The neurotoxic effects of cerebrospinal fluid (CSF) from patients with amyotrophic lateral sclerosis (ALS) have been reported by various authors who have attributed this neurotoxicity to the glutamate in CSF-ALS.
Materials and methodsCultures of rat embryonic cortical neurons were exposed to CSF from ALS patients during an incubation period of 24hours. Optical microscopy was used to compare cellular changes to those elicited by exposure to 100μm glutamate, and confocal microscopy was used to evaluate immunohistochemistry for caspase-3, TNFα, and peripherin.
ResultsIn the culture exposed to CSF-ALS, we observed cells with nuclear fragmentation and scarce or null structural modifications to the cytoplasmic organelles or to plasma membrane maintenance. This did not occur in the culture exposed to glutamate. The culture exposed to CSF-ALS also demonstrated increases in caspase-3, TNFα, and in peripherin co-locating with caspase-3, but not with TNFα, suggesting that TNFα may play an early role in the process of apoptosis.
ConclusionsCFS-ALS cytotoxicity is not related to glutamate. It initially affects the nucleus without altering the cytoplasmic membrane. It causes cytoplasmic apoptosis that involves an increase in caspase-3 co-located with peripherin, which is also overexpressed.
Los efectos neurotóxicos del líquido cefalorraquídeo (LCR) procedentes de pacientes con esclerosis lateral amiotrófica (ELA) han sido descritos por varios autores que han atribuido esta neurotoxicidad al efecto de glutamato del LCR-ELA.
Material y métodosSe han expuesto cultivos de neuronas embrionarias corticales de rata con un incubación de 24h y el LCR procedente de pacientes con ELA, valorando las alteraciones celulares a través de microscopía óptica en comparación con aquellas que produce con 100mM de glutamato y la inmunohistoquímica de caspasa-3, TNF y periferina a través de microscopia confocal.
ResultadosEn el cultivo expuesto a LCR-ELA se observan células con fragmentación del núcleo con escasa o nula modificación estructural de los organelos citoplasmáticos y mantenimiento de la membrana plasmática, lo que no ocurre con la exposición a glutamato. Se observa un aumento de caspasa-3 y de TNFα y un incremento de periferina que co-localiza con caspasa-3 pero no con TNFα, lo hace sugerir que puede tener un papel precoz en el desarrollo de la apoptosis.
ConclusionesLa citotoxicidad por LCR-ELA no se relaciona con el glutamato, que provoca una afectación nuclear precoz sin alteración de la membrana citoplasmática produciendo una apoptosis citoplasmática que conlleva un incremento de caspasa-3 que co-localiza con sobreexpresión anómala de periferina.
One specific trait of cerebrospinal fluid from patients with amyotrophic lateral sclerosis (CSF-ALS) is its ability to decrease cell survival in motor neuron cell cultures. This is referred to as the cytotoxic effect.1 This effect is limited to the CSF2–8 and it has been demonstrated by repeated studies.9–13 In a previous study,14 we observed this mechanism in neuronal cell cultures and found it to be glutamate-independent after exposing cultures to different antagonists. The purpose of this study is to determine which cellular changes occur in neuronal cell cultures exposed to CSF-ALS.
Materials and methodsUsing lumbar puncture, we extracted CSF from 3 patients diagnosed with ALS according to the El Escorial/Airlie House Diagnostic Criteria.15 We extracted 1.5 to 3cc and centrifuged, quantised, and stored the sample at −80° until it was exposed to the culture. We also extracted CSF from 3 controls who had undergone lumbar puncture as part of the diagnostic procedure after headache or epileptic attack. All patients and controls signed an informed consent form.
Extraction and maintenance procedures for cultures of motor neurons from embryonic rats followed the protocol previously described by Yáñez et al.14 Firstly, cultures were exposed to CSF-ALS, CSF-control and 100μM glutamate, and we also kept a baseline culture. They were incubated for 24hours and analysed with an optical microscope and immunohistochemical methods.
Immunohistochemical studies after cell fixation involved using anti-caspase-3 antibodies (1:200, Millipore, 04-1090), anti-TNF antibodiesα (1:100, Abcam ab66579), anti-peripherin antibodies (1:500, Millipore, AB9282), and the FluoroPan Neuronal Marker to label all cells of neural origin (1:100, Millipore MAB2300×). Cells were then incubated with pertinent secondary antibodies (Alexa 488, 555, or 647, 1:500, Invitrogen). Nuclei were later contrasted with DRAQ5 (1:3000, Abcam, ab108410). Phase contrast and immunofluorescence imaging were performed with an Olympus inverted microscope attached to an Olympus FV1000® confocal microscopy system. Quantitative analysis was performed using ImageJ analysis software (version 1.42) (http://rsbweb.nih.gov/ij/). The measurements were made following a double-blind method. We used GraphPad Prism 5 software for statistical analysis and the values are presented as mean±standard error of the mean (SEM).
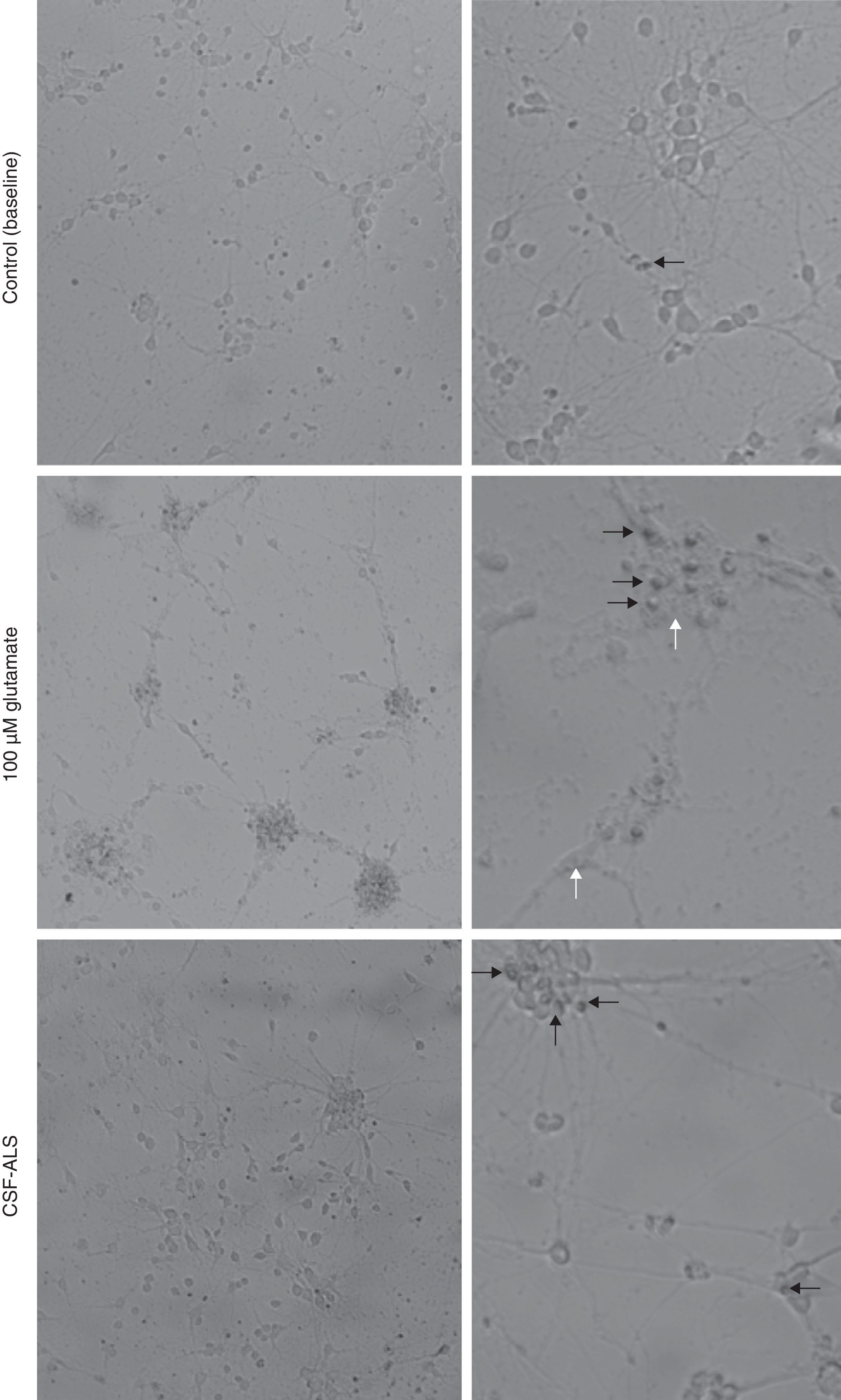
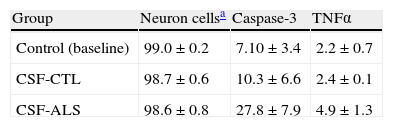
ResultsCellular changes observed under the optical microscope and provoked in the motor neuron cell cultures after exposure to glutamate (100μM) present signs of autophagic degradation, characterised by autophagic vacuolation of the cytoplasm and granulovacuolar degeneration, in addition to pyknotic cells and obvious condensation of chromatin. On the contrary, in the culture exposed to CSF-ALS, we observed cells with shortened cellular projections, decreased cellular volume, nuclear fragmentation (karyorrhexis), or chromatin condensation (pyknosis). Cells may show a little to no structural modification of cytoplasmic organelles, plasma membrane blebbing or maintenance of the plasma membrane (Fig. 1). Comparison of the two images showed that cellular changes provoked by CSF-ALS and glutamate were different.
Cellular changes in motor neuron cell culture in control (baseline) and after exposure to CSF-control, 100μM glutamate, and CSF-ALS. In the control image, the black arrow indicates pyknotic cells. In the culture exposed to glutamate, white arrows indicate autophagic vacuoles and black arrows show chromatin condensation. In the culture exposed to CSF-ALS, black arrows indicate maintenance of the cytoplasmic membrane.
When we compared the number of cells marked using immunohistochemical methods between the control (baseline) culture, those exposed to CSF-control, and cultures exposed to CSF-ALS, we observed a significant increase in caspase-3 and TNFα in the latter (Table 1). No differences were observed between the control (baseline) culture and CSF-control. Caspase-3 labelling yielded a mean±SD of 27.8±7.9 when exposed to CSF-ALS vs. 7.1±3.4 and 10.3±6.6 in the other 2 cultures. Mean TNFα±SD after exposure to CSF-ALS was 4.9±1.3 vs. 2.2±0.7 and 2.4±0.1 for the other groups. Comparison of these data therefore shows that exposure to CSF-ALS provokes an increase in caspase-3 and TNFα at 24hours of incubation.
Immunohistochemistry values for caspase-3 and TNFα in the 3 types of cultures.
| Group | Neuron cellsa | Caspase-3 | TNFα |
| Control (baseline) | 99.0±0.2 | 7.10±3.4 | 2.2±0.7 |
| CSF-CTL | 98.7±0.6 | 10.3±6.6 | 2.4±0.1 |
| CSF-ALS | 98.6±0.8 | 27.8±7.9 | 4.9±1.3 |
Mean±SD data expressed as percentages.
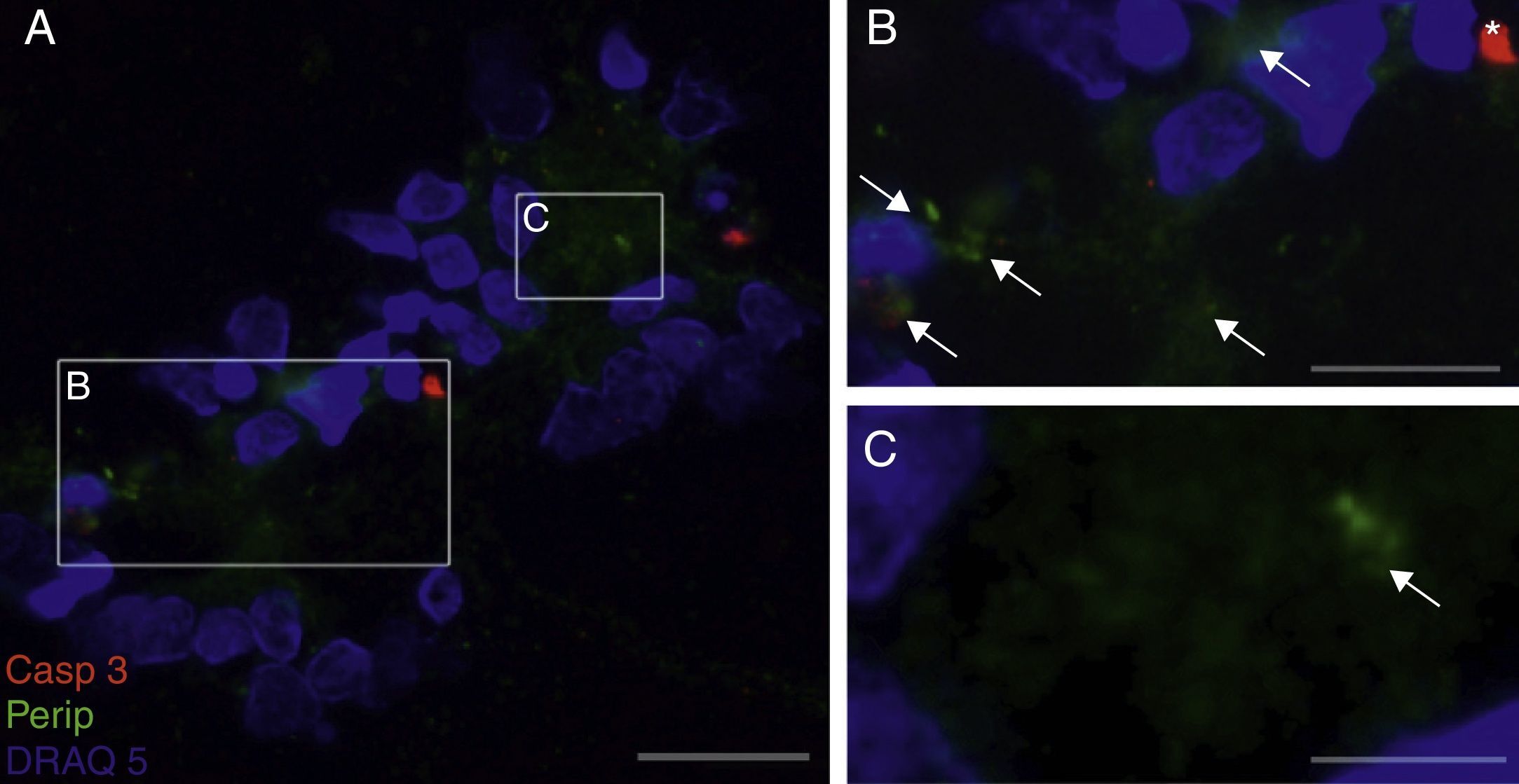
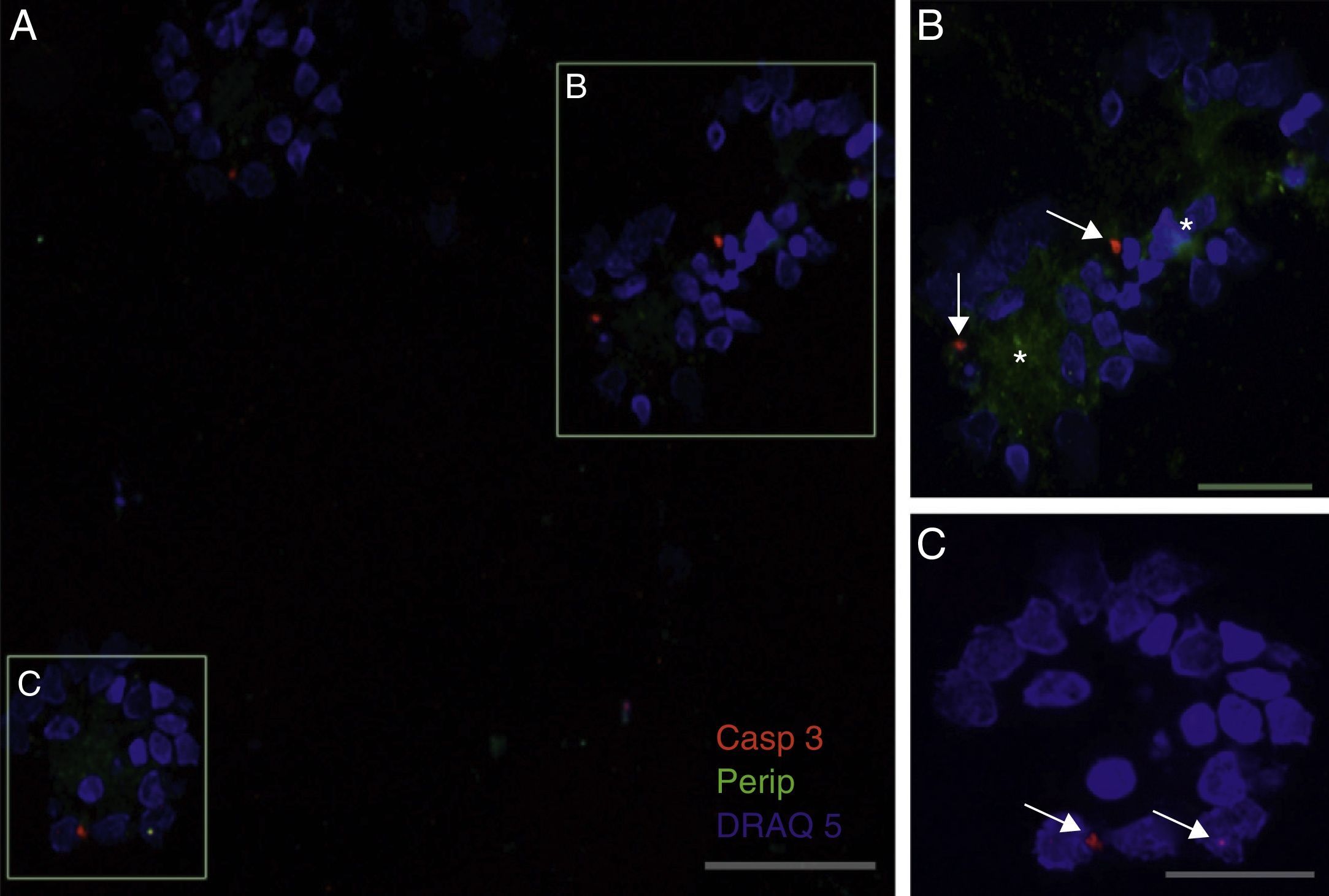
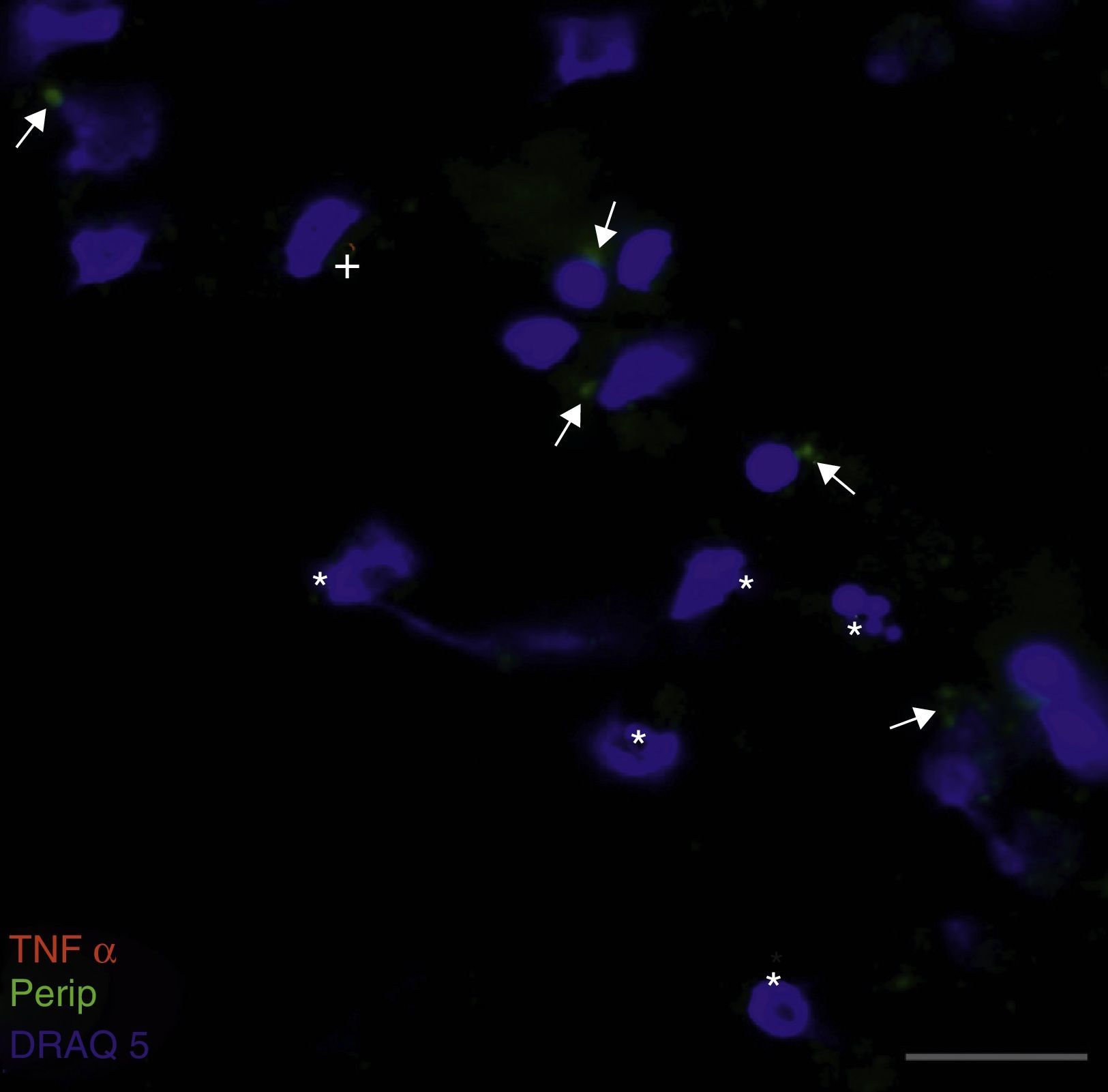
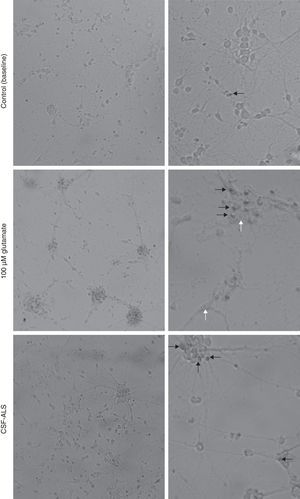
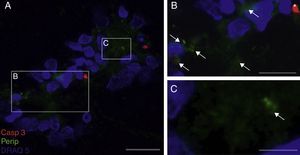
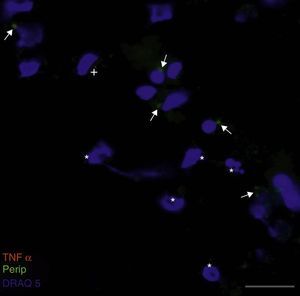
Peripherin expression was observed only in the culture exposed to CSF-ALS and not in other cultures. Expression of this protein as small cytoplasmic precipitates was observed in the group of cells exposed to CSF-ALS (Fig. 2). We observed cells expressing caspase-3 that did not co-express peripherin as well as caspase-3 positive cells that did express peripherin (Fig. 3). No TNFα labelling can be observed in some cells expressing peripherin, which leads us to suggest that peripherin expression may precede the increase in TNFα (Fig. 4). Consequently, comparison of these cultures shows that exposure to CSF-ALS induces overexpression of peripherin that precedes the increase in TNFα at 24hours after exposure.
Co-expression of caspase-3 and peripherin in the culture exposed to CSF-ALS (A–C). Labelling of caspase-3 (red), peripherin (green, arrows) and nuclei (blue) (A–C). Peripherin expression was observed as small cytoplasmic precipitates (arrows, B and C) and caspase-3 positive cells (asterisk). Bar: (A) 30 microns, (B) 15 microns, and (C) 5 microns.
Co-expression of caspase-3 and peripherin in the culture exposed to CSF-ALS (A–C). Labelling of caspase-3 (red-asterisk), peripherin (green-arrows) and nuclei (blue) (A–C). Image B shows inclusions that express peripherin (asterisks) and expression of caspase-3 marked with arrows (B and C). Bar: (A) 60 microns, (B) 40 microns, and (C) 20 microns.
Cytotoxicity in primary motor neuron cells exposed to CSF-ALS has historically been based on laboratory techniques which have varied between different research groups. For example, researchers have used intracellular LDH determination,16 ChAT expression,17 cell viability by MTT assay or a similar technique,14,17 live cell count,9,10,17 intracellular calcium levels,14 or expression of phosphorylated neurofilaments.18 Our study confirms that, according to cell viability by MTT assay, cytotoxicity is associated with an increase in caspase-3 and therefore correlates with the process of apoptosis.
Our study shows that cellular changes observed as a result of exposing a motor neuron cell culture to CSF-ALS differ from those caused by exposure to 100μM glutamate. These changes lead to apoptosis with an increase in TNFα. Some authors report that glutamate-induced excitotoxicity is one of the mechanisms in ALS and the mutant SOD1 mouse model of ALS.19,20 This idea is supported by the fact that excitatory neurotransmission by spinal motor neurons are especially dependent on AMPA receptors. This may explain the specific vulnerability of these cells.21This mechanism is also supported by the fact that riluzole, the only drug to have shown a discrete protective effect by prolonging the patient's life by a few months,22,23 blocks presynaptic glutamate release, inhibits calcium entry, regulates AMPA receptors, and inhibits the effect of NMDA and AMPA receptors.24–26
There are little data in the literature regarding cellular changes produced by cytotoxic CSF from patients with ALS. Researchers have described changes ranging from astrogliosis27–29 in in vivo models or mixed neuron-astrocyte cultures, vacuoles,30,31 pre- or pro-apoptotic signs,32–34 or signs of cellular death.35 Our data show that changes differ from those observed after cultures are exposed to glutamate, thereby supporting our conclusions from a previous study that demonstrated that the CSF-ALS mechanism is glutamate-independent by exposing cultures to different antagonists.14
Observed cellular changes suggest that when cells are exposed to CSF-ALS, nuclear impairment occurs earlier than impairment of the cell membrane or cytoplasmic organelles. This phenomenon has already been observed in the disease,36 the transgenic mouse model of ALS,37 and in the autosomal dominant form of familial ALS (ALS8).38,39 The latter produces a mutant protein which contributes to the formation of cytoplasmic ER membranes with observable nuclear envelope defects. This blocks transport of nucleoporins to the nuclear envelope, and these proteins remain sequestered in the cytoplasm.40 Our findings suggest that the cytotoxic mechanism might initially penetrate motor neurons without damaging the cell membrane, a process that is probably also due to anomalous accumulation of protein in the cytoplasm. This would explain why the apoptosis mechanism shown by the increase of caspase-3 would be associated with increased TNFα.35 Expression would probably have been even higher if the culture had been incubated for more than 24hours.
Peripherin is a neuronal intermediate-filament protein that predominates in the peripheral nervous system, although it has also been found in some neuron populations of the central nervous system. Overexpression of the protein has been described in multiple neurodegenerative disorders. In the case of ALS, peripherin gene mutations are normally sporadic and gene overexpression results in motor neuron disease in mice.41 Mizuno et al.42 have described presence of this protein in Bunina bodies, which are typical of those forms of the disease not related to SOD1.43 A mouse model developed with a TDP-43 mutation shows clinical signs of frontotemporal degeneration similar to those of ALS and presents peripherin overexpression.44 Presence of this protein in cultures exposed to CSF-ALS supports the hypothesis presented by Mizuno et al.42 stating that peripherin could play a role in the development of ALS. Considering that it reduces the effect of BDNF45 and that riluzole acts on BDNF receptors, this mechanism might explain how this drug could act independently of glutamate. The increase in peripherin preceding the increase in TNFα suggests that the protein may play an early role in the process of apoptosis. This supports the observation that it co-localises with caspase-3.
In summary, our study supports the idea that cytotoxic CSF-ALS is not related to glutamate, which causes early nuclear impairment without damaging the cytoplasmic membrane. This provokes cytoplasmic apoptosis that results in an increase in caspase-3 which is co-localised with anomalously overexpressed peripherin.
FundingThe research behind our study was funded within the project “Vulnerabilidad selectiva de la motoneurona a los efectos neurotóxicos del líquido cefalorraquídeo de pacientes en esclerosis lateral amiotrófica (ELA)” by Fundación Mutua Madrileña 2008, 2009.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Gomez-Pinedo U, Yáñez M, Matías-Guiu J, Galán L, Guerrero-Sola A, Benito-Martin MS, et al. Cambios celulares producidos por la citotoxicidad del líquido cefalorraquídeo de pacientes con esclerosis lateral amiotrófica sobre cultivos de neuronas motoras. Neurología. 2014;29:346–352.