before 31 March 2017.
Data sourcesAn electronic search was performed on the following databases: Medline (http://www.ncbi.nlm.nih.gov/pubmed [accessed 31.03.17]), CENTRAL (http://www.cochrane.org/ [accessed 31.03.17]), LILACS (http://lilacs.bvsalud.org/es/ [accessed 31.03.17]), and CINAHL (http://search.ebscohost.com [accessed 31.03.17]); we also reviewed such other sources as Google Scholar (https://scholar.google.cl/).
Search strategiesThe search on the Medline database followed the highly sensitive search strategy proposed by Cochrane.28 PubMed was used to obtain search terms from the MeSH thesaurus; the terms used included Alzheimer and omega-3. The search strategy was as follows:
- 1.
Alzheimer Disease.
- 2.
Omega3 Fatty.
- 3.
Fatty Acids.
- 4.
Fatty Acids omega3.
- 5.
(#2) OR (#3) OR (#4).
- 6.
(#1) AND (#5).
- 7.
Randomized Clinical trial.
- 8.
Clinical trial.
- 9.
(#7) OR (#8).
- 10.
Humans.
- 11.
Animals.
- 12.
(#10) NOT (#11).
- 13.
(#6) AND (#9) AND (#12).
For the remaining databases (CENTRAL, CINAHL, LILACS, and Google Scholar), the search strategy combined the terms mentioned above in the advanced search tool.
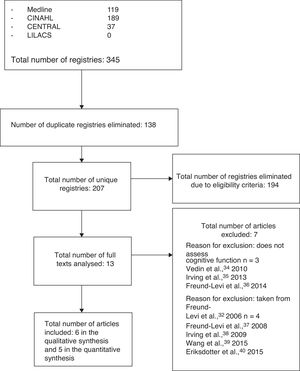
Study selectionThree authors (FAQ, HGE, and ABV) performed the search independently; after eliminating duplicate results, the preliminary search yielded a total of 207 studies. After the first screening, we eliminated 194 articles that did not meet the eligibility criteria.
Selection criteriaThe 13 articles selected were read in full text and evaluated according to the following criteria:
Inclusion criteria- –
RCTs evaluating the effectiveness of oral supplementation with omega-3 fatty acids and their derivatives (e.g., DHA and EPA) with comparison against placebo.
- –
RCTs evaluating different dimensions of cognitive function, such as memory, praxis, or language skills.
- –
RCTs including patients with non-Alzheimer cognitive impairment.
Three researchers (FAQ, MPY, and LFL) independently used a standardised form to collect data on the RCTs. In the event of disagreement/discrepancies, the article was submitted for analysis by an independent rater and a decision was made regarding its inclusion through discussion and consensus.
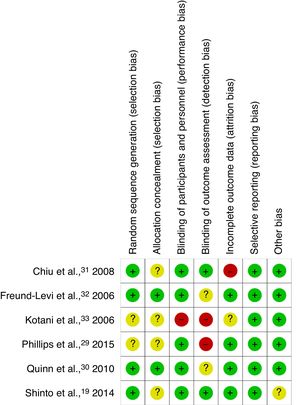
Risk of bias in the individual studiesThe risk of bias in the individual studies included was evaluated according to the recommendations of the Cochrane Handbook.28 The studies were assessed qualitatively and rated according to whether the criteria were met (green: low risk of bias), were not met (red: high risk of bias), or whether this was unclear (amber: unclear risk [insufficient information/uncertainty]). The 7 criteria evaluated were sequence generation, allocation sequence concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other potential sources of bias.
Synthesis of resultsThe RevMan 5 software was used for data analysis and synthesis. Measures of results were analysed as continuous variables; with the random effects model, the point estimates used for continuous variables were the mean difference (MD) and the standardised mean difference (SMD) and their respective 95% confidence intervals (CI). Results may only be combined to calculate point estimates when they are homogeneous; statistical homogeneity was evaluated with the chi-square test and the I2 test. We considered data to be admissible for the meta-analysis when they showed a low level of heterogeneity, defined as P>.1 for the chi-square test and an I2 value equal to or less than 40%.
ResultsStudy selectionThirteen articles met the eligibility criteria for our systematic review, with 6 finally being included after application of the selection criteria.19,29–33 The flow diagram in Fig. 1 shows the phases of the review and the reasons for exclusion of the other 7 studies.34–40
Study characteristicsThe preliminary search identified 345 articles potentially eligible for inclusion; after applying the eligibility and selection criteria, the final sample included 6 articles.19,29–33 Together, these studies included a total of 758 patients (mean, 142 per study), with a mean age of 71.8 years (range, 67-76.7). Table 1 summarises each article's clinical characteristics, methodology, and results.
Characteristics of the articles included.
| Author/year | Patient characteristics | Intervention | Follow-up/outcome measures | Results |
|---|---|---|---|---|
| Kotani et al.,33 2006 | Patients with mild cognitive impairment (subgroup with AD but not dementia)Rand.: not mentionedN=21EG: n=8Age: 67 years (SD, 6.3)CG: n=9Age: 69.7 years (SD, 5.2) | EG: 6 capsules per day, total dose of ARA and DHA of 240mg/day; duration, 90 daysCG: 6 capsules per day, total dose 240mg of olive oil; duration, 90 days | Follow-up at 90 daysOutcome measures:5 cognitive function domains of the RBANS– Immediate memory– Visuospatial/constructional– Language– Attention– Delayed memory | No significant differences were observed between EG and CG on any of the cognitive function domains at 90 days (P>.05). |
| Freund-Levi et al.,32 2006 | Patients with mild to moderate AD under treatment with AChE inhibitors for at least 3 monthsRand.: block randomisation, generated electronically; sealed envelopes were used for concealment.n=174EG: n=89Age: 72.6 years (SD, 9)CG: n=85Age: 72.9 years (SD, 8.6) | EG: 4 capsules per day, total dose of 1.7g DHA and 0.6g EPA; 4mg vitamin E was also administered; duration, one yearCG: 4 capsules per day of corn oil (including 0.6g linoleic acid) for 6 months, then 6 months on the same treatment as the EG; 4mg vitamin E was also administered | Follow-up at 6 and 12 monthsOutcome measures:Cognitive function– MMSE– ADAS-CogGlobal function– CDR– Arterial blood pressure– Tolerability | The global analysis identified no significant differences between EG and CG in cognitive function at 6 or 12 months (P>.05).In the subgroup analysis, only patients with very mild AD (MMSE>27) showed significant differences in MMSE score between control and treatment groups (P<.05). |
| Chiu et al.,31 2008 | Patients with mild to moderate ADRand.: not mentionedn=46EG: n=24Age: 74 years (95% CI, 70.1–77.8)CG: n=22Age: 76.5 years (95% CI, 71.8–81.1) | EG: 3 capsules twice per day, total dose 1g EPA and 0.7g DHA; duration, 24 weeksCG: 3 capsules twice per day of olive oil esters; duration, 24weeks | Follow-up at 24 weeksOutcome measures:– CIBIC-plus– ADAS-Cog– MMSE– HDRS– Adherence– Adverse reactions | No significant differences were observed between groups for cognitive function at 6, 12, 18, or 24 weeks (P>.05).Subgroup analysis showed significant differences in ADAS-Cog scores in patients with mild AD (P=.03). |
| Quinn et al.,30 2010 | Patients with mild to moderate AD under treatment with AChE inhibitors for at least 3 monthsRand.: block randomisation, generated electronicallyn=402EG: n=238Age: 76 years (SD, 9.3)CG: n=164Age: 76 years (SD, 7.8) | EG: 2 capsules daily, total dose of 2g DHA; duration, 18 monthsCG: 2 capsules daily of corn and soy oil; duration, 18 months | Follow-up at 6, 12, and 18 monthsOutcome measures:Cognitive function:– ADAS-Cog– CDR– MMSEActivities of daily living:– ADCS-ADL– NPIQuality of life:– QLADSAdverse reactions | No significant differences in cognitive function were observed between groups at 6, 12, or 18 months.At the end of the treatment period, differences were:– ADAS-Cog: P=.41– CDR: P=.68– MMSE: P=.88– ADCS-ADL: P=.38– NPI: P=.11 |
| Shinto et al.,19 2014 | Patients with mild to moderate ADRand.: block randomisation, generated electronicallyn=39EG1: n=13Age: 75.9years (SD, 8.1)EG2: n=13Age: 76.7 years (SD, 10.6)CG: n=13Age: 75.2 years(SD, 10.8) | EG1: 3 capsules per day, total dose of 675mg DHA and 975mg EPA; duration, one yearEG2: one tablet per day of 600mg ALA plus 3 capsules per day with a total dose of 675mg DHA and 975mg EPA; duration, one yearCG: 3 capsules per day of excipients; duration, one year | Follow-up at 6 and 12 monthsOutcome measures:Cognitive function:– ADAS-Cog– MMSEActivities of daily living:– ADCS-ADLBiomarkers of oxidative stressAdverse reactions | At 12 months, differences in cognitive function were as follows:– ADAS-Cog: CG vs. EG1, P=.86; CG vs. EG2, P=.98– MMSE: CG vs. EG1, P=.80; CG vs. EG2, P<.01 |
| Phillips et al.,29 2015 | Patients with AD and patients with cognitive impairment but not dementia; all patients were receiving AChE inhibitorsRand.: not mentionedn=76EG: n=37Age: 71.1 years(SD, 8.6)CG: n=39Age: 71.1 years(SD, 9.5) | EG: total daily dose of 600mg EPA and 625mg DHA; duration, 4 monthsCG: olive oil; duration, 4 months | Follow-up at 1 and 4 monthsOutcome measures:– MMSE– Immediate verbal memory– Delayed verbal memory– Recognition verbal memory– Mood | No significant differences were found between groups at 1 or 4 months.At the end of the treatment period, differences were:– MMSE: P=.576– Immediate verbal memory: P=.499– Delayed verbal memory: P=.463– Recognition verbal memory: P=.463– Mood: P=.548 |
AChE: acetylcholinesterase; ADAS-Cog: Alzheimer's Disease Assessment Scale – Cognitive Subscale; ADCS-ADL: Alzheimer's Disease Cooperative Study – Activities of Daily Living; ARA: arachidonic acid; CDR: Clinical Dementia Rating scale; CIBIC-plus: Clinician Interview-Based Impression of Change, plus carer interview; SD: standard deviation; DHA: docosahexaenoic acid; AD: Alzheimer disease; EPA: eicosapentaenoic acid; CG: control group; EG: experimental group; HDRS: Hamilton Depression Rating Scale; ALA: alpha-lipoic acid; Rand.: randomisation method; MMSE: Mini–Mental State Examination; NPI: Neuropsychiatric Inventory; QLADS: Quality of Life Alzheimer's Disease Scale; RBANS: Repeatable Battery for the Assessment of Neuropsychological Status.
The authors independently evaluated the risk of bias of all the articles included (Figs. 2 and 3). Most studies described the methods of randomisation and allocation concealment used; however, the studies by Kotani et al.33 and Phillips et al.29 do not provide this information, which limits their internal validity. Most studies were classed as having low risk of bias for the blinding of participants and personnel, with the exception of the study by Kotani et al.,33 which did not provide this information. Blinding of raters was not applied in the articles by Kotani et al.33 and Phillips et al.,29 and was not described in the studies by Quinn et al.30 and Freund-Levi et al.32; blinding raters is essential for avoiding over- or underestimation of results. While the majority of studies did report drop-outs during the treatment and/or follow-up periods, they performed intention-to-treat analyses to prevent attrition bias. Only the RCT by Chiu et al.31 did not include an intention-to-treat analysis. Currently, protocols are not registered on any registry system for any of the studies. This makes it very difficult to establish the existence of selective reporting. However, the studies include all the expected results and all studies are free of other sources of bias.
Outcome measuresThe outcome measures most frequently used in the RCTs reviewed were cognitive deterioration (Mini–Mental State Examination [MMSE]), cognitive dysfunction (Alzheimer's Disease Assessment Scale-Cognitive Subscale [ADAS-Cog]), level of dementia (Clinical Dementia Rating [CDR] scale), and neuropsychiatric status (Neuropsychiatric Inventory [NPI]).
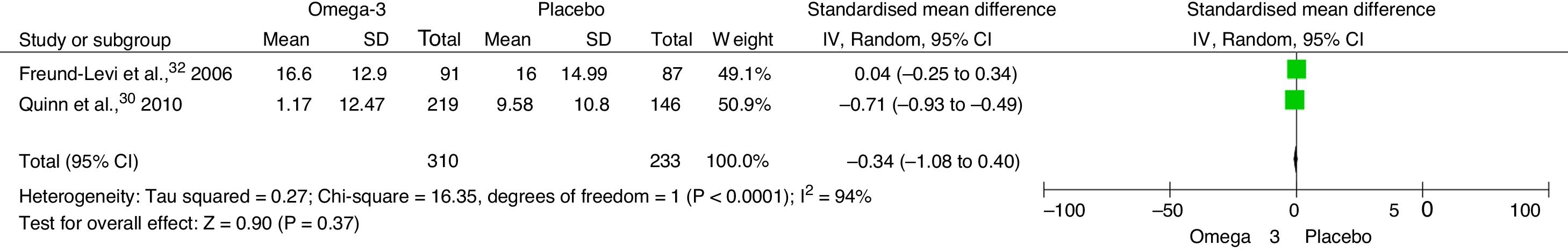
Synthesis of resultsWe analysed the results of the 6 studies that met the eligibility criteria for inclusion in the systematic review.19,29–33 Based on point estimates, it was only possible to combine the results of 5 studies to analyse the effectiveness of oral supplementation with DHA plus EPA in improving some aspect of cognitive function as compared to placebo.19,29–32
The meta-analysis found an SMD of −0.34 (95% CI, −1.08 to 0.40; P=.37) in NPI score at 6 months between patients receiving omega-3 and those receiving placebo (Fig. 4). The SMD for MMSE score at 6 months was 0.58 (95% CI, −0.43 to 1.60; P=.26) (Fig. 5). The SMD for ADAS-Cog score at 6 months was 1.10 (95% CI, −1.03 to 3.23; P=.31) (Fig. 6).
We aimed to determine the effectiveness of oral supplementation with omega-3 as compared to placebo for improving cognitive function in patients with AD. We synthesised the available evidence in a systematic review of RCTs, including 6 studies that met our eligibility criteria, and considered it viable to perform a meta-analysis of 5 of the selected studies. Although some epidemiological studies report that the consumption of fish rich in omega-3 fatty acids reduces the risk of cognitive impairment,12,25 systematic reviews studying omega-3 supplementation in patients with AD conclude that the treatment only improves certain aspects of cognitive function in patients with cognitive impairment not associated with dementia; these findings cannot be extrapolated to patients with AD.24–26 Our own results are consistent with these findings.
Numerous factors may explain our results; these include variability in the dose of DHA or DHA/EPA (which ranged from 240mg to 2.3g per day) and in the duration of the treatment period (90 days to 1.5 years). A second factor is the ratio of DHA to EPA administered. These factors constitute an obstacle for the comparison and extrapolation of study results. Another factor not addressed by any of the studies was polymedication associated with the chronic diseases present in a majority of older adults: some drugs may interact with the dietary supplement, preventing proper absorption.41,42
We should also take into account the diets of individual patients. Animal studies have shown that diets high in sugars may abrogate the reported benefits of omega-3.43 A diet rich in fibre and chelating agents may interfere with the proper absorption of lipids from the intestinal lumen, while supplementation with foods of animal origin may promote absorption.
In terms of the mechanism of action, omega-3 fatty acids (and especially DHA) are beneficial in controlling inflammatory processes.39 They modulate the inflammatory response, modifying the fluidity and composition of cell membranes through direct effects on receptor function and the conductance of ion channels involved in immune activation.44 Recent studies have demonstrated that deficient production of anti-inflammatory mediators plays an important role in cognitive impairment in patients with AD.39,45–47 Furthermore, evidence from epidemiological studies suggests the existence of a critical period of 2 or more years before the onset of dementia in which an increase is observed in pro-inflammatory mediators in the brain, which may affect the progression of AD.32–48 In the light of the above, researchers including Freund-Levi et al.32 report that supplementation with omega-3 may be beneficial for preventing or slowing the progression of the disease but not for treating it after the onset of dementia.
Regarding adverse reactions to the treatment, and taking into account the characteristics of the intervention studied, only Chiu et al.31 and Quinn et al.30 report treatment-related adverse effects, which are classified as severe or non-severe. Severe adverse reactions included hospitalisation, deep vein thrombosis, and death, while non-severe reactions (which were more frequent) included diarrhoea, constipation, gastrointestinal symptoms, and urinary tract infections. Neither study reports statistically significant differences in the incidence of these events between experimental and control groups; the authors therefore consider omega-3 supplementation to be a safe intervention.
Our systematic review does present some limitations, such as the decision not to include the grey literature (biomedical information not indexed on academic databases), which was necessary in the search for the studies included. This represents a source of publication bias and should be taken into account when interpreting our findings, as it is likely that some studies will have been excluded from our review due to indexing problems. Finally, we would like to thank the authors of the review for the information provided for the development of our investigation.
ConclusionAccording to the results of our systematic review, there is no consistent evidence that omega-3 supplementation is effective for improving cognitive function in patients with AD in the short to medium term.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Araya-Quintanilla F, Gutiérrez-Espinoza H, Sánchez-Montoya U, Muñoz-Yañez MJ, Baeza-Vergara A, Petersen-Yanjarí M, et al. Efectividad de la suplementación de ácidos grasos omega-3 en pacientes con enfermedad de Alzheimer: revisión sistemática con metaanálisis. Neurología. 2020;35:105–114.