SARS-CoV-2 was first detected in December 2019 in the Chinese city of Wuhan and has since spread across the world. At present, the virus has infected over 1.7 million people and caused over 100000 deaths worldwide. Research is currently focused on understanding the acute infection and developing effective treatment strategies. In view of the magnitude of the epidemic, we conducted a speculative review of possible medium- and long-term neurological consequences of SARS-CoV-2 infection, with particular emphasis on neurodegenerative and neuropsychiatric diseases of neuroinflammatory origin, based on the available evidence on neurological symptoms of acute SARS-CoV-2 infection.
DevelopmentWe systematically reviewed the available evidence about the pathogenic mechanisms of SARS-CoV-2 infection, the immediate and lasting effects of the cytokine storm on the central nervous system, and the consequences of neuroinflammation for the central nervous system.
ConclusionsSARS-CoV-2 is a neuroinvasive virus capable of triggering a cytokine storm, with persistent effects in specific populations. Although our hypothesis is highly speculative, the impact of SARS-CoV-2 infection on the onset and progression of neurodegenerative and neuropsychiatric diseases of neuroinflammatory origin should be regarded as the potential cause of a delayed pandemic that may have a major public health impact in the medium to long term. Cognitive and neuropsychological function should be closely monitored in COVID-19 survivors.
La infección por el coronavirus SARS-CoV-2 originada en diciembre de 2019 en la región china de Wuhan ha adquirido proporciones pandémicas. A día de hoy ha ocasionado más de 1,7 millones de contagios y más de 100.000 muertes en todo el mundo. La investigación científica actual se centra en el mejor conocimiento de la infección aguda y de sus estrategias terapéuticas. Dada la magnitud de la epidemia, planteamos una revisión especulativa sobre las posibles consecuencias en patología neurológica a medio/largo plazo, con especial atención a enfermedades neurodegenerativas y neuropsiquiátricas con base neuroinflamatoria, teniendo en cuenta la evidencia directa de afectación neurológica a causa de la infección aguda.
DesarrolloRevisamos de forma sistemática lo conocido sobre los mecanismos patogénicos de la infección por SARS-CoV-2, la repercusión de la tormenta de citoquinas sobre el sistema nervioso central y su persistencia en el tiempo y las consecuencias que la neuroinflamación puede tener sobre el sistema nervioso central.
ConclusionesEl SARS-CoV-2 es un virus neuroinvasivo capaz de provocar una tormenta de citoquinas que podría convertirse en persistente en población seleccionada. Aunque nuestra hipótesis tiene alto componente especulativo, la repercusión que esta situación puede tener en la puesta en marcha y progresión de enfermedades neurodegenerativas y neuropsiquiátricas con base neuroinflamatoria debe ser considerada como posible germen de una pandemia demorada que podría tener un gran impacto en salud pública a medio o largo plazo. Se hace necesario un estrecho seguimiento de la salud cognitiva y neuropsiquiátrica de los pacientes supervivientes a infección COVID-19.
In December 2019, a new coronavirus was detected in the Chinese city of Wuhan in association with a life-threatening severe acute respiratory syndrome (SARS).1–3 The World Health Organization (WHO) named this novel virus SARS-CoV-2 due to its similarities with SARS-CoV in terms of virology and clinical expression. The latter virus caused a syndrome of similar characteristics and also originated in Chinese animal markets, in 2003.
SARS-CoV-2 rapidly spread across the world; as a result, the WHO declared the associated disease a pandemic on 11 March 2020. At the time of writing this article, SARS-CoV-2 has infected over 1.7 million people in more than 200 countries and has caused over 100000 deaths; however, these figures are probably underestimated.4 According to the WHO, the official name of the disease caused by SARS-CoV-2 is coronavirus disease (COVID-19).
Human coronaviruses whose natural reservoir or source of transmission is wild animals (e.g., bats) can be classified as low-pathogenic (HCoV-229E, HCoV-NL63, HCoV-OC43, and HCoV-HKU1) or highly pathogenic. The latter category includes SARS-CoV, which caused the 2003 outbreak; MERS-CoV, which caused the 2012 outbreak of Middle East respiratory syndrome (MERS); and the recently described SARS-CoV-2. The last 3 viruses are categorised as ßCoV.
ßCoV have become a major public health problem due to their high pathogenicity and infectivity. During the 2002–2003 outbreak, SARS-CoV infected approximately 8400 people, with a mortality rate of 9.6%.5 MERS-CoV infected a total of 1936 people and caused 690 deaths, which corresponds to a mortality rate of 36%.6
SARS-CoV-2 infection can be asymptomatic, but certain risk groups may develop severe, highly lethal syndromes characterised by severe respiratory involvement; bilateral atypical pneumonia associated with severe respiratory dysfunction secondary to diffuse alveolar damage constitutes the pathological hallmark in these cases. SARS-CoV-2 can also infect other organs and cell types over the course of the disease, including intestinal mucosa cells, renal tubular cells, lymphatic cells, reticuloendothelial cells, and nervous system cells.7
The virus’ capacity to infect the nervous system is the subject of the present review. We address the hypothesis that SARS-CoV-2 infection may cause neurological symptoms in the medium term and analyse its potential impact on neurodegenerative processes.
Stages of the clinical expression of CoV infectionThe clinical expression of SARS-CoV infection, which has served as a model for understanding SARS-CoV-2 infection due to the similar infectivity mechanisms (e.g., angiotensin-converting enzyme 2 [ACE2]), comprises 3 stages. The initial stage is characterised by high viral replication, with fever, cough, and general discomfort of several days’ duration. The second stage is associated with high fever, hypoxaemia, and progression of respiratory symptoms to bilateral pneumonia, although laboratory analyses reveal lower rates of viral replication toward the end of this stage.8 In the third stage, approximately 20% of patients develop SARS, which is frequently fatal.9,10 As viral replication progressively decreases during the final stage, the pathogenesis of SARS is thought to be explained by an exacerbated inflammatory response in the host (the so-called “cytokine storm”), which causes diffuse alveolar damage and severe hypoxaemia, promoting the development of fatal secondary sepsis.
To understand the inflammatory response to CoV infection, we must first understand the human immune response to viral infection. Due to the very recent appearance of SARS-CoV-2, there is a total lack of immunological memory of this antigen in the community: the human immune system had never previously encountered this type of coronavirus. In these situations, the innate immune system is the first line of defence. In fact, it is an excessive, dysregulated innate immune response that may explain the severity of the associated symptoms.11,12
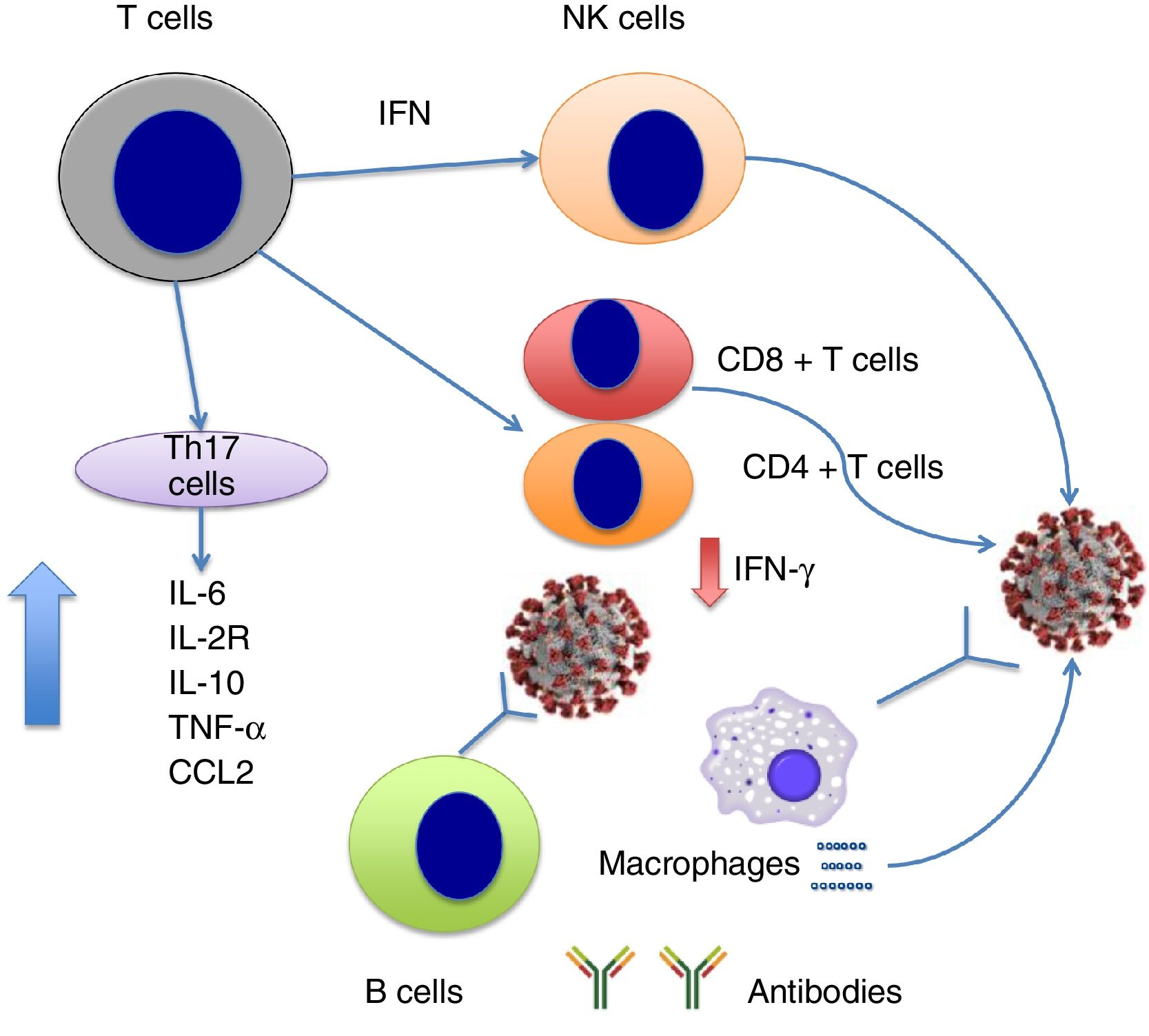
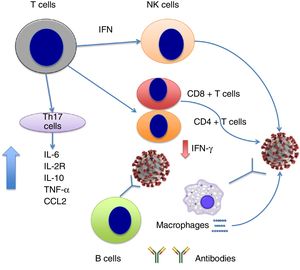
SARS-CoV invades such haematopoietic stem cells as dendritic cells, monocytes, and macrophages, inducing low-level expression of antiviral cytokines (eg, IFN-aß) and upregulation of pro-inflammatory cytokines (TNF, IL-6) and inflammatory chemokines (CCL3, CCL5, CCL2, CXCL10).13 In contrast, patients with SARS-CoV infection and SARS show extremely low levels of anti-inflammatory cytokines such as IL-1014 and high levels of IFN-a and IFN-? and inflammatory chemokines CXCL10 and CCL2 compared to patients with pneumonia, which suggests that IFN plays a role in the immunopathogenesis of SARS in humans.14
At the time of writing, few published studies have addressed the dynamics of the cytokine storm in the context of SARS-CoV-2 infection. Very recently, Chen et al.15 reported that severe SARS-CoV-2 infection is associated with lymphocytopaenia (with low levels of CD4+ and CD8+ T cells but normal B cell levels); increased levels of cytokines IL-6, IL-2R (soluble IL-2 receptor), IL-10, TNF-a, and CCL2; and reduced expression of IFN-? in CD4+ T cells. IL-6, IL-2R, IL-10, and TNF-a levels are slightly elevated or remain within the normal range in patients with moderate symptoms of COVID-19, but are high in severe cases (Fig. 1). The researchers suggest that the cytokine storm is associated with severe COVID-19, similarly to the observations reported for SARS-CoV infection.
Persistence of the neuroinflammatory responseAn important question is whether the neuroinflammatory response is a short-term occurrence, or rather it persists under certain circumstances. Immune dysregulation may persist after infection in the form of persistent inflammation, immunosuppression, and catabolism syndrome (PICS). This immune state is thought to be triggered by a cytokine storm during acute infection, such as COVID-19, and results from continuous release of endogenous alarmins or danger-associated molecular patterns (DAMP) from damaged organs. This leads to chronic systemic inflammation and a shift from production of bone marrow stem cells to myeloid cells, which promotes chronic anaemia and lymphocytopaenia.16
For obvious reasons, conclusive data on the possibility of persistent inflammation in COVID-19 survivors are not available; however, this hypothesis is plausible considering that PICS is associated with severity of acute infection.
The cytokine storm and the nervous systemThe cytokine storm has devastating effects on the respiratory system, and is largely responsible for the fatal outcomes of the disease. From a neurological viewpoint, however, it would be interesting to determine whether the cytokine storm occurring during the final stage of the disease may cause acute or subacute central nervous system (CNS) involvement. Poyiadji et al.17 recently reported the case of a woman with COVID-19 who, after several days with fever, cough, and altered mental status, presented acute necrotising haemorrhagic encephalopathy; this rare condition is associated with intracranial cytokine storm and blood-brain barrier disruption, but without direct viral invasion.
The available evidence suggests that chronic neuroinflammation associated with high levels of cytokines/chemokines is involved in the pathogenesis of such neurodegenerative diseases as multiple sclerosis (MS), Parkinson's disease, Alzheimer disease, Huntington disease, and amyotrophic lateral sclerosis.18 The immune mechanisms that trigger the cytokine storm typical of SARS are frequently involved in the pathogenesis and progression of a wide range of neurodegenerative diseases.
In Alzheimer disease, for example, the presence of proinflammatory cytokines (mainly IL-1 and IL-6) inhibits beta amyloid (Aß) phagocytosis by microglial cells, which leads to pathogenic Aß deposition.19 This observation supports the hypothesis that the persistence and accumulation of amyloid plaques in vivo may be a direct consequence of neuroinflammation. Furthermore, patients with Alzheimer disease display greater numbers of IL-1a microglial cells in cortical layers presenting amyloid plaque deposition.20
In patients with Parkinson's disease, inflammatory mediators (including TNF, IL-1ß, IL-6, and IFN-?) have been found in the CSF and in brain tissue studied post mortem.21,22 In another study, monkeys with MPTP-induced parkinsonism continued to show elevated TNF levels one year after MPTP administration, which suggests that TNF may play a role in neuronal death in toxic parkinsonism.23
Post mortem studies reveal increased expression of proinflammatory cytokines (IFN-?, TNF, IL-2, and IL-22) and molecules involved in sustained B-cell activity and lymphoid neogenesis (CXCL13, CXCL10, LTa, IL-6, and IL-10) in the meninges and CSF of patients with MS displaying high levels of meningeal inflammation. A similar proinflammatory pattern (increased levels of CXCL13, TNF, IFN-?, CXCL12, IL-6, IL-8, and IL-10) has been observed in the CSF of patients with MS showing severe grey matter damage at the time of diagnosis. These findings may support the role of neuroinflammation in the neurodegenerative phase of MS.24
Interestingly, neuroinflammation has also been found to play a major role in the pathogenesis of psychiatric diseases. Neuroinflammation can induce changes in neurotransmitter metabolism, cause hypothalamic-pituitary-adrenal axis dysregulation, activate the microglia, alter neuroplasticity, and trigger structural and functional changes in the brain that may affect cognition and behaviour. Proinflammatory cytokine dysregulation constitutes the starting point for the disruption of these systems, explaining the pathogenesis of affective disorders and such other disorders as schizophrenia, bipolar disorders, and substance abuse, especially alcohol abuse.25
The hypothesis that neuroinflammation plays a major role in the pathophysiology of acute neurological symptoms is increasingly accepted. For instance, the involvement of neuroinflammation in the pathogenesis of epileptic seizures and epileptic syndromes is widely acknowledged; in this case, it is secondary to microglial and astrocyte activation through the release of such inflammatory markers as IL-1ß, IL-6, and TNF in various brain regions, including the hippocampus and the neocortex.26
Lastly, neuroinflammation is known to play a role in the pathogenesis of a wide range of neurological diseases, including immune-mediated or postinfectious encephalitis, cerebrovascular disease, and peripheral nervous system disorders. Strikingly, however, the immune response varies with age among patients with SARS-CoV-2 infection, with older individuals displaying greater severity. The innate immune response is less efficient in older age, which makes these patients more vulnerable to infection.27 Numerous studies suggest that a proinflammatory environment promotes the development of age-related diseases, and an association has been demonstrated between immunosenescence, neuroinflammation, and neurodegeneration, as is observed in Alzheimer disease, where microglial dysfunction results in Aß accumulation and the loss of peripheral immune response.28
Given all this evidence, the nervous system seems to be a target for neuroinflammatory mechanisms. In the light of the above, special emphasis should be placed on understanding the effects of SARS-CoV-2 infection on the development and progression of neurodegenerative diseases.
We should also be aware of the possibility that in the long term, the infection may be associated with cognitive and psychiatric disorders in children and adolescents, despite its seemingly mild course in these population groups. The immunological alterations associated with SARS-CoV-2 infection may alter synaptic pruning during childhood and adolescence, causing problems that will only become apparent in adulthood.
Direct neuroinvasive potential of SARS-CoV-2The question of whether SARS-CoV-2 is neuroinvasive is an important line of research. We are beginning to see published evidence on the subject.
The molecular mechanisms underlying cell invasion by SARS-CoV-2 are associated with the virus’ capacity to bind to ACE2 receptors, similarly to SARS-CoV. ACE2 receptors are highly expressed in epithelial cells of the respiratory and digestive systems,29–32 as well as in neurons and glial cells in the CNS, which makes the CNS a potential target for SARS-CoV-2.33 Furthermore, SARS-CoV has been shown to induce neuronal death in mice after invading the CNS via the cribriform plate of the ethmoid bone and subsequently the olfactory neuroepithelium.34
SARS-CoV-2 may also enter the CNS via the haematogenous route. The virus has been detected in the general circulation. In the light of this, and given the slow flow of blood through the cerebral microcirculation, the virus may interact with ACE2 receptors expressed in the capillary endothelium.30 Interestingly, this mechanism may also be involved in the endothelial damage and subsequent cerebral bleeding observed in patients with acute COVID-19.
Furthermore, preliminary evidence suggests that the neuroinvasive potential of SARS-CoV-2 may have prognostic implications even in the acute phase. It has been hypothesised that death due to severe respiratory involvement is at least partially mediated by central hypoventilation syndrome secondary to invasion of the CNS.35
A retrospective review of 214 consecutive patients with COVID-19 from Wuhan reports neurological symptoms in 36.4% of the sample; most of the patients with neurological manifestations had severe COVID-19, and the most frequently reported neurological symptoms were impaired consciousness, acute stroke (both ischaemic and haemorrhagic), and skeletal muscle damage.36
Some patients with SARS-CoV-2 infection have subsequently displayed evidence of CNS invasion. Using genome sequencing, researchers at Beijing Ditan Hospital detected SARS-CoV-2 in the CSF of a patient with COVID-19 and clinical encephalitis.37
It is yet to be determined whether SARS-CoV-2 remains in the CNS in the medium or long term. However, viral latency in the CNS has been described for other viruses, including some coronaviruses. For instance, HCoV-OC43 was detected in the CNS over a year after inoculation in a murine model of coronavirus encephalitis.38
The question of how the virus is able to persist in the CNS is subject to debate. It has been suggested that the cytolytic and inflammatory strategies that are effective in controlling viral infection of other organs cannot be employed by the immune system in the brain due to their potentially devastating consequences. As a result, a different type of immune response is induced in the CNS, favouring viral latency in the CNS and reactivation in favourable situations.39
We cannot rule out the possibility that SARS-CoV-2 may remain latent in the CNS. If it does, we may observe delayed-onset neurological symptoms due to viral reactivation in the medium or long term, which may result in reactivation of the series of neuroinflammatory processes associated with neurodegenerative disease.
Indirect effects of SARS-CoV-2 on the CNSSARS-CoV-2 infection may have an indirect effect on the CNS, and specifically on the development of neurodegenerative diseases. In recent years, a strong association has been described between the gut microbiota, neuroinflammation, and CNS diseases. SARS-CoV-2 is known to infect intestinal mucosa cells, triggering inflammation and intestinal dysbiosis, potentially resulting in short- and long-term alterations in the gut microbiota that may contribute to neuroinflammation, leading to neurodegeneration and the development of neurodegenerative diseases.40
ConclusionsThe COVID-19 pandemic represents the greatest public health problem in recent history, not only due to its high spreadability and lethality but also because of its potential health consequences in the medium and long term.
There is evidence that the nervous system may not escape infection, and could become a major focus in the future.
A recent Spanish-language review on the topic focused on conditions directly associated with SARS-CoV-2 infection.41 Our study provides complementary data, addressing the incidence of other neurological diseases of neuroinflammatory origin.
SARS-CoV-2 infection triggers a cytokine storm whose effects on the CNS may have unpredictable consequences in both the short and the long term. Numerous neurological diseases are known to have a neuroinflammatory component involving the same factors that are stimulated during the final stage of COVID-19. A matter of particular interest is the impact that these molecular mechanisms may have on the development and progression of such neurodegenerative diseases as Alzheimer disease, Parkinson's disease, or the neurodegenerative phase of MS, as well as on psychiatric disorders, especially affective disorders, whose pathogenesis has recently been found to involve neuroinflammatory mechanisms. Surviving patients with severe COVID-19 should undergo close cognitive and psychiatric follow-up; this is particularly important considering that neuroinflammatory dysregulation may persist in severe cases, leading to PICS.
Furthermore, SARS-CoV-2 has a direct neuroinvasive potential. Previous experience with SARS-CoV may help us to understand the mechanisms by which SARS-CoV-2 may infect the CNS. Special emphasis should be placed on understanding the virus’ behaviour in the CNS. If SARS-CoV-2 remains latent in the CNS, as occurs with other coronaviruses, we cannot rule out the persistence or exacerbation of the inflammatory cascade, which may promote the development and progression of neurodegenerative diseases.
We are aware that this review is highly speculative. However, should the hypotheses presented here prove to be true, COVID-19 may lead to a neurological pandemic in the medium term: CNS infection may accelerate the onset of neurodegenerative diseases in genetically or environmentally predisposed individuals and cause direct effects on the CNS as a result of the neuroinflammatory response.
We must not consider the pandemic to be irrelevant to the field of neurology. On the contrary, COVID-19 will have a direct impact on our specialty in the near future, affecting both clinical and research work.
Neuro-RECA has developed a prospective multicentre research project aimed at gathering cognitive, psychiatric, virological, neurophysiological, and quality of life data from patients surviving severe COVID-19 during the first year after hospital discharge. This project endeavours to answer some of the questions posed in this article.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Neuro-RECA is a research network for clinical and translational neurology funded by the Andalusian regional government's Department of Health and Family Services (financing programme for biomedical and health science research, development, and innovation; project no. RIC-0111-2019). It includes over 90 researchers from 18 neurology healthcare and clinical research centres and preclinical neuroscience research groups from the region of Andalusia. Neuro-RECA is supported by the Andalusian Molecular Biology and Regenerative Medicine Centre (CABIMER), the Andalusian Network for the Design and Translation of Advanced Therapies, the Spanish Society of Family and Community Medicine, and the Spanish Society of Primary Care Physicians.
More information on Neuro-RECA is available in Appendix A.
Please cite this article as: Serrano-Castro PJ, Estivill-Torrús G, Cabezudo-García P, Reyes-Bueno JA, Ciano Petersen N, Aguilar-Castillo MJ, et al. Influencia de la infección SARS-CoV-2 sobre enfermedades neurodegenerativas y neuropsiquiátricas: ¿una pandemia demorada? Neurología. 2020;35:245–251.