Though uncommon, ischaemic stroke due to temporal arteritis carries serious difficulties for diagnosis and subsequent management and requires a high level of suspicion.
MethodsWe analysed a series of 6 patients with biopsy-proven temporal arteritis presenting with ischaemic stroke. We discuss clinical characteristics, difficulties of assessment, short- and long-term progression, treatment, and the usefulness of new diagnostic techniques.
ResultsOur sample of 6 patients had a mean age of 68.3 years; 50% were women. The majority of patients showed systemic symptoms. Anterior and posterior circulation were affected similarly. MRI angiography, Doppler sonography, and PET-CT proved to be very useful for diagnosis and treatment. Mean follow-up time was 26 months. Clinical outcomes were far from good: 33% scored ≥ 3 on the modified Rankin scale, including one death. Two patients had recurrent stroke despite treatment with full doses of corticosteroids, and 2 underwent angioplasty.
ConclusionsStroke caused by giant cell arteritis is a serious and potentially severe condition which requires a high level of suspicion and early treatment with corticosteroids. New diagnostic techniques contribute to refining patient assessment and identifying the optimal treatment. Endovascular treatment may be a valid therapeutic option in selected patients.
Aunque la asociación de arteritis de la temporal e ictus isquémico no es frecuente, su diagnóstico y su manejo posterior plantean serias dificultades, requiriendo un alto índice de sospecha.
MétodosAnalizamos una serie de 6 pacientes con arteritis de la temporal confirmada por biopsia con ictus isquémico como forma de presentación. Discutimos las características clínicas y las dificultades en su valoración, su evolución a corto y largo plazo, el tratamiento realizado y la utilidad de las nuevas pruebas diagnósticas.
ResultadosNuestra serie de 6 pacientes tenía una edad media de 68,3 años y el 50% eran mujeres. La mayoría de los pacientes asociaba síntomas sistémicos. No hubo diferencias en la afectación de territorio anterior y posterior. La RM y angio, el Doppler y la PET-TAC fueron muy útiles en el diagnóstico y la orientación terapéutica de los pacientes. El seguimiento medio fue de 26 meses y la evolución distó de ser benigna: el 33% quedó con Rankin ≥ 3, falleciendo un paciente. Además, 2 pacientes, tratados con corticoides a dosis plena, tuvieron nuevos ictus. Dos pacientes fueron tratados con angioplastia.
ConclusionesEl ictus provocado por la arteritis de células gigantes es una enfermedad potencialmente grave, que requiere una alta sospecha diagnóstica y un tratamiento corticoideo temprano. Los nuevos métodos diagnósticos ayudan a refinar la correcta evaluación de los pacientes y a determinar el tratamiento óptimo. El tratamiento endovascular puede ser una opción terapéutica válida en casos seleccionados.
Giant cell arteritis (GCA), a granulomatous vasculitis affecting medium- and large-calibre blood vessels, is the most common form of vasculitis in adults.1 The condition most frequently involves branches of the external carotid artery; failure to provide early treatment results in vision loss.2
Stroke is a potentially severe complication, though it is rarely attributed to GCA; the prevalence of GCA in a register of over 4000 patients with stroke was 0.15%.3 In a series of 2000 consecutive stroke patients from Spanish hospitals, temporal arteritis was an infrequent diagnosis among ischaemic stroke of unusual cause (5.7% of cases, 4 of 70 patients).4
In another study including 287 cases of biopsy-proven temporal arteritis, only 3% of patients experienced a stroke within 4 weeks of diagnosis.5 However, stroke significantly worsens prognosis, as it is responsible for 10% of all deaths due to biopsy-proven GCA.6
Temporal arteritis continues to be a diagnostic challenge. Early diagnosis is essential: the condition may cause irreversible vision loss if corticosteroid therapy is not administered early. GCA also constitutes a challenge to vascular neurologists: the condition requires early diagnosis and treatment given that strokes associated with temporal arteritis may have a fatal outcome.7
Temporal artery biopsy continues to be the gold standard for diagnosing GCA; however, results may be negative given that pathological changes are not consistently present throughout the artery. In addition to tests for acute-phase reactants, new diagnostic tools have been developed in recent years to support clinical suspicion. While they are non-specific, these tools are of great assistance in diagnosis, and include Doppler ultrasound (which reveals the halo sign, useful both for diagnosis and for selecting the vessel for a biopsy),8 MRI and MRI angiography,9 and PET-CT imaging.10
We analyse a series of 6 patients with biopsy-proven temporal arteritis presenting as ischaemic stroke. We describe the characteristics of these patients and address difficulties in assessment, and the progression and treatment of the condition, as well as the usefulness of new diagnostic tools.
Patients and methodsWe present 6 cases of ischaemic stroke secondary to biopsy-proven temporal arteritis, recorded between July 2009 and July 2015. Mean age was 68.3 years; 50% were women. All patients underwent MRI angiography and Doppler ultrasound, 4 patients also underwent PET-CT. Patient characteristics are summarised in Table 1.
Patient characteristics.
| Age (years) | Sex | Headache | ESR>50mm/h | CRP>8mg/L | Halo sign | CA involvement | VA involvement | AGP | PET-CT | Follow-up (months) | 3m mRS | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | 59 | W | No | Yes | Yes | No | Yes | Yes | No | Yes (+) | 31 | 0 |
| Patient 2 | 72 | W | Yes | Yes | – | Yes | Yes | Yes | Yes | Yes (−) | 16 | 2 |
| Patient 3 | 69 | M | Yes | No | Yes | Yes | Yes | Yes | None | None | 71 | 1 |
| Patient 4 | 73 | W | Yes | Yes | Yes | Yes | Yes | No | No | No | 3 | 6 |
| Patient 5 | 64 | M | No | Yes | Yes | Yes | No | Yes | No | Yes (+) | 19 | 1 |
| Patient 6 | 73 | M | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes (−) | 17 | 3 |
| Total | 68.3 | 50% W | 66.7% | 83.3% | 100% | 83.3% | 83.3% | 83.3% | 33.3% | 50% | 26.2 |
3m mRS: modified Rankin scale score at 3 months; AGP: angioplasty; CA: carotid artery; CRP: C-reactive protein; PET-CT: positron emission tomography-computed tomography; ESR: erythrocyte sedimentation rate; M: man; VA: vertebrobasilar artery; W: woman.
Woman, aged 59 years, with history of dyslipidaemia and arthrosis, and a premorbid mRS score of 0. She visited the emergency department due to recurrent, self-limited episodes of right upper limb weakness lasting several minutes, occurring on a nearly daily basis for several weeks. During one episode, she showed dysarthria and facial droop. She also presented diffuse myalgia and arthralgia of several months’ duration, which she attributed to arthrosis. She reported no headache, vision loss, or jaw claudication. The examination at the emergency department detected distal weakness in the right arm.
A blood analysis revealed a CRP level of 77.8mg/L, ESR of 97mm/h, and a haemoglobin level of 10.7g/dL.
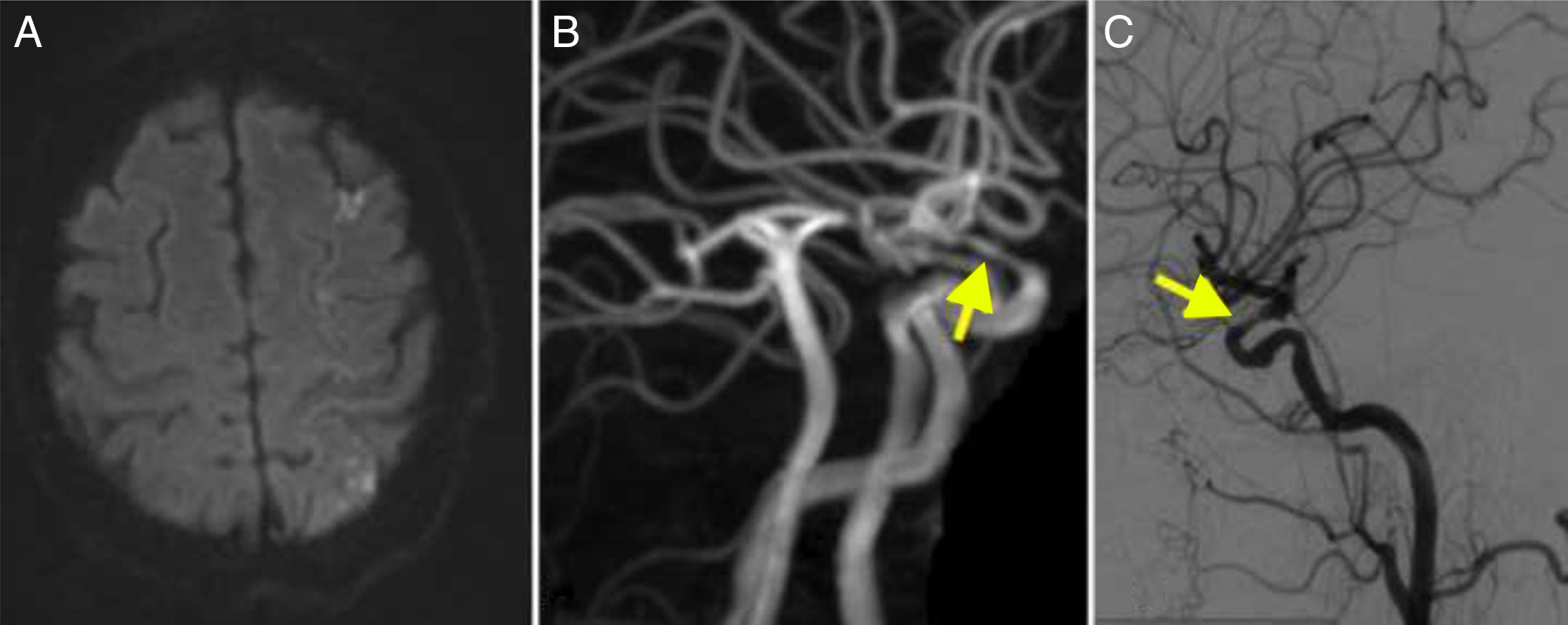
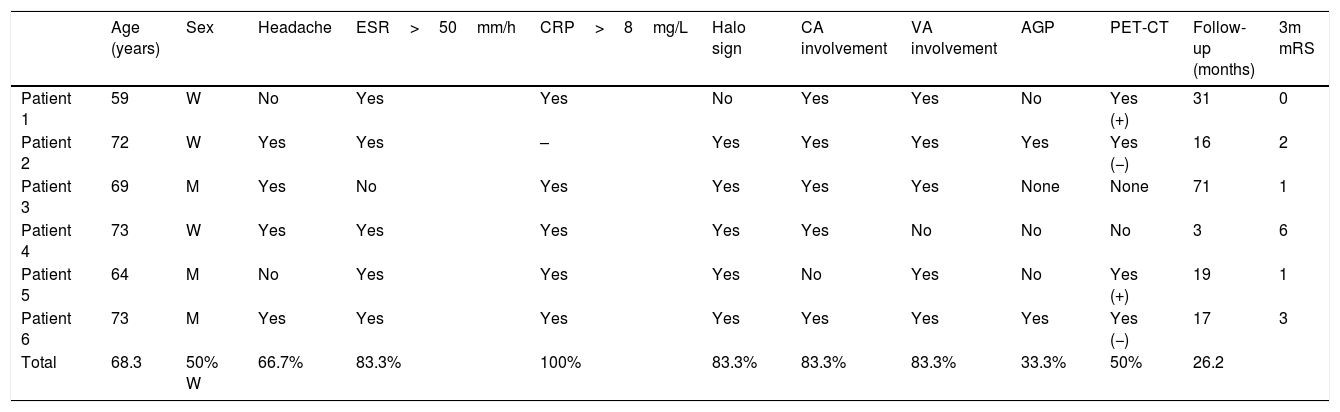
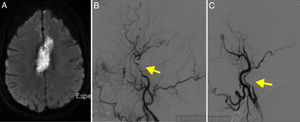
MRI angiography and digital intravenous angiography system (DIVAS) assessment confirmed vascular watershed infarction of the left middle cerebral artery and intracranial carotid and vertebral artery stenoses (Fig. 1).
(A) Diffusion-weighted imaging showed watershed infarctions in the territory of the left middle cerebral artery. (B) MRI angiography showing stenosis in the supraclinoid segment of the carotid artery (arrow). (C) Cerebral angiography confirming stenosis in the ophthalmic segment of the carotid artery (arrow).
Power Doppler ultrasound did not show the halo sign.
PET-CT revealed linear, continuous hypermetabolism in medium-size supra-aortic vessels, and linear metabolic activity in the subclavian and carotid arteries, more prominently on the left side.
In addition to acetylsalicylic acid, the patient received a corticosteroid megadose for 5 days (methylprednisolone 1g/day), followed by prednisone dosed at 60mg/day; symptoms resolved and blood alterations normalised progressively. The patient is currently asymptomatic, receiving prednisone at 2.5mg/day after 31 months of follow-up.
Temporal artery biopsy revealed signs of GCA.
Patient 2Woman, aged 72 years, admitted due to dizziness and vision loss. She had arterial hypertension and no drug habits, and had undergone surgery for morbid obesity. She had a 3-month history of pulsating headache and dizziness, followed by vision loss in the left eye. She visited another hospital for neurology consultation; a blood analysis revealed a fasting ESR of 50mm/h and a fibrinogen level of 570mg/dL. Symptoms resolved with empirical treatment with prednisone dosed at 60mg/day. Temporal artery biopsy was not performed. The patient presented dizziness on the first day after changing to 45mg prednisone; on 1 May she attended our hospital due to vision loss in the right eye accompanied by left-sided pulsatile headache.
The examination revealed right inferior quadrantanopia.
A blood analysis revealed a CRP level of 0.6mg/L and an ESR of 12mm/h.
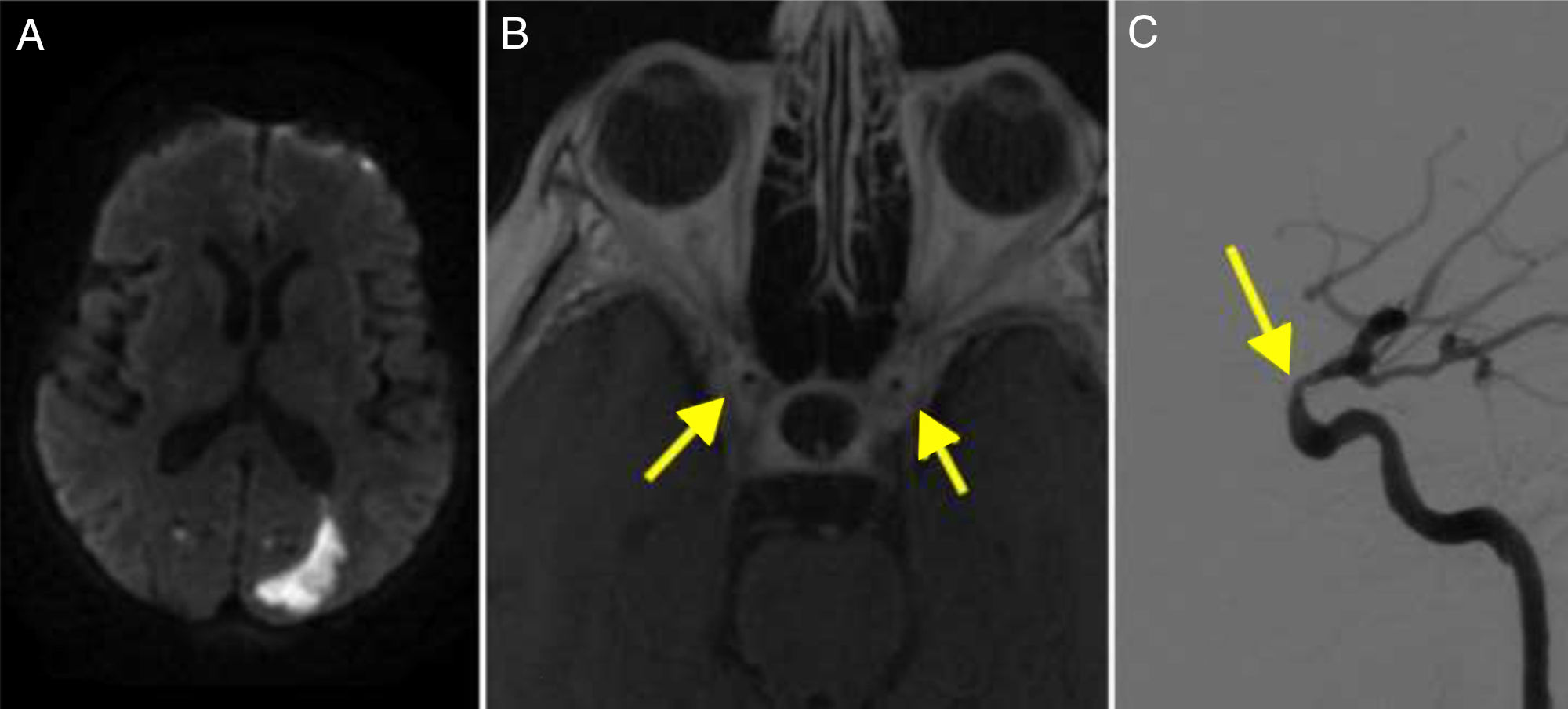
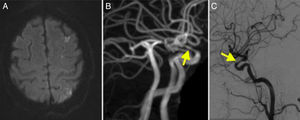
Diffusion-weighted imaging (DWI) revealed punctiform watershed infarctions bilaterally, which were more marked in the left hemisphere, and occipital infarction (Fig. 2A).
(A) Diffusion-weighted imaging revealed an infarction in the left occipital region. (B) T1-weighted MRI scan showing contrast enhancement in the walls of the internal carotid arteries (arrows). (C) Cerebral angiography confirming stenosis in the ophthalmic segment of the carotid artery (arrow).
DIVAS revealed bilateral carotid siphon stenosis, particularly on the left side (Fig. 2C), and occlusion of both vertebral arteries.
PET-CT detected no metabolic activity in the walls of large- and medium-size vessels.
Suspecting haemodynamic stroke, on 18 May we dilated the carotid-ophthalmic segment of the internal carotid artery, significantly improving stenosis.
During admission, the patient received antiplatelets, a corticosteroid megadose, and prednisone dosed at 60mg/day.
A temporal artery biopsy revealed GCA. The patient was left with vision loss. She came to a follow-up consultation at 16 months and is currently receiving prednisone dosed at 15mg/day.
Patient 3Man, aged 69 years, admitted to the neurology department due to vertebrobasilar stroke. He had type 2 diabetes mellitus, which was well controlled. He was a former smoker (3-4 packs per day over 30 years previously). For one and a half months, he had experienced vertigo, nausea, sweating, headache, and tinnitus in the right ear. He was admitted due to considerable worsening of these symptoms, with vomiting and severe instability preventing him from walking or standing. The patient reported losing 10kg of weight in the previous month, and had anorexia and asthenia. He had no history of jaw claudication, but did report joint soreness.
Examination revealed spontaneous horizontal nystagmus in all gaze positions, particularly to the left. He showed right upper limb dysmetria and dysdiadochokinesia during the finger-to-nose test.
A blood analysis revealed a CRP level of 28mg/L (normal range, 0–8) and an ESR of 21mm/h.
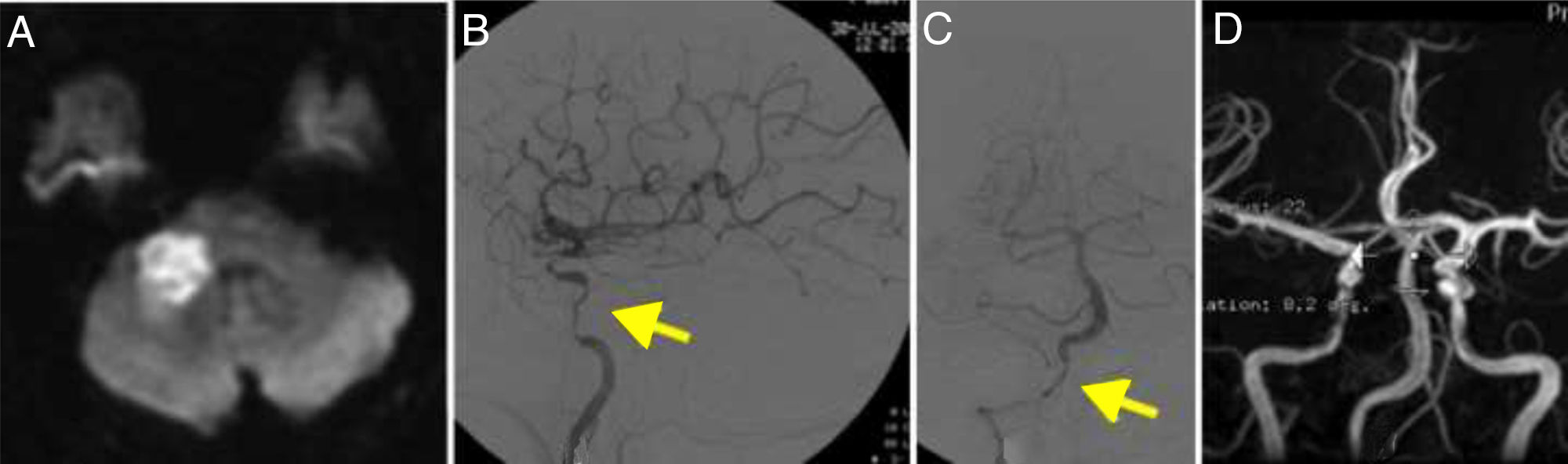
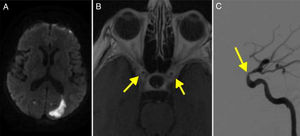
DWI detected a recent right cerebellar infarction and multiple irregularities in the vertebral arteries, with a filiform left vertebral artery; these findings were confirmed by DIVAS (Fig. 3). Multiple stenoses were also detected in the left internal carotid artery.
(A) Diffusion-weighted imaging showed an infarction in the right cerebellar peduncle. (B) Cerebral angiography of the left carotid artery revealing multiple stenoses in the internal carotid artery (arrow). (C) Angiography showing stenosis of the extracranial segments of the right vertebral artery (arrow). (D) MRI angiography performed at 2 years of follow-up, showing resolution of the stenoses.
Doppler ultrasound revealed patent temporal arteries, surrounded by an extensive hypoechoic halo, which was more marked on the right side.
The patient received full doses of corticosteroids and was followed up for 71 months; he is currently receiving 5mg prednisone on alternate days. A follow-up MRI angiography study revealed near-complete resolution of intracranial stenoses (Fig. 3).
Patient 4Woman, aged 73 years, admitted to the neurology department due to a space-occupying lesion. She had obesity, arterial hypertension, diabetes mellitus, and dyslipidaemia, and a premorbid mRS score of 0. She had a one-year history of headache and anaemia; the latter was treated with blood transfusions. The patient also reported arthralgia. Symptoms intensified one and a half months before admission, with extreme fatigue and focal weakness (resolved by the time of admission), which were attributed to heart failure. A blood transfusion improved symptoms.
The examination performed at the emergency department revealed bradypsychia, dysarthria, disinhibition, right supranuclear facial palsy, right limb claudication, right faciobrachicrural hypoaesthesia, and right extensor plantar reflex.
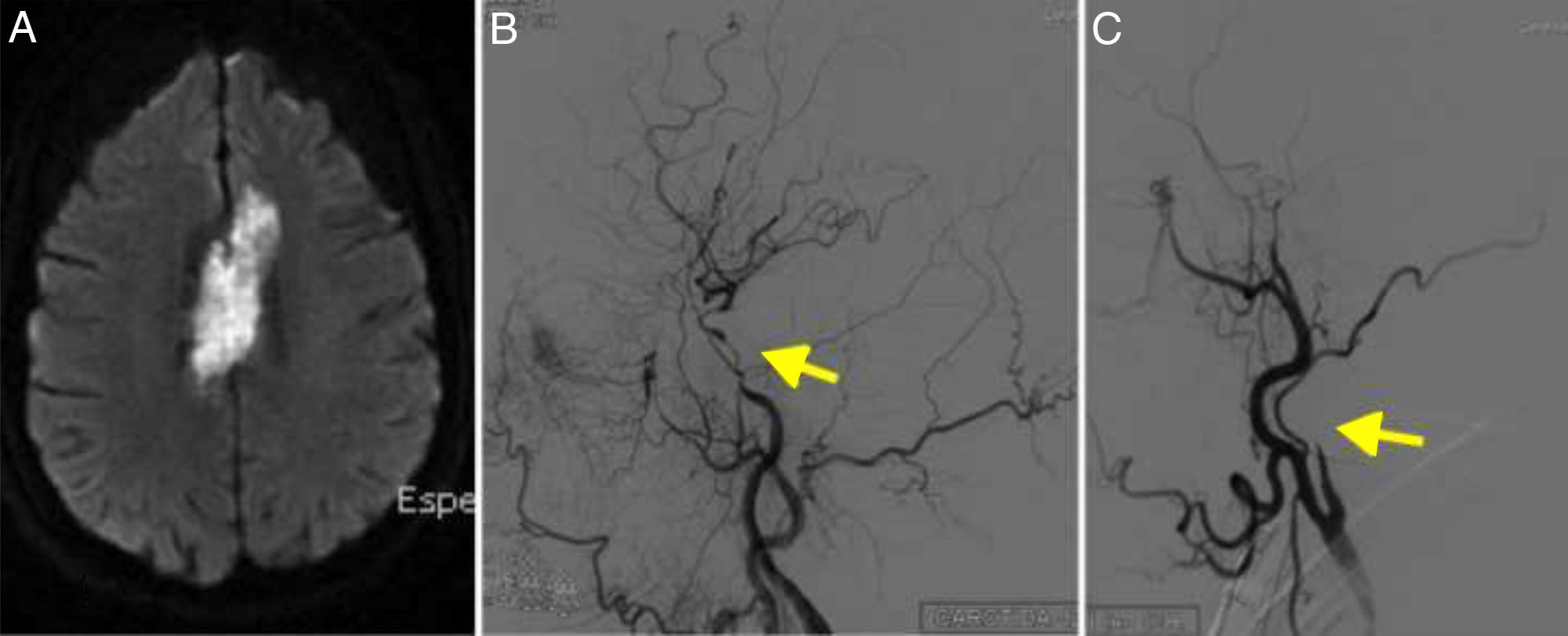
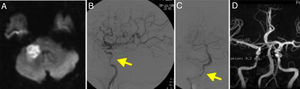
A head CT scan showed a hypodense lesion in the corpus callosum, extending towards both sides of the midline; the lesion was bright on DWI sequences (Fig. 4A). Imaging also revealed 2 small acute watershed ischaemic infarctions in the territory of the left middle cerebral artery.
(A) Diffusion-weighted imaging showed an infarction in the territory of both anterior cerebral arteries. (B) Cerebral angiography of the left carotid artery showing multiple stenoses in the internal carotid artery (arrow). (C) Angiography revealed right internal carotid artery occlusion, resembling dissection (arrow).
The patient worsened significantly during the first days after admission, showing left crural paresis and reduced verbal fluency despite anticoagulation therapy. GCA was suspected due to presence of ischaemic lesions in different vascular territories and high ESR; megadose corticosteroid therapy led to a slight clinical improvement, with greater right leg mobility and improved verbal fluency.
MRI angiography revealed complete carotid artery occlusion bilaterally and signs of internal carotid artery dissection (Fig. 4B and C); these findings were confirmed by DIVAS. No vertebrobasilar artery stenosis was observed.
The patient was discharged and transferred to a long-stay hospital with an mRS score of 4, and was on a tapering regimen of corticosteroids. She died 2 months later due to sepsis.
Patient 5Man, aged 64 years, with history of arterial hypertension, dyslipidaemia, and tobacco use. He was followed up by the rheumatology department due to polymyalgia rheumatica: 4 years before admission, he had displayed symptoms of polyarthralgia and an ESR of 80-100mm/h, which improved with oral corticosteroids. He had a premorbid mRS score of 0. The patient was receiving methylprednisolone dosed at 1mg/day. He was admitted to the stroke unit due to sudden-onset paraesthesia and numbness affecting the left side of the face; he later developed poor coordination in the left side of the body and frequent falls while walking. He improved the following day. The examination performed by the emergency department revealed left-sided facial hypoaesthesia, mild left facial involvement, and mild dysdiadochokinesia in the left arm. The patient did not present dysmetria and had normal strength. He displayed truncal ataxia, with increased base of support and inability to walk in tandem. He scored 3 on the NIHSS.
A blood analysis revealed a CRP level of 49.1mg/L and a fasting ESR of 80mm/h.
Brain MRI revealed left lateral bulbar infarction.
Brain CT angiography showed left vertebral artery occlusion.
B-mode ultrasound showed swelling of the temporal arteries. The halo sign appeared in some segments on power Doppler ultrasound.
PET-CT of the supra-aortic trunks and the aortic arch revealed intense metabolic activity in medium-size vessels, particularly in the supra-aortic vessels (subclavian and common carotid arteries bilaterally) and to a lesser extent in the iliac axes.
A biopsy of the temporal artery confirmed the diagnosis of GCA.
During hospitalisation, the patient received intravenous methylprednisolone dosed at 1g for 5 days, followed by 1mg/kg prednisone, which was subsequently reduced. At 19 months of follow-up, the patient is taking 2.5mg/day prednisone and remains asymptomatic, with an mRS score of 1.
Patient 6Man, aged 73 years, with history of arterial hypertension, diabetes mellitus, and tobacco use. He was admitted to the neurology department due to a 3-day history of gait instability, dysarthria, and poor coordination of the left limbs. He also reported a 6-month history of headache with no other symptoms. A CT scan performed at the emergency department revealed bilateral infarction in the territory of the posterior inferior cerebellar artery.
A blood analysis revealed a CRP level of 49.5mg/L and an ESR of 68mm/h.
Brain MRI confirmed the diagnosis of acute infarction in both cerebellar hemispheres and the left middle cerebellar peduncle. A vascular study revealed bilateral intracranial vertebral artery stenosis and narrowing of the cavernous segments of both internal carotid arteries.
Positive halo sign in Doppler ultrasound strongly suggested temporal arteritis despite negative results from a temporal artery biopsy; symptoms improved with full-dose corticosteroid therapy. The patient was readmitted one month later due to recurrent vertebrobasilar ischaemic stroke despite corticosteroid therapy. DWI revealed new infarcts in the cerebellum.
An additional MRI angiography study revealed distal occlusion of the V2 segment of the left vertebral artery, and right V2 occlusion. The V4 segment was markedly irregular bilaterally. CRP levels and ESR were within the normal range.
A temporal artery biopsy revealed GCA.
PET-CT showed reduced metabolic activity in the axillary vessels.
DIVAS confirmed these findings. Angioplasty reduced the left vertebral artery stenosis. At 17 months of follow-up, the patient had an mRS score of 3 and was taking prednisone dosed at 5mg/day.
DiscussionWe present 6 cases of stroke secondary to biopsy-proven temporal arteritis. Diagnosis of this entity is complex and requires a high level of suspicion since pathognomonic signs are currently only detectable with biopsy. New diagnostic tools assist in evaluating these patients and optimising treatment. Prognosis is far from benign.
Although intracranial arteries usually remain intact in GCA, 3%-6% of patients also present ischaemic stroke or vascular dementia due to involvement of the intracranial branches of the carotid and vertebral arteries.5,11–13
The causes of stroke in the context of GCA are much debated. Given the ages of affected patients and the high prevalence of vascular risk factors, stroke was initially attributed to underlying atherosclerosis. However, more recent studies report the presence of intracranial vasculitic changes, which have been found to be more severe than initially thought.9 Involvement of intradural vessels is even rarer. In a pioneering article, Wilkinson and Russell14 hypothesise that GCA may be triggered by an autoimmune reaction against arterial elastic tissue; this would explain why intradural arteries are rarely involved, as they contain practically no elastic tissue.
Arterial inflammation causes intimal thickening, lumen irregularities, stenosis, and ultimately occlusion, which causes infarction or hypoperfusion in watershed territories.15 Distal occlusion or embolisation may also occur as a result of thrombosis16,17 or artery dissection.15 Temporal arteritis, other types of inflammatory vasculitis, cortical infarcts, and watershed infarcts may also cause lacunar stroke.18
Greater involvement of the posterior territory is reported in stroke secondary to GCA.5,19 In our series, however, both territories are similarly involved: 4 patients showed involvement of both the carotid and vertebrobasilar arteries, one showed carotid artery involvement only, and the remaining one vertebrobasilar involvement only.
Imaging findings from patient 4 (Fig. 4C) were typical of carotid artery dissection. Parra et al.15 described the case of a patient with carotid arteritis and intracranial dissections. This makes it even more difficult to diagnose the condition; vasculitis should always be considered in the differential diagnosis of ischaemic stroke.
GCA requires a high level of suspicion. Systemic symptoms occurring before stroke, though frequently non-specific (headache, arthralgia, weight loss), may guide diagnosis, which should be based on alterations in inflammatory marker levels. Determining levels of these inflammatory markers is essential for diagnosis and treatment monitoring. CRP has been found to be more sensitive than the ESR; however, normal CRP levels do not rule out diagnosis, as some patients with temporal arteritis may have normal levels of inflammatory markers.20
Doppler ultrasound guides diagnosis, with the halo sign revealing temporal artery inflammation, and helps select a vessel for biopsy.8 The technique also helps assess treatment response: the halo sign disappears when temporal artery inflammation resolves.
GCA is the most frequent type of vasculitis in adults.1 Diagnosis of vasculitis has improved with MRI: intra- and extracranial artery enhancement is a frequent finding of GCA, reflecting mural inflammation.9 The diagnostic criteria for intracranial vasculitis are intramural contrast uptake and frequently artery wall thickening (Fig. 2B).21
PET-CT has recently emerged as a complementary imaging technique for diagnosing GCA.22 In our series, the technique supported diagnosis and therapeutic decision-making. Patient 5 had multiple vascular risk factors and lateral bulbar infarction secondary to vertebral artery occlusion. PET-CT revealed intense metabolic activity in the subclavian and carotid arteries, confirming the vasculitic origin of stroke.
As described in the literature, some patients (patients 2 and 6 in our series) continue to present strokes despite corticosteroid therapy.1,7,19 In these cases, disease activity should be determined before we administer other potentially harmful medications, such as immunosuppressants (whose effectiveness for the treatment of GCA has not been proven). In our case, disease activity was determined by measuring the inflammatory markers CRP and ESR, which yielded normal results. In patients 2 and 6, PET-CT results were negative at that stage of the disease, confirming the resolution of inflammation. Symptoms were mainly attributed to hypoperfusion, given that both patients showed alterations in blood flow (receiving full-dose corticosteroid therapy) that resolved with angioplasty, improving blood flow in the territory affected.23 In our series, 33% of patients received reperfusion therapy.
Patients were followed up for a mean of 26 months (Table 1). Prognosis is far from benign, particularly in cases of treatment delay; this was the case with patient 4, who died, demonstrating the fatal nature of GCA. Unfortunately, diagnosis was delayed for up to a year, which contributed to the fatal vascular onset,7 associated with multiple irreversible lesions. Patient 6 was left with severe sequelae from recurrent strokes despite early diagnosis and treatment.
Recurrence of intracranial artery stenoses has been reported after corticosteroid therapy.24,25 In our series, patient 3 had severe stenosis in the C2 segment of the carotid artery bilaterally. The vascular study confirmed the resolution of intracranial artery stenoses (Fig. 3D).
Stroke secondary to GCA is potentially fatal and requires a high level of suspicion. New imaging techniques play an essential role in diagnosis and treatment. Endovascular treatment is a valid option in some cases.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Lago A, Tembl JI, Fortea G, Morales L, Nieves C, Campins M, et al. Arteritis temporal e ictus: análisis de 6 casos. Neurología. 2020;35:75–81.