Most of the cases of ischaemic stroke in our setting are of atherothrombotic origin. Detecting intracranial and cervical carotid artery stenosis in patients with ischaemic stroke is therefore essential. Ultrasonography has become the tool of choice for diagnosing carotid artery stenosis because it is both readily accessible and reliable. However, use of this technique must be validated in each laboratory. The purpose of this study is to validate Doppler ultrasound in our laboratory as a means of detecting severe carotid artery stenosis.
Patients and methodsWe conducted an observational descriptive study to evaluate diagnostic tests. The results from transcranial and cervical carotid Doppler ultrasound scans conducted by neurologists were compared to those from carotid duplex scans performed by radiologists in patients diagnosed with stroke. Arteriography was considered the gold standard (MR angiography, CT angiography, or conventional arteriography).
ResultsOur sample included 228 patients. Transcranial and cervical carotid Doppler ultrasound showed a sensitivity of 95% and specificity of 100% for detection of carotid artery stenosis >70%, whereas carotid duplex displayed a sensitivity of 87% and a specificity of 94%. Transcranial carotid Doppler ultrasound achieved a sensitivity of 78% and a specificity of 98% for detection of intracranial stenosis.
ConclusionsDoppler ultrasound in our neurosonology laboratory was found to be a useful diagnostic tool for detecting cervical carotid artery stenosis and demonstrated superiority to carotid duplex despite the lack of B-mode. Furthermore, this technique was found to be useful for detecting intracranial stenosis.
La detección de estenosis arterial cervical e intracraneal es fundamental en el estudio del ictus isquémico, al ser el origen aterotrombótico el más prevalente en nuestro entorno. La ultrasonografía se ha convertido, por su accesibilidad y fiabilidad, en la técnica de elección para la primera aproximación al diagnóstico de esta patología, y debe ser validada en cada laboratorio en particular. El objetivo del presente trabajo es validar la técnica Doppler practicada en nuestro laboratorio para la detección de estenosis carotídea crítica.
Pacientes y métodosSe diseñó un estudio descriptivo observacional de evaluación de pruebas diagnósticas. Se compararon los resultados obtenidos sobre pacientes con diagnóstico de ictus mediante la técnica integrada Doppler carotídeo y transcraneal realizada por neurología, con los proporcionados por la técnica dúplex carotídeo, dependiente del servicio de radiología. El gold standard fue el resultado obtenido por una técnica arteriográfica (angio-RM, angio-TC o arteriografía convencional).
ResultadosN=228 pacientes. En la detección de estenosis carotídea >70%, el Doppler cervical y transcraneal tenía una sensibilidad y especificidad del 96 y el 100%, respectivamente, frente al 87 y el 94% obtenidos por el dúplex exclusivamente cervical. Para las estenosis intracraneales detectadas mediante Doppler transcraneal, esos parámetros fueron del 78 y el 98%, respectivamente.
ConclusionesEl estudio Doppler realizado en el laboratorio de neurosonología quedó validado como herramienta diagnóstica útil para la detección de estenosis carotídea cervical, siendo superior al dúplex cervical aislado, pese a la ausencia de modo B. Demostró, además, un valor adicional como técnica de detección de estenosis arterial intracraneal.
Stroke has become the second leading cause of death (the first in women) and dementia and the leading cause of disability in adults in our setting.1,2 The IBERICTUS study reports incidence rates of 118 and 29 cases per 100000 person-years for a first episode of ischaemic stroke and transient ischaemic attack, respectively. According to this study, the most frequent aetiological subtype of ischaemic stroke is atherosclerotic (35%), including cervical carotid artery stenosis (CCAS), followed by cardioembolic (20%), and small vessel occlusion (18%).3
The presence of atheromatous plaques in arteries with intracranial segments increases the risk of stroke. A European meta-analysis published in 2010 reported prevalence figures for critical CCAS of 3.1% and 0.9% in men and women older than 50, respectively.4 The annual risk of cerebrovascular events in patients with CCAS > 80% is estimated to be between 1.7% and 18%.5 Regarding the prevalence of intracranial stenosis (ICS), the AsIA study, conducted in a cohort from Barcelona (Spain), detected moderate-to-severe lesions at 3.3% in asymptomatic subjects with vascular risk factors.6
The above data demonstrate the importance of detecting atherothrombotic diseases presenting a risk to the brain, which is even more important in the case of CCAS since in this context, preventive treatment can require mechanical revascularisation techniques (endarterectomy or angioplasty/stenting).7 For this reason, examinations to diagnose extracranial carotid artery stenosis are highly significant in the management of atherothrombotic stroke. These techniques currently include ultrasound imaging (Doppler or duplex), magnetic resonance angiography (MR angiography), computed tomography angiography (CT angiography), and cerebral angiography (Ax). Due to its innocuity and greater accessibility, ultrasound has become the main approach technique, with neurosonology laboratories (NSL), distributed across Spain and directed by vascular neurologists, showing the best performance. In fact, reference tables for velocimetry values and accessory criteria for the detection and grading of stenoses have been published in the main national reference guidelines.8,9
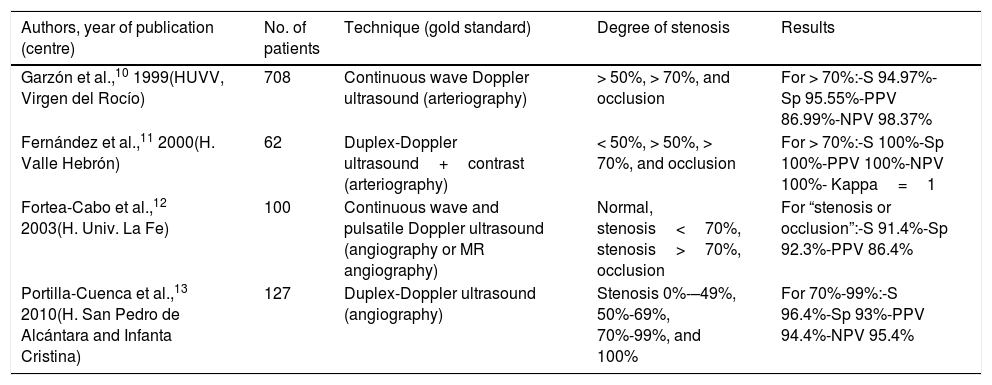
However, given variability in both the examiner and the equipment used, all authors agree on the need for every NSL to validate its aptitude in detecting and grading stenoses. In Spain, only 4 groups have published their results; all report very favourable validation results (Table 1).10–13
Summary of Spanish validation studies indexed on Medline for the detection of CCAS by ultrasound.
| Authors, year of publication (centre) | No. of patients | Technique (gold standard) | Degree of stenosis | Results |
|---|---|---|---|---|
| Garzón et al.,10 1999(HUVV, Virgen del Rocío) | 708 | Continuous wave Doppler ultrasound (arteriography) | > 50%, > 70%, and occlusion | For > 70%:-S 94.97%-Sp 95.55%-PPV 86.99%-NPV 98.37% |
| Fernández et al.,11 2000(H. Valle Hebrón) | 62 | Duplex-Doppler ultrasound+contrast (arteriography) | < 50%, > 50%, > 70%, and occlusion | For > 70%:-S 100%-Sp 100%-PPV 100%-NPV 100%- Kappa=1 |
| Fortea-Cabo et al.,12 2003(H. Univ. La Fe) | 100 | Continuous wave and pulsatile Doppler ultrasound (angiography or MR angiography) | Normal, stenosis<70%, stenosis>70%, occlusion | For “stenosis or occlusion”:-S 91.4%-Sp 92.3%-PPV 86.4% |
| Portilla-Cuenca et al.,13 2010(H. San Pedro de Alcántara and Infanta Cristina) | 127 | Duplex-Doppler ultrasound (angiography) | Stenosis 0%-–49%, 50%-69%, 70%-99%, and 100% | For 70%-99%:-S 96.4%-Sp 93%-PPV 94.4%-NPV 95.4% |
NPV: negative predictive value; PPV: positive predictive value; S: sensitivity; Sp: specificity.
The main aim of this study is to validate the diagnostic yield of integrated cervical and transcranial Doppler ultrasound (ICTD, that is, when both studies are performed at once by the same examiner in the same patient), a technique developed by our NSL in the detection of significant CCAS, and to compare it with cervical duplex ultrasound, the main technique used at our centre. As secondary objectives, our study aims to analyse whether the set of parameters used in the NSL increases the diagnostic yield of the ICTD technique, also providing reliable data from intracranial arteries. Finally, we aim to assess whether performance of a neurovascular study by a neurologist improves the diagnostic yield.
Patients and methodsWe conducted an ambispective, observational, descriptive study evaluating diagnostic tests: data gathering was retrospective from January 2009 (laboratory opening) to December 2012 and prospective from that date to July 2013. The study population included patients from the cerebrovascular disease and emergency neurological care clinics.
- –
Inclusion criteria: the sample included patients aged 18 or older who had been diagnosed with a cerebral ischaemic event in the anterior territory of the carotid artery by a neurologist.
- –
Exclusion criteria:
- 1.
Possible or probable cardioembolic aetiology
- 2.
Suspected “other determined aetiology” based on well-documented clinical history
- 3.
The neurologist performing the Doppler ultrasound study knew the results of the radiological tests before performing the Doppler ultrasound.
- 1.
We conducted a comparative analysis of the CCAS ranges estimated with our ICTD technique and with those used in everyday practice (cervical duplex ultrasound and/or angiography studies: conventional angiography, MR angiography, or CT angiography), performed and interpreted by the department of diagnostic radiology, which does not have the means to perform transcranial ultrasound. Estimations of ICS were also analysed, since in addition to their intrinsic value, they are an indispensable part of the neurosonological study as they provide indirect data on CCAS when stenosis is significant, as occurs in the study of the ophthalmic arteries. Angiography studies were considered the gold standard; when more than one angiography study was performed in the same patient, these were prioritised in the order of reliability traditionally used by our centre's radiology department: Ax>CT angiography>MR angiography.14
The different techniques were performed using the following equipment: DWL Multi-Dop-X Digital (Compumedics, Germany) for Doppler ultrasound; Siemens Acuson Antares for duplex ultrasound; Siemens Magnetom Symphony (normally with gadolinium) for MR angiography; Philips Brilliance 64 CT scanner for CT angiography; and Siemens Artis Zee Flat Panel Bi-panel system for angiography.
The Doppler studies were preferentially performed in the NSL; however, the scanner was moved to the patient's bedside when necessary. In all cases, studies were performed by a neurologist certified in neurosonology by the Spanish Society of Neurology.
Once the various studies were performed, data from the relevant reports were grouped in a data collection sheet.
The variables recorded included sex, age, place of ICTD performance, difficulties (e.g., a short and thick neck, patient agitation), acoustic window (considered “poor” when there was difficulty identifying any 2 intracranial arteries or at least one of the 2 middle cerebral arteries), delay between patient arrival and performance of the Doppler ultrasound study or between this study and the tests performed by the department of radiology in admitted patients, normality or degree of ICS according to the Doppler ultrasound study or the radiological tests performed, normality or stenosis of the intracranial arteries, and gross velocimetry values for each artery together with the corresponding systolic and diastolic indexes of cervical carotid arteries.
We conducted exploratory, descriptive, and inferential analyses, and where relevant created graphs with the SPSS statistical software for Windows, the online Centre for Evidence-Based Medicine Statistics Calculator (Canadian Institute of Health Research, Toronto), and the EPIDAT 3.1 (Programa para Análisis Epidemiológico de Datos Tabulados) software from the Regional Government of Galicia. For the analysis of qualitative variables, we created contingency tables and applied the chi-square test with continuity correction or the Fisher exact test (for 2×2 tables with little information). The t test or the Mann–Whitney U test were used to compare numerical variables, depending on whether or not data were normally distributed. We calculated sensitivity, specificity, positive and negative predictive values (PPV/NPV), and positive/negative likelihood ratios (PLR/NLR) of the different diagnostic studies. The diagnostic validity of the tests was compared by creating ROC curves for each test and calculating the corresponding areas under the curve.
ResultsThe neurosonological study was performed in 228 patients (112 between December 2012 and July 2013); 140 were men. Mean age was 66±13 years.
A total of 156 patients (68.4% of the total sample) underwent a cervical duplex ultrasound, whereas 97 (42.5%) underwent a cervical angiography study and 101 (44.3%) an intracranial angiography study.
Mean delay in the performance of the ICTD study was one day, with 3 additional days’ delay in the performance of any type of radiological study.
The transcranial window for ultrasound was considered poor in 37 cases (16%). There was difficulty performing the study in 35% of cases; the scanner was moved to the patient's bedside (outside the NSL) in 28% of the cases during the prospective period.
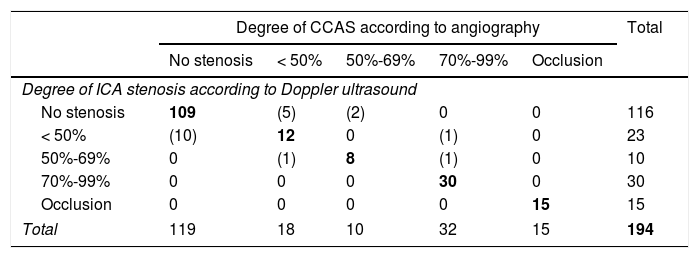
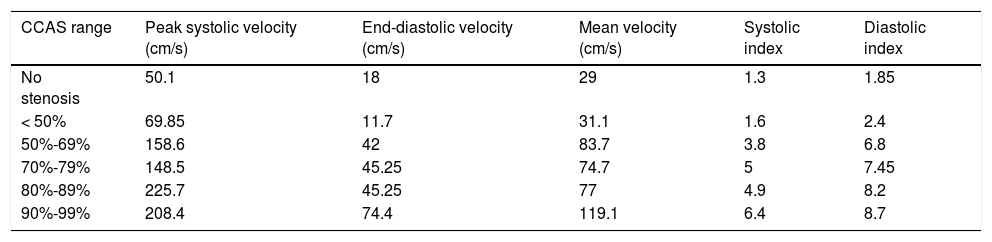
Seventy-four patients (32.5%) presented signs of CCAS in the Doppler ultrasound study. Coincidences and discrepancies with reference to the standard test are listed in Table 2, whereas mean velocimetry values and indices from our NSL are shown in Table 3. Twenty-nine patients (13%) presented evidence of ICS in the Doppler ultrasound study.
Contingency table for the classification of the degree of CCAS detected with Doppler ultrasound vs the reference test.
| Degree of CCAS according to angiography | Total | |||||
|---|---|---|---|---|---|---|
| No stenosis | < 50% | 50%-69% | 70%-99% | Occlusion | ||
| Degree of ICA stenosis according to Doppler ultrasound | ||||||
| No stenosis | 109 | (5) | (2) | 0 | 0 | 116 |
| < 50% | (10) | 12 | 0 | (1) | 0 | 23 |
| 50%-69% | 0 | (1) | 8 | (1) | 0 | 10 |
| 70%-99% | 0 | 0 | 0 | 30 | 0 | 30 |
| Occlusion | 0 | 0 | 0 | 0 | 15 | 15 |
| Total | 119 | 18 | 10 | 32 | 15 | 194 |
Coincidences in bold and discrepancies in parentheses.
Mean velocimetry values obtained in each CCAS range.
| CCAS range | Peak systolic velocity (cm/s) | End-diastolic velocity (cm/s) | Mean velocity (cm/s) | Systolic index | Diastolic index |
|---|---|---|---|---|---|
| No stenosis | 50.1 | 18 | 29 | 1.3 | 1.85 |
| < 50% | 69.85 | 11.7 | 31.1 | 1.6 | 2.4 |
| 50%-69% | 158.6 | 42 | 83.7 | 3.8 | 6.8 |
| 70%-79% | 148.5 | 45.25 | 74.7 | 5 | 7.45 |
| 80%-89% | 225.7 | 45.25 | 77 | 4.9 | 8.2 |
| 90%-99% | 208.4 | 74.4 | 119.1 | 6.4 | 8.7 |
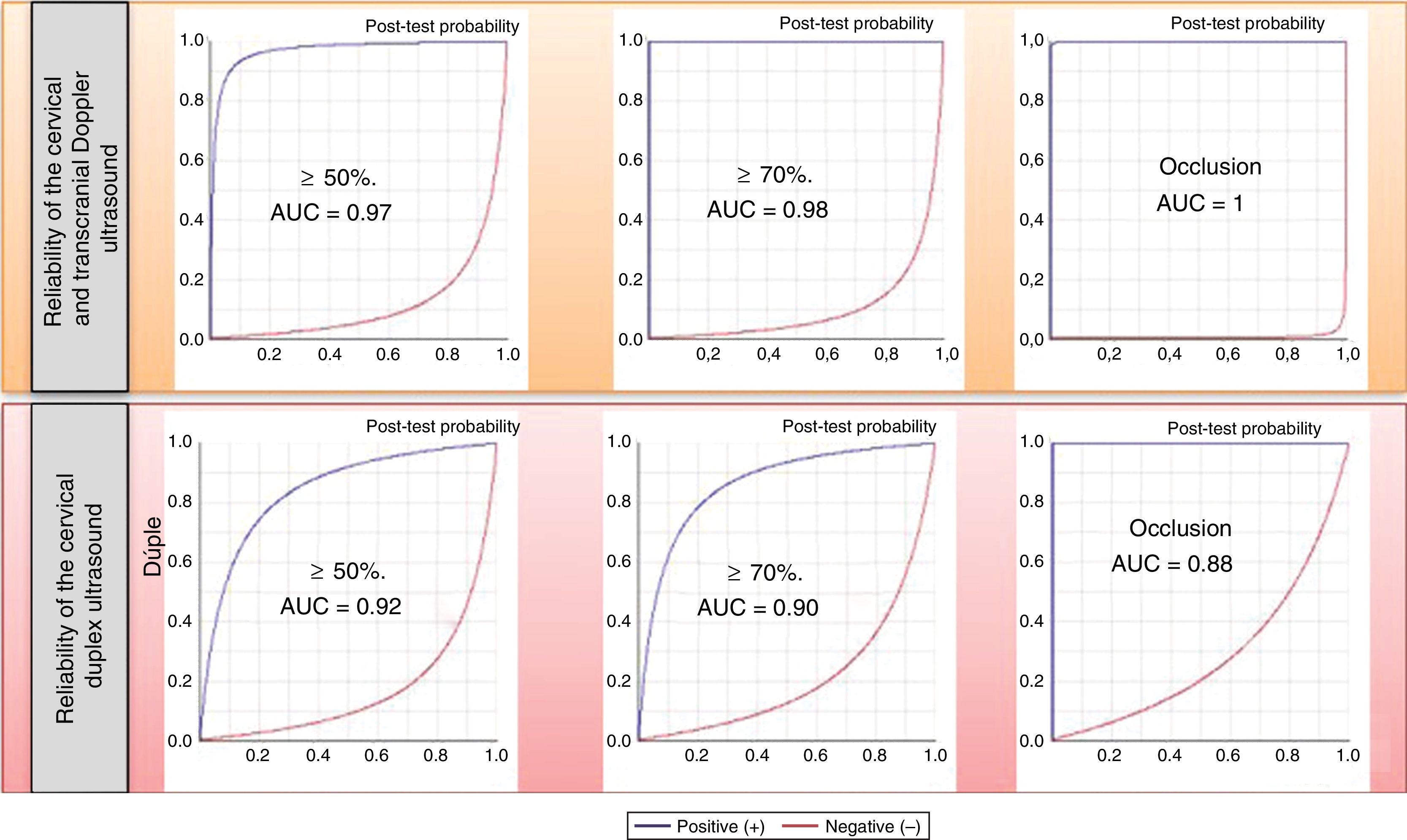
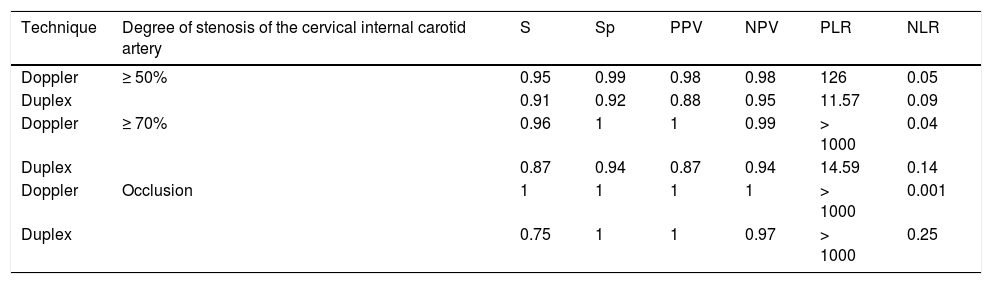
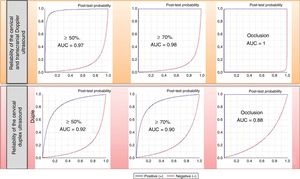
The parameters of diagnostic reliability for the detection of significant CCAS are listed in Table 4, both for ICTD and cervical duplex ultrasound. The comparative analysis was performed by calculating the area under the ROC curve (Fig. 1).
Diagnostic reliability parameters for ICTD vs cervical duplex ultrasound in the detection and grading of CCAS.
| Technique | Degree of stenosis of the cervical internal carotid artery | S | Sp | PPV | NPV | PLR | NLR |
|---|---|---|---|---|---|---|---|
| Doppler | ≥ 50% | 0.95 | 0.99 | 0.98 | 0.98 | 126 | 0.05 |
| Duplex | 0.91 | 0.92 | 0.88 | 0.95 | 11.57 | 0.09 | |
| Doppler | ≥ 70% | 0.96 | 1 | 1 | 0.99 | > 1000 | 0.04 |
| Duplex | 0.87 | 0.94 | 0.87 | 0.94 | 14.59 | 0.14 | |
| Doppler | Occlusion | 1 | 1 | 1 | 1 | > 1000 | 0.001 |
| Duplex | 0.75 | 1 | 1 | 0.97 | > 1000 | 0.25 |
NLR: negative likelihood ratio; NPV: negative predictive value; PLR: positive likelihood ratio; PPV: positive predictive value; S: sensitivity: Sp: specificity.
Reliability in the detection of ICS was lower, with combined values for all intracranial arteries yielding a sensitivity of 78%, specificity of 98%, a PPV of 60%, and a NPV of 99%. These results improved when calculated for the anterior, middle, and posterior cerebral arteries exclusively, with sensitivity of 84%, specificity of 98%, PPV of 92%, and NPV of 99%; they further improved when calculated exclusively for the middle cerebral artery, reaching values of 88%, 97%, 72%, and 99%, respectively. Results for the posterior cerebral artery were more modest (78%, 96%, 50%, and 99%); for the remaining vessels, the number of stenoses was insufficient to obtain assessable individual results.
DiscussionThe overall results obtained in our study are very similar to those obtained in other previously published Spanish studies. Our sample initially included 228 patients; in addition to the Doppler ultrasound study, 97 underwent a carotid angiography study and were therefore included in the validation. The study by Garzón et al.,10 the largest study in the Spanish literature, included 708 individuals, whereas the study by Portilla Cuenca et al.13 included 127 patients, the study by Fortea-Cabo et al.12 100 patients, and Fernández et al.11 included 62. Regarding the intracranial study, 101 of our patients underwent an intracranial angiography study, vs 100 patients in Fortea-Cabo et al.12 and 49 in Palomeras et al.15 Regarding the number of stenoses detected in the patient sample, 32.5% of our patients presented CCAS diagnosed with a Doppler ultrasound study; Garzón et al.10 report a very similar percentage (31.2%). While our study reported 13% of patients with some ICS detected with ultrasound, Fortea-Cabo et al.12 reported 14%. Lastly, the percentage of individuals considered to have a poor transcranial Doppler window was higher in our study: 16% of patients, vs the 12.1% reported by Fortea-Cabo et al.12 and the 10.2% reported by Palomeras et al.15; these differences may be explained by the different definition of the concept “poor transcranial Doppler window,” which was probably stricter in our case, as well as by the older age of the patients in our study (mean age of 66 vs 65.4 in the study by Fortea-Cabo et al.12 and 62.7 reported by Palomeras et al.15). Although the difference in age with the sample in the Fortea-Cabo et al.12 study is smaller, the effect of a better window might also be influenced by the lower percentage of women included in that study (34% vs 39%).
Analysis of all the published validation studies suggests a downward trend in the use of conventional angiography as the gold standard test from the earliest studies to the most recent, with an increase in the use of CT angiography and MR angiography, which involve lower risk. It also shows that, although Doppler ultrasound is still the subject of validation studies in ICS, it is being substituted by duplex ultrasound in CCAS.
Regarding the main result of our comparative study, we believe that the higher diagnostic capacity of ICTD vs cervical duplex ultrasound alone is probably explained by the additional information provided by the transcranial examination in the integrated study. This additional information probably also helps explain why the peak carotid velocities obtained in our study, which were slightly slower than the reference ranges established by the most widely used manuals in our setting,8,9 did not lead to a significant margin of diagnostic error. This reduction in velocities is observed not only in areas with stenosis, but also in the unaffected arteries, which demonstrates the consistency of the overall results, obtained from a determined sample with a specific ultrasound scanner and by a specific examiner. This highlights the above-mentioned need for every NSL to validate its results. Our results also support the idea that it is not appropriate to draw conclusions on the degree of stenosis of an artery based exclusively on the peak systolic velocity obtained for that artery, but also on a complete set of additional haemodynamic data when preparing a report.
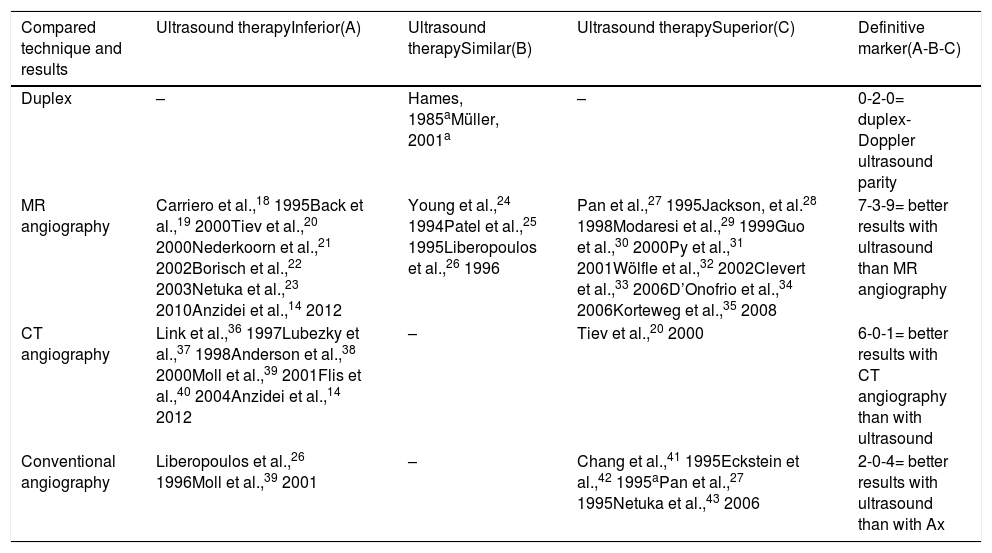
Another interesting consideration arises from analysing the results of studies comparing different diagnostic techniques in the detection of CCAS (Table 5).14,16–43 It is surprising that only 2 studies compared the duplex and Doppler ultrasound techniques, both with similar results. Also, when performed by experts, ultrasound studies might even surpass such theoretically superior techniques as MR angiography or even Ax.
Summary of studies comparing different techniques for the detection and grading of cervical carotid artery stenosis, and the resulting hierarchy.
| Compared technique and results | Ultrasound therapyInferior(A) | Ultrasound therapySimilar(B) | Ultrasound therapySuperior(C) | Definitive marker(A-B-C) |
|---|---|---|---|---|
| Duplex | – | Hames, 1985aMüller, 2001a | – | 0-2-0= duplex-Doppler ultrasound parity |
| MR angiography | Carriero et al.,18 1995Back et al.,19 2000Tiev et al.,20 2000Nederkoorn et al.,21 2002Borisch et al.,22 2003Netuka et al.,23 2010Anzidei et al.,14 2012 | Young et al.,24 1994Patel et al.,25 1995Liberopoulos et al.,26 1996 | Pan et al.,27 1995Jackson, et al.28 1998Modaresi et al.,29 1999Guo et al.,30 2000Py et al.,31 2001Wölfle et al.,32 2002Clevert et al.,33 2006D’Onofrio et al.,34 2006Korteweg et al.,35 2008 | 7-3-9= better results with ultrasound than MR angiography |
| CT angiography | Link et al.,36 1997Lubezky et al.,37 1998Anderson et al.,38 2000Moll et al.,39 2001Flis et al.,40 2004Anzidei et al.,14 2012 | – | Tiev et al.,20 2000 | 6-0-1= better results with CT angiography than with ultrasound |
| Conventional angiography | Liberopoulos et al.,26 1996Moll et al.,39 2001 | – | Chang et al.,41 1995Eckstein et al.,42 1995aPan et al.,27 1995Netuka et al.,43 2006 | 2-0-4= better results with ultrasound than with Ax |
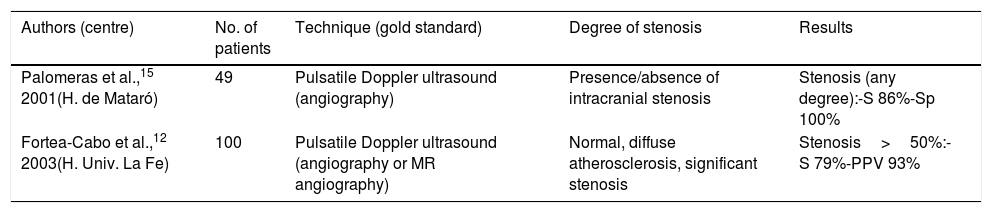
Furthermore, the validation of a transcranial study is even more interesting in the light of the fact that of all the equivalent studies indexed on PubMed, only 2 were conducted in Spain (Table 6). Design and results are more heterogeneous than those of carotid artery studies, and diagnostic reliability is lower, as we have also observed in our study. However, it should be noted that the results of our study may be biased by the fact that we included cases of stroke in the acute phase, which showed a mean delay of 3 days between transcranial Doppler ultrasound and intracranial angiography. In fact, several studies have shown that the haemodynamic behaviour of intracranial emboli may resemble that of stenoses at certain moments during early progression,44 with spontaneous resolution occurring during the first 48hours in > 70% of cases.45 Similarly, doctoral theses by Segura et al.46 and Calleja Puerta47 show that many ICSs detected in the acute phase resolve completely, sometimes very early. Therefore, the correlation between intracranial findings from Doppler ultrasound and from Ax is clearly better when the interval between the tests does not exceed 120min. We cannot then rule out that some of the false positives in our study are due to the resolution of the arterial thrombus, given the difference of 3 days between the sonological study and the gold standard test in our sample. According to Gerriets et al.,48 limiting the time interval between the ultrasound and the reference test might be one of the main explanations for the difficulty in obtaining homogeneous results in ICS validation studies.
Summary of Spanish validation studies indexed in Medline for the detection of intracranial arterial stenosis by ultrasound.
| Authors (centre) | No. of patients | Technique (gold standard) | Degree of stenosis | Results |
|---|---|---|---|---|
| Palomeras et al.,15 2001(H. de Mataró) | 49 | Pulsatile Doppler ultrasound (angiography) | Presence/absence of intracranial stenosis | Stenosis (any degree):-S 86%-Sp 100% |
| Fortea-Cabo et al.,12 2003(H. Univ. La Fe) | 100 | Pulsatile Doppler ultrasound (angiography or MR angiography) | Normal, diffuse atherosclerosis, significant stenosis | Stenosis>50%:-S 79%-PPV 93% |
PPV: positive predictive value; S: sensitivity; Sp: specificity.
Lastly, we should highlight the study of event-test latencies, which shows another benefit of the NSL: earlier diagnosis. The ICTD result was obtained after one day, whereas the first radiological study was performed 3 days later. These differences arise from the greater effort of neurosonologists, who on many occasions have to perform the study in the difficult conditions inherent to the acute phase of stroke (35%), or even outside the NLS (28%) at the patient's bedside. Despite all the difficulties that ultimately led to greater diagnostic speed, reliability was not compromised. Furthermore, this factor suggests implications for clinical management.
Regarding the limitations of our study, it should be noted that data were incomplete in many cases: for 36 patients (16%), Doppler ultrasound was the only blood flow test (including cases from our normal clinical practice, such as tourists or early deaths). Furthermore, transcranial Doppler ultrasound was the only endocranial blood flow test for 127 patients (56%); radiological test results for carotid flow were normal in 68 patients, and therefore the possibility of an ICS was ruled out (territorial omission) according to our centre's diagnostic protocol. In that Doppler group, we identified 9 cases of CCAS. Therefore, a considerable number of patients, for whatever reason, would not undergo some study, or the study would be incomplete if there is no NSL. Finally, we should mention that the Doppler ultrasound study was not repeated to assess whether intracranial recanalisation had occurred in the cases of discrepancies against the reference test; therefore, it was not possible to demonstrate the suggested overrepresentation of false positives.
In conclusion, we consider that the ICTD technique performed in the NSL is validated as a reliable diagnostic tool for the detection of haemodynamically significant CCAS, and is more effective than isolated cervical duplex ultrasound for this purpose, despite the lack of a mode B. Most of its reliability is very likely to be explained by the integration of cervical and transcranial studies. Certainly, when the ultrasound study includes intracranial haemodynamics and the findings of the complete study are interpreted by a duly qualified examiner, the diagnostic certainty of the technique increases. In Spain, only neurologists are currently trained to perform integrated extra- and intracranial ultrasound studies.
Furthermore, the NSL has shown faster diagnostic speed in our study, which results in shorter waiting times, with unquestionable clinical and economic advantages.
In this context, we believe that the results of our study point to the need to consolidate the NSL at hospitals that habitually treat patients with stroke.
FundingThis study has received no private or public funding of any kind.
Conflicts of interestNone.
To all those neurologists who selflessly train other professionals in neurosonology techniques.
Please cite this article as: de la Cruz Cosme C, Dawid Milner MS, Ojeda Burgos G, Gallardo Tur A, Márquez Martínez M, Segura T. Validación de un laboratorio básico de neurosonología para la detección de estenosis carotídea cervical. Neurología. 2019;34:367–375.
The preliminary results of this study were presented as an oral communication at the 66th Annual Meeting of the Spanish Society of Neurology.