Cervical artery dissection (CAD) is the cause of 2% to 3% of ischaemic strokes and 10% to 25% of the ischaemic strokes in young people. Our objective is to evaluate whether the implementation of a comprehensive stroke centre (CSC) improves the diagnosis and modifies the prognosis of patients with acute stroke due to CAD.
Patients and methodsRetrospective study of a registry of consecutive patients with acute stroke due to CAD. They were classified according to the period of care at our centre: pre-CSC (October 2004 to March 2008, 42 months) or post-CSC (April 2008 to June 2012, 51 months). We compared baseline characteristics, methods of diagnosis, treatment and outcome of these patients in both periods.
ResultsNine patients were diagnosed with CAD in the pre-CSC and 26 in the post-CSC, representing 0.8% and 2.1% of all ischaemic strokes treated in each period, respectively. The diagnosis of CAD was made within the first 24hours in 42.3% of the patients in the post-CSC versus 0% in the pre-CSC, through the use of urgent cerebral angiography as a diagnostic test in 46.2% of cases in the second period compared to 0% in the first. The severity of stroke (median NIHSS score 11 vs. 3, P=.014) and time to neurological care (265min vs. 148, P=.056) were higher in the post-CSC period. Endovascular treatment was performed in 34.3%, all in the post-CSC. The functional outcome was comparable in both periods.
ConclusionsThe implementation of a CSC increases the frequency of the diagnosis of CAD, as well as the treatment options for these patients in the acute phase of stroke.
La disección de arterias cervicales (DAC) es la causa del 2-3% de ictus isquémicos y del 10-25% en pacientes jóvenes. Nuestro objetivo es evaluar si la implementación de un centro terciario de ictus (CTI) facilita el diagnóstico y modifica el pronóstico de los pacientes con ictus agudo por DAC.
Pacientes y métodosEstudio retrospectivo de un registro de pacientes consecutivos con ictus agudo por DAC. Se clasificaron según el periodo de atención: pre-CTI (octubre 2004-marzo 2008, 42 meses) o post-CTI (abril 2008-junio 2012, 51 meses). Se compararon las características basales, el método diagnóstico, el tratamiento y la evolución de estos pacientes entre ambos periodos.
ResultadosSe diagnosticó a 9 pacientes con DAC en el periodo pre-CTI y 26 en el post-CTI, representando el 0,8 y el 2,1% de los ictus isquémicos atendidos en cada periodo. El diagnóstico de DAC se realizó en las primeras 24h en el 42,3% de pacientes en el periodo post-CTI frente al 0% en el pre-CTI, gracias al uso de la arteriografía cerebral urgente como prueba diagnóstica en el 46,2% de los casos en el segundo periodo frente al 0% en el primero. La gravedad del ictus (mediana puntuación escala NIHSS 11 vs. 3, p=0,014) y el tiempo hasta la atención neurológica (265 minutos vs. 148, p=0,056) fueron mayores en la fase post-CTI. Se realizó tratamiento endovascular en el 34,3%, todos en el periodo post-CTI. El pronóstico funcional fue comparable en ambos periodos.
ConclusionesLa implementación de un CTI incrementa la frecuencia en el diagnóstico de DAC y aumenta las opciones terapéuticas en la fase aguda del ictus en estos pacientes.
Cervical artery dissection (CAD) is responsible for 2% to 3% of all ischaemic strokes in the general population and 10% to 25% of ischaemic strokes in patients younger than 50 years.1,2 Arteriography has traditionally been the diagnostic tool of choice for CAD. However, new and less invasive techniques, including magnetic resonance imaging (MRI), computerised tomography (CT) angiography, and ultrasonography studies, have replaced conventional arteriography in daily clinical practice. Despite the above, none of these new techniques has proved more sensitive than another for diagnosing CAD. As a result, conventional arteriography is still considered the gold standard.3,4
Intravenous thrombolysis is a safe treatment in the acute phase of CAD-related strokes.5–7 However, CAD-related ischaemic stroke patients treated with systemic thrombolysis show poorer clinical progress due to frequent tandem intracranial internal carotid artery/middle cerebral artery occlusions.6,8 Experience with endovascular treatment (EVT) in CAD-related stroke patients is limited,9–13 and it remains uncertain whether EVT provides greater clinical benefits than systemic thrombolysis.14,15
Comprehensive stroke centres (CSC) provide 24-hour diagnostic care with multimodal neuroimaging tests of viable ischaemic tissue, brain angiography studies, and mechanical thrombectomy.16
Our purpose was to determine whether caring for patients in a CSC facilitates diagnosing CAD and affects patients’ functional outcomes.
Patients and methodsWe retrospectively studied patients with CAD-related acute ischaemic stroke diagnosed at Hospital Germans Trias i Pujol (HGTiP) and included in the Spanish Society of Neurology's prospective stroke registry between October 2004 and June 2012.17
In March 2008, the HGTiP implemented a 24-hour on-call team consisting of an interventional neuroradiologist, a neurologist specialised in cerebrovascular disease, specialised nursing staff, and an anaesthetist. A neuro-angioradiology room was available at all times. Specific protocols were also developed for diagnosing acute-phase stroke and administering EVT.16 The hospital was certified as a CSC by the Catalan Regional Ministry of Health.
Patients were classified into 2 groups according to the period when they received care: pre-CSC status (October 2004 to March 2008; 42 months) or post-CSC status (April 2008 to June 2012; 51 months).
Clinical assessmentAll patients were assessed by neurologists in the emergency department and admitted to a stroke unit. We gathered demographic data, vascular risk factors, baseline vital signs, and time elapsed from symptom onset (or the last time patients were asymptomatic in cases of wake-up stroke or uncertain onset time) to neurological care. Stroke severity was evaluated using the National Institutes of Health Stroke Scale (NIHSS). Neurological improvements were labelled as ‘early’ when patients showed a decrease of 4 points or reached a score of 0 to 1 on the NIHSS (baseline score <4) within the first 24hours; a decrease of 10 points or attaining a score of 0 or 1 (baseline score 4-10) within that same lapse was labelled a ‘dramatic’ neurological improvement. Therefore, patients scoring 0 or 1 on the initial assessment were not included in our analysis in order for us to be able to evaluate early and dramatic neurological improvement. The variable ‘degree of neurological improvement at 7 days’ was defined as the difference between baseline NIHSS score and the score at 7 days. The patients’ functional prognosis was determined at discharge and at 90 days after stroke using the modified Rankin Scale (mRS). Scores ≤2 were regarded as indicative of a good functional prognosis.
Diagnostic and therapeutic protocolAcute stroke was managed according to the recommendations of the European Stroke Organisation.18 Patients attended within the therapeutic window for systemic thrombolysis underwent a simple cranial CT and an echo Doppler study of the transcranial and supra-aortic trunks. Patients attended out of the therapeutic window were studied with multimodal MRI and intracranial angio-MRI, or else simple cranial CT and echo Doppler sonography of the supra-aortic trunks if the MRI scanner was unavailable.
Until November 2008, patients with no contraindications were treated with intravenous thrombolytics at a standard dose of 0.9mg/kg when time since onset was less than 3hours. The time window was increased to 4.5hours in December 2008, after the results of the ECASS-3 study were published.19
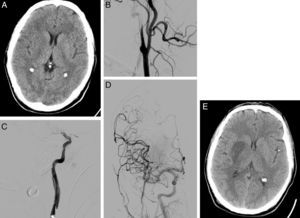
After implementation of the CSC (March 2008), diagnostic angiography and EVT were indicated for patients with large artery occlusion who were not eligible for intravenous thrombolysis, or patients in whom intravenous rtPA had failed to achieve arterial recanalisation, provided that viable ischaemic tissue had been demonstrated by a prior imaging study (ASPECTS>7 in CT or lesion covering <50% of the affected arterial territory in a DWI MRI sequence and not seen in FLAIR). Mechanical thrombectomy was performed in patients with occlusion of an accessible intracranial artery. In those patients with tandem occlusions (extracranial artery/intracranial artery), we initially attempted to perforate the proximal occlusion/stenosis to subsequently perform mechanical thrombectomy of the intracranial artery occlusion and then place a stent in the proximal occlusion/stenosis when necessary (Fig. 1). EVT was not administered to patients with extracranial carotid artery occlusion and no intracranial tandem occlusion.
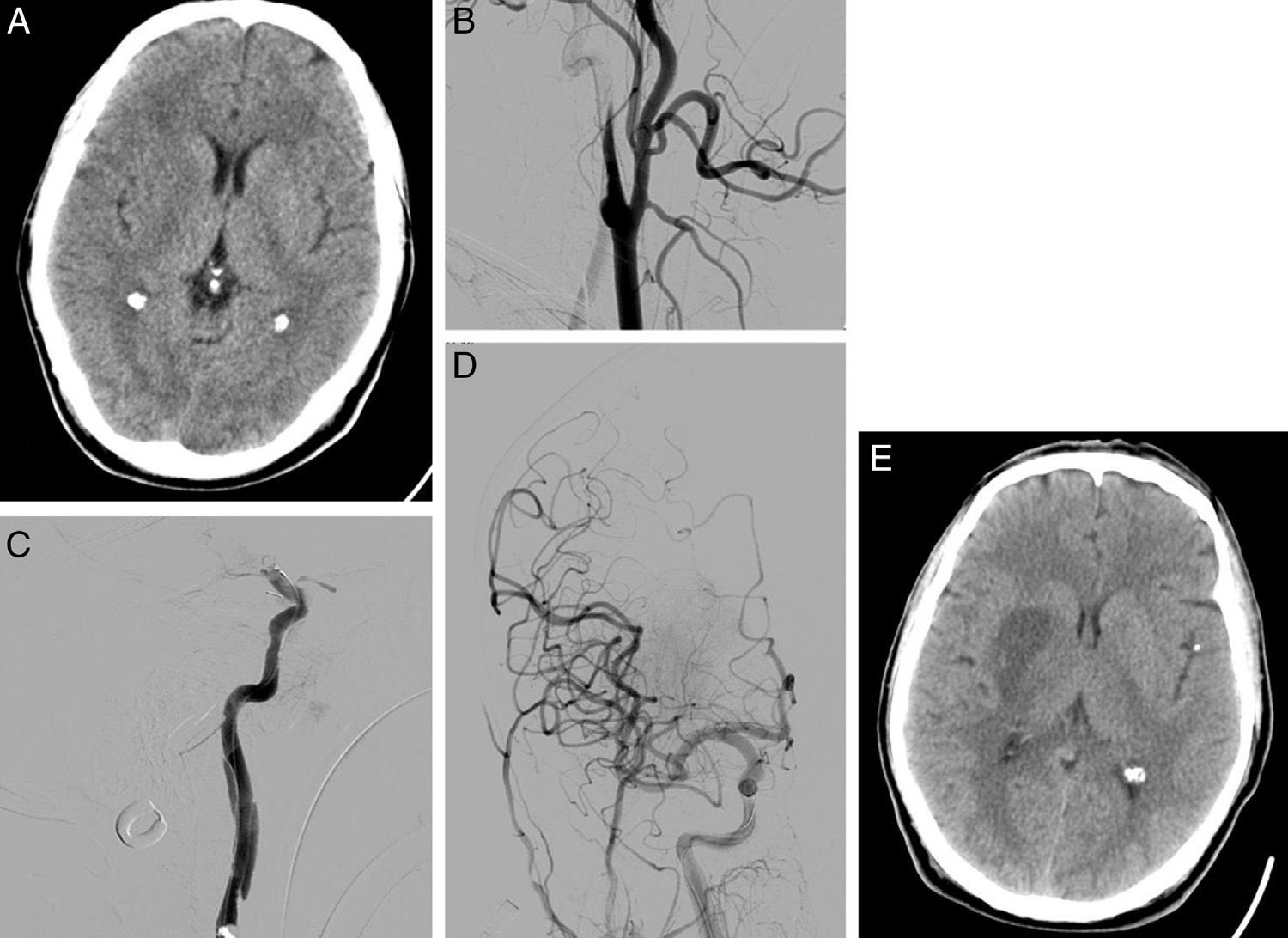
58-year-old man with dysarthria, left hemiparesis, and an NIHSS score of 14. (A) Cranial CT shows effacement of the lentiform nucleus and right insula; the patient scored 8 on ASPECTS. (B) The initial arteriography shows dissection of the right internal carotid artery, with typical flute-beak-shaped occlusion. (C) On the other side of the carotid occlusion, an occlusion was observed in the M1 segment of the right middle cerebral artery. (D) A mechanical thrombectomy was performed in the intracranial occlusion and a stent was placed in the carotid stenosis, achieving complete recanalisation as shown by the follow-up arteriography. (E) Follow-up cranial CT (at 24hours) displays infarction of the deep territory of the right middle cerebral artery.
Patients who were not eligible for revascularisation (intravenous or endovascular) were treated with either antiplatelet or anticoagulant antithrombotics, depending on their clinical characteristics, neurological severity, and size of the cerebral infarct.
Conventional arteriography or brain MRI studies were performed to confirm the clinical suspicion of CAD as the cause of stroke. Findings that confirmed diagnosis included intimal flap, double lumen image, ‘flute-beak’ or ‘flame-shaped’ stenosis/occlusion, irregular narrowing of the artery or dissecting aneurysm in arteriography studies or brain MRI angiographic sequences, and a crescent-shaped false lumen adjacent to the vascular lumen in axial T1-weighted fat-suppressed cranial MRI sequences. CAD was considered to have been diagnosed in the acute phase when the neuroimaging study confirming the diagnosis was performed within 24hours of symptom onset or during the diagnostic arteriography study before revascularisation therapy.
Statistical analysisNormally distributed continuous variables were expressed as means±SD, or as medians and quartiles for variables that were not normally distributed. Categorical variables were expressed as percentages. After conducting a descriptive analysis of all the studied variables, we estimated statistical significance for differences between groups using the chi-square and Fisher exact tests for categorical variables, and the Mann–Whitney, ANOVA, Kruskal–Wallis, and t tests for continuous variables. P values <.05 were considered statistically significant.
ResultsA total of 2853 patients were recorded as having an initial stroke between October 2004 and June 2012. Of this number, 35 (1.23%) experienced a CAD-related ischaemic stroke. Nine (0.8%) of all ischaemic stroke cases diagnosed in the pre-CSC period (1094), and 26 (2.1%) of the ischaemic strokes diagnosed in the post-CSC period (1245) were CAD-related (P=.008). The percentage of CAD-related ischaemic strokes was also higher in the post-CSC period in patients younger than 50 years (4.7% vs 12%; P=.068).
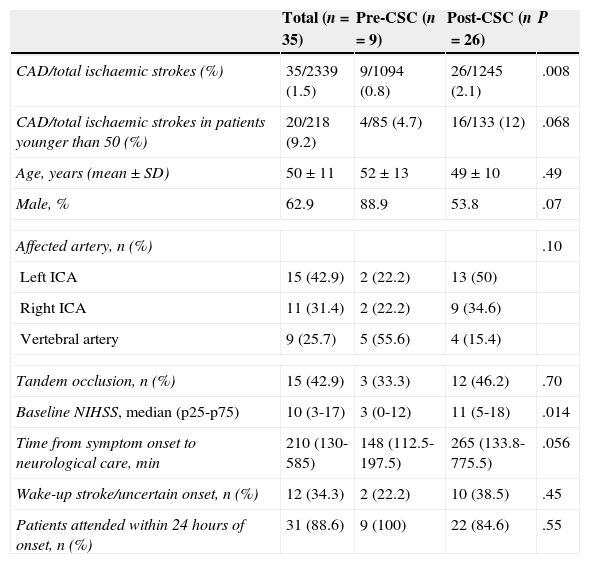
Table 1 lists clinical characteristics of the total study population and of pre-CSC and post-CSC subgroups. The 2 subgroups displayed no statistically significant differences with regard to age, sex, type of cervical artery affected by dissection, or percentage of patients with tandem occlusion. However, the percentage of vertebral artery dissections was higher in the pre-CSC subgroup. Baseline stroke severity (baseline NIHSS score) and time from symptom onset to neurological care at HGTiP were greater in the post-CSC subgroup.
Baseline characteristics of patients with CAD broken down by study period.
| Total (n=35) | Pre-CSC (n=9) | Post-CSC (n=26) | P | |
|---|---|---|---|---|
| CAD/total ischaemic strokes (%) | 35/2339 (1.5) | 9/1094 (0.8) | 26/1245 (2.1) | .008 |
| CAD/total ischaemic strokes in patients younger than 50 (%) | 20/218 (9.2) | 4/85 (4.7) | 16/133 (12) | .068 |
| Age, years (mean±SD) | 50±11 | 52±13 | 49±10 | .49 |
| Male, % | 62.9 | 88.9 | 53.8 | .07 |
| Affected artery, n (%) | .10 | |||
| Left ICA | 15 (42.9) | 2 (22.2) | 13 (50) | |
| Right ICA | 11 (31.4) | 2 (22.2) | 9 (34.6) | |
| Vertebral artery | 9 (25.7) | 5 (55.6) | 4 (15.4) | |
| Tandem occlusion, n (%) | 15 (42.9) | 3 (33.3) | 12 (46.2) | .70 |
| Baseline NIHSS, median (p25-p75) | 10 (3-17) | 3 (0-12) | 11 (5-18) | .014 |
| Time from symptom onset to neurological care, min | 210 (130-585) | 148 (112.5-197.5) | 265 (133.8-775.5) | .056 |
| Wake-up stroke/uncertain onset, n (%) | 12 (34.3) | 2 (22.2) | 10 (38.5) | .45 |
| Patients attended within 24hours of onset, n (%) | 31 (88.6) | 9 (100) | 22 (84.6) | .55 |
ICA: internal carotid artery.
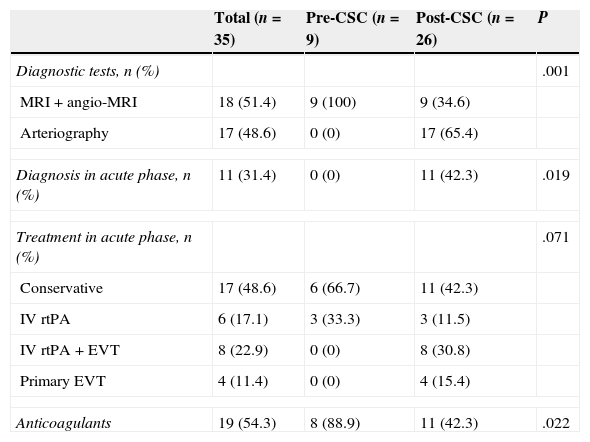
Table 2 shows the diagnostic procedures and revascularisation therapies applied broken down by subgroup. CAD confirmation diagnosis in the pre-CSC subgroup was conducted with MRI in all cases and never took place in the acute phase of stroke. In the post-CSC subgroup, however, diagnosis of CAD was confirmed with arteriography in 17 patients (65.4%), and during the acute phase in 11 (42.3%). Likewise, launching the CSC entailed changing the management of CAD-related stroke: 57.7% of patients in the post-CSC group were treated with some type of cerebral revascularisation therapy in the acute phase vs 33.3% in the pre-CSC subgroup. Furthermore, EVT was administered to 46.2% of post-CSC patients vs none of the pre-CSC patients. Anticoagulation treatment as secondary prevention after a CAD-related stroke was significantly less common after the launch of the CSC.
Variables related to diagnosis and treatment of CAD in each period.
| Total (n=35) | Pre-CSC (n=9) | Post-CSC (n=26) | P | |
|---|---|---|---|---|
| Diagnostic tests, n (%) | .001 | |||
| MRI+angio-MRI | 18 (51.4) | 9 (100) | 9 (34.6) | |
| Arteriography | 17 (48.6) | 0 (0) | 17 (65.4) | |
| Diagnosis in acute phase, n (%) | 11 (31.4) | 0 (0) | 11 (42.3) | .019 |
| Treatment in acute phase, n (%) | .071 | |||
| Conservative | 17 (48.6) | 6 (66.7) | 11 (42.3) | |
| IV rtPA | 6 (17.1) | 3 (33.3) | 3 (11.5) | |
| IV rtPA+EVT | 8 (22.9) | 0 (0) | 8 (30.8) | |
| Primary EVT | 4 (11.4) | 0 (0) | 4 (15.4) | |
| Anticoagulants | 19 (54.3) | 8 (88.9) | 11 (42.3) | .022 |
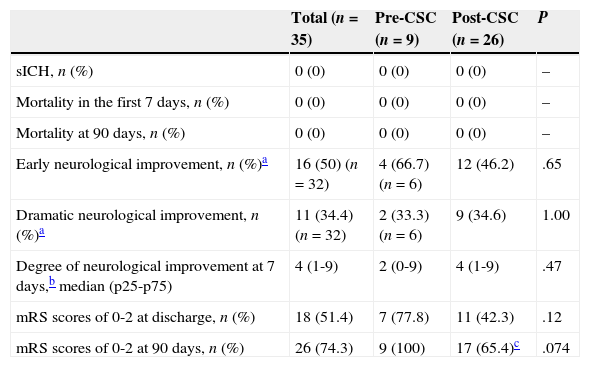
Table 3 summarises the main safety and efficacy variables in the pre-CSC and post-CSC periods. Although initial neurological severity was significantly greater in post-CSC patients, there were no significant intergroup differences regarding rate of symptomatic haemorrhagic transformation, early and late mortality, percentage of patients displaying functional independence at discharge or at 90 days, or the degree of neurological improvement at 7 days.
Safety and functional prognosis of CAD patients in each period.
| Total (n=35) | Pre-CSC (n=9) | Post-CSC (n=26) | P | |
|---|---|---|---|---|
| sICH, n (%) | 0 (0) | 0 (0) | 0 (0) | – |
| Mortality in the first 7 days, n (%) | 0 (0) | 0 (0) | 0 (0) | – |
| Mortality at 90 days, n (%) | 0 (0) | 0 (0) | 0 (0) | – |
| Early neurological improvement, n (%)a | 16 (50) (n=32) | 4 (66.7) (n=6) | 12 (46.2) | .65 |
| Dramatic neurological improvement, n (%)a | 11 (34.4) (n=32) | 2 (33.3) (n=6) | 9 (34.6) | 1.00 |
| Degree of neurological improvement at 7 days,b median (p25-p75) | 4 (1-9) | 2 (0-9) | 4 (1-9) | .47 |
| mRS scores of 0-2 at discharge, n (%) | 18 (51.4) | 7 (77.8) | 11 (42.3) | .12 |
| mRS scores of 0-2 at 90 days, n (%) | 26 (74.3) | 9 (100) | 17 (65.4)c | .074 |
sICH: symptomatic intracranial haemorrhage.
Of the 35 patients diagnosed with CAD, 17 were managed conservatively, 6 were treated with systemic thrombolysis, and EVT was indicated for 12 (as rescue treatment after systemic thrombolysis in 8 and as primary treatment in 4). Lastly, 4 patients were treated conservatively after the initial arteriography study showed either intracranial occlusion in distal branches or good compensation of intracranial circulation with no tandem occlusion. Two patients were not candidates for EVT since their occlusions were non-accessible (a catheter could not be placed in the true lumen). Clinical characteristics of patients with CAD, broken down by type of treatment, are listed in Table 4. Initial severity levels were similar in patients treated with isolated systemic thrombolysis and in patients undergoing EVT (median NIHSS score of 12 [25/75 percentile limits, 7-18] vs. 15 [11-20]; P=.28). These treatment groups showed no differences in early gains (at discharge) or in late neurological improvement (at 90 days).
Baseline characteristics and prognostic variables in the total population, broken down by treatment received.
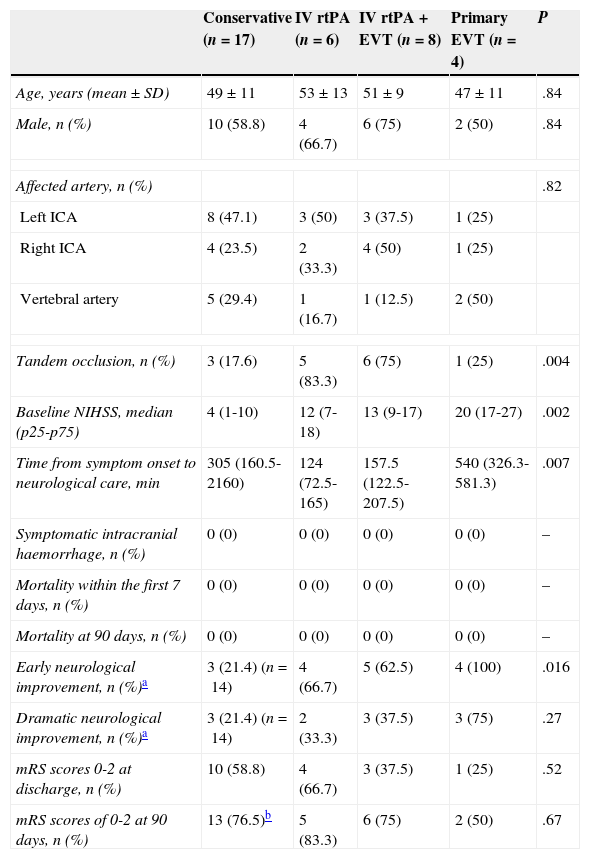
| Conservative (n=17) | IV rtPA (n=6) | IV rtPA+EVT (n=8) | Primary EVT (n=4) | P | |
|---|---|---|---|---|---|
| Age, years (mean±SD) | 49±11 | 53±13 | 51±9 | 47±11 | .84 |
| Male, n (%) | 10 (58.8) | 4 (66.7) | 6 (75) | 2 (50) | .84 |
| Affected artery, n (%) | .82 | ||||
| Left ICA | 8 (47.1) | 3 (50) | 3 (37.5) | 1 (25) | |
| Right ICA | 4 (23.5) | 2 (33.3) | 4 (50) | 1 (25) | |
| Vertebral artery | 5 (29.4) | 1 (16.7) | 1 (12.5) | 2 (50) | |
| Tandem occlusion, n (%) | 3 (17.6) | 5 (83.3) | 6 (75) | 1 (25) | .004 |
| Baseline NIHSS, median (p25-p75) | 4 (1-10) | 12 (7-18) | 13 (9-17) | 20 (17-27) | .002 |
| Time from symptom onset to neurological care, min | 305 (160.5-2160) | 124 (72.5-165) | 157.5 (122.5-207.5) | 540 (326.3-581.3) | .007 |
| Symptomatic intracranial haemorrhage, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | – |
| Mortality within the first 7 days, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | – |
| Mortality at 90 days, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | – |
| Early neurological improvement, n (%)a | 3 (21.4) (n=14) | 4 (66.7) | 5 (62.5) | 4 (100) | .016 |
| Dramatic neurological improvement, n (%)a | 3 (21.4) (n=14) | 2 (33.3) | 3 (37.5) | 3 (75) | .27 |
| mRS scores 0-2 at discharge, n (%) | 10 (58.8) | 4 (66.7) | 3 (37.5) | 1 (25) | .52 |
| mRS scores of 0-2 at 90 days, n (%) | 13 (76.5)b | 5 (83.3) | 6 (75) | 2 (50) | .67 |
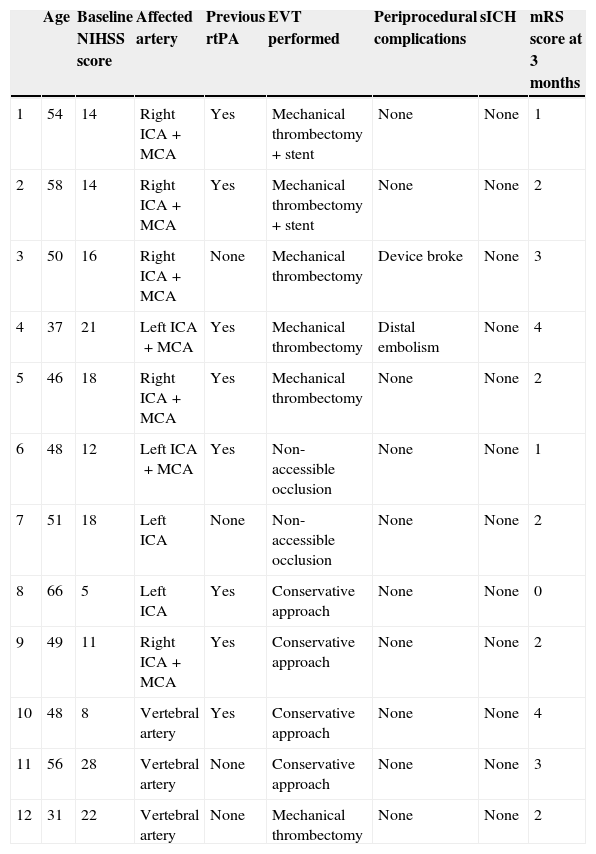
The 6 patients finally treated with EVT underwent mechanical thrombectomy of the occlusion distal to the CAD. In 2 cases, a stent was also placed in the proximal artery affected by the CAD. Two patients experienced complications associated with the procedure (distal embolism in the territory of the anterior cerebral artery and the device breaking). Neurological progress was favourable in 4 patients who achieved functional independence at 3 months. Four of the 6 patients with catheter-inaccessible occlusion or managed conservatively showed favourable progression at 90 days (Table 5).
Patients with CAD undergoing primary or rescue EVT at our CSC.
| Age | Baseline NIHSS score | Affected artery | Previous rtPA | EVT performed | Periprocedural complications | sICH | mRS score at 3 months | |
|---|---|---|---|---|---|---|---|---|
| 1 | 54 | 14 | Right ICA+MCA | Yes | Mechanical thrombectomy+stent | None | None | 1 |
| 2 | 58 | 14 | Right ICA+MCA | Yes | Mechanical thrombectomy+stent | None | None | 2 |
| 3 | 50 | 16 | Right ICA+MCA | None | Mechanical thrombectomy | Device broke | None | 3 |
| 4 | 37 | 21 | Left ICA+MCA | Yes | Mechanical thrombectomy | Distal embolism | None | 4 |
| 5 | 46 | 18 | Right ICA+MCA | Yes | Mechanical thrombectomy | None | None | 2 |
| 6 | 48 | 12 | Left ICA+MCA | Yes | Non-accessible occlusion | None | None | 1 |
| 7 | 51 | 18 | Left ICA | None | Non-accessible occlusion | None | None | 2 |
| 8 | 66 | 5 | Left ICA | Yes | Conservative approach | None | None | 0 |
| 9 | 49 | 11 | Right ICA+MCA | Yes | Conservative approach | None | None | 2 |
| 10 | 48 | 8 | Vertebral artery | Yes | Conservative approach | None | None | 4 |
| 11 | 56 | 28 | Vertebral artery | None | Conservative approach | None | None | 3 |
| 12 | 31 | 22 | Vertebral artery | None | Mechanical thrombectomy | None | None | 2 |
sICH: symptomatic intracerebral haemorrhage.
The launch of the CSC was associated with an increase in the rate of diagnosis of CAD-related ischaemic stroke. The incidence of CAD-related stroke during the post-CSC period (2.1% of all patients and 12% of patients younger than 50) is in line with reports in the literature.1,2 This finding indicates that our hospital may have underdiagnosed CAD-related stroke during the pre-CSC period. The increased number of CAD diagnoses in the post-CSC period might be explained by the integration of a 24-hour on-call interventional neuroradiology team and increased use of conventional arteriography in the acute phase with reduced use of other less sensitive diagnostic tools.3
Furthermore,16 the post-CSC period was associated with specialised care for more severe CAD-related strokes and longer times since onset. This may be explained by the unit's implementation of multimodal neuroimaging diagnostic protocols to determine suitability for revascularisation therapy in the acute phase among patients with wake-up stroke or stroke of uncertain onset.20,21
No significant differences were observed between the 2 subgroups in terms of percentage of patients with early neurological improvement, symptomatic intracranial haemorrhage, mortality rates, or functional outcome. However, post-CSC patients did show longer progression times from symptom onset and greater initial stroke severity. Implementing the CSC was associated with an increased number of revascularisation therapies conducted in the acute phase in patients with CAD-related stroke, which may have contributed to better outcomes in patients with more severe strokes.
Throughout the entire study period, patients undergoing revascularisation therapy achieved early neurological improvement more frequently than patients on conservative treatment. The small sample size, the greater baseline neurological severity, and the greater percentage of tandem occlusions in patients undergoing revascularisation therapy may have had a negative impact on other progress-related variables, with the result that no significant differences could be detected between these patients and those treated conservatively.
In our series, EVT did not yield clear clinical benefits compared to isolated intravenous thrombolysis, unlike in other studies.14 Nonetheless, we should highlight that CAD patients undergoing primary EVT in the CSC were treated more than 4.5hours after symptom onset, and therefore would have been managed conservatively in the pre-CSC period.
EVT may pose greater technical difficulties in CAD-related strokes than in strokes of other aetiologies. As a result, the occlusion could not be accessed due to technical problems in only 3.9% of patients with ischaemic stroke receiving EVT (n=231), while this occurred in 2 out of the 8 patients with CAD-related stroke (25%). The difficulty of treating patients with CAD-related stenosis or occlusion resides in correctly identifying and catheterising the true lumen. This aspect has not explicitly been addressed in the literature, although some studies do mention failure of the technique without explaining the cause.13,22 Therefore, taking into account the added technical difficulties of this procedure in patients with CAD-related stroke, it may be preferable to adopt a conservative approach when the intracranial artery is permeable or appears inaccessible and the patient's neurological severity is mild to moderate. In our series, favourable neurological outcomes were observed in 2 out of the 4 patients who were treated conservatively after a diagnostic arteriography revealed good compensation of intracranial circulation or an intracranial occlusion at a distal location.
Our study has some methodological limitations. Firstly, our imaging protocol does not include using multimodal CT in the acute phase of stroke, which is not the case in many centres in Spain. This may have contributed to failing to diagnose CAD within 24hours of stroke onset during the pre-CSC period. Secondly, the small sample size results in low statistical power and makes it more difficult to find potential differences between subgroups. And lastly, the observational study design did not permit us to draw conclusions as to whether there was a causal link between treatment received and functional prognosis, since baseline prognostic characteristics were not homogeneous in the 2 groups.
In conclusion, our findings suggest that implementing a CSC increases the number of patients diagnosed with CAD as the aetiology of ischaemic stroke and expands the range of treatment options in the acute phase. EVT is a safe and viable option in the acute phase of CAD-related stroke; however, as reported in some studies of ischaemic strokes with other aetiologies,23–25 its benefits are similar to those of systemic thrombolysis. Further controlled trials are necessary to evaluate the clinical impact of EVT on these patients.
Conflict of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Almendrote M, Millán M, Prats LA, Pérez de la Ossa N, López-Cancio E, Gomis M, et al. Impacto de un centro terciario de ictus en la atención de pacientes con ictus isquémico agudo por disección de arterias cervicales. Neurología. 2015;30:331–338.
This study was presented orally at the 64th Annual Meeting of the Spanish Society of Neurology, held in Barcelona in November 2012.