Currently, concussion considers a problem of great magnitude, adolescents and young people being the population at risk, since it is in the process of maturation. Our goal has been to compare the effectiveness of different interventions (exercise therapy, vestibular rehabilitation and rest) in adolescents and young people with concussion.
DevelopmentA bibliographic search was carried out in the main databases. Once the inclusion/exclusion criteria and the PEDro methodological scale were applied, 6 articles were reviewed. The results support the use of exercise and vestibular rehabilitation in the initial stages to reduce post-concussion symptoms. According to most authors, therapeutic physical exercise and vestibular rehabilitation report greater benefits, although a protocol that unifies assessment scales, study variables and analysis parameters would be needed to be able to make the inference in the target population.
ConclusiónFrom the moment of hospital discharge, the combined application of exercise and vestibular rehabilitation could be the best option to reduce post-concussion symptoms.
Actualmente la conmoción cerebral se considera un problema de gran magnitud, siendo los adolescentes y jóvenes la población de riesgo, ya que se encuentran en proceso de maduración. Nuestro objetivo ha sido comparar la eficacia de diferentes intervenciones (ejercicio físico terapéutico, terapia vestibular y descanso) en adolescentes y jóvenes con conmoción cerebral.
DesarrolloSe realizó una búsqueda bibliográfica en las principales bases de datos. Una vez aplicados los criterios de inclusión/exclusión y la escala metodológica Physiotherapy Evidence Database PEDro, fueron revisados seis artículos. Los resultados apoyan la utilización del ejercicio y la terapia vestibular en las etapas iniciales para disminuir los síntomas posconmoción. Según la mayoría de los autores, el ejercicio físico terapéutico y la terapia vestibular reportan mayores beneficios, aunque se necesitaría un protocolo que unificara escalas de valoración, variables de estudio y parámetros de análisis para poder realizar la inferencia en la población diana.
ConclusiónDesde el momento del alta hospitalaria del paciente, la aplicación combinada de ejercicio físico y terapia vestibular, podría considerarse como la mejor opción para disminuir los síntomas posconmoción.
Traumatic brain injury (TBI) involves both primary lesions caused by an external mechanical force and secondary lesions resulting from the physiological and biomolecular changes caused by brain ischaemia and intracranial hypertension.1–3
Mild TBI is an acute brain lesion caused by an external force that prevents the individual from living a normal life in the social, psychological, and physical spheres.4,5
Concussion is a pathophysiological alteration of the brain caused by a mechanical force transmitted to the brain and resulting in transient neural dysfunction, with no evidence of structural damage in neuroimaging studies.6–8
According to the United States Centers for Disease Control and Prevention, the most common causes of TBI-related death are road traffic collisions in individuals younger than 15 years and falls in individuals older than 65.9
Concussion may present with a wide range of symptoms, with the most important being headache and loss of consciousness after the lesion.8,10 Broglio et al.11 describe dizziness as a predictor of prolonged recovery.
Among men, the sports with the highest incidence of concussion are American football, ice hockey, and lacrosse; among women, these are ice hockey, soccer, and basketball.12,13
TBI is one of the best known health conditions and has a major impact worldwide, affecting millions of people of all ages every year. It is regarded as one of the most complex disabling injuries, resulting in a 15.7% loss of productivity, 14 times as much as that associated with spinal cord injury.14
Around 80%–90% of concussions resolve within 7–10 days; however, in special populations (children and adolescents), recovery may take longer.7,15 Several awareness campaigns for TBI have been developed; in Spain, however, it is not widely known.8,16–18
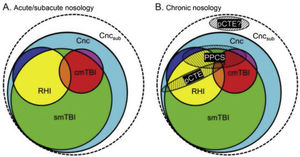
Diagnosis and treatmentGreat advances have been made in diagnostic criteria over the past 10 years. TBI was traditionally defined as a “head injury,” comprising a broad diagnostic spectrum. Although the introduction of the term TBI has not improved the conceptualisation of the injury, it has been very helpful from an epidemiological, treatment, and research viewpoint. When different levels of severity were established, the concept of mild TBI began to be used. The universally used diagnosis of mild TBI is mainly based on the development of signs and symptoms resulting from an external impact against the head, causing alterations in brain function. However, mild TBI is underdiagnosed, and its diagnostic criteria have been modified and expanded over time.19,20 In their study, Mayer et al.20 describe the current and the ideal diagnostic nosology of mild TBI and its different stages.
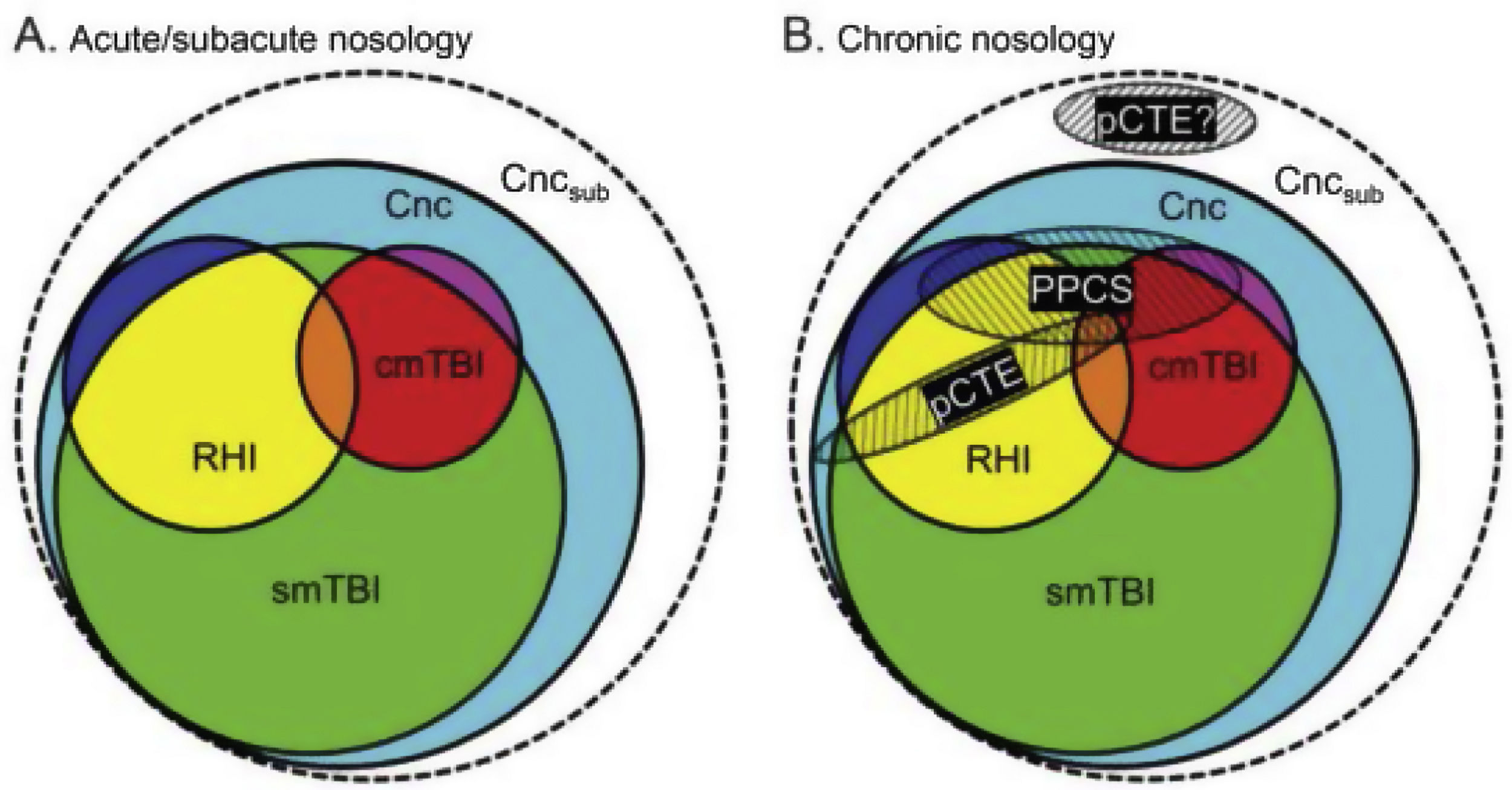
According to their description of the current diagnostic nosology of this entity (Fig. 1), concussion (Cnc, in cyan) is the mildest form of injury and encompasses all other diagnoses. Single mild TBI (smTBI, in green) falls under the diagnosis of concussion, although no diagnostic criteria are currently available that differentiate single mild TBI from concussion. Complicated mild TBI (cmTBI, in red, orange, and purple) is a head injury associated with positive neuroimaging findings. Repetitive head injury (RHI, in yellow, orange, and blue) may occur in either of the previous diagnostic categories. Subconcussive blows (Cncsub, in white) are speculative and not officially recognised. Prolonged postconcussive symptoms (PPCS) may present in any acute diagnostic entity and are currently diagnosed as major or mild neurocognitive disorder due to TBI. Probable chronic traumatic encephalopathy (pCTE) occurs following smTBI. PPCS and pCTE present within 3 months of acute diagnosis.
Current diagnostic nosology of mild traumatic brain injury. cmTBI: complicated mild traumatic brain injury; Cnc: concussion; Cncsub: subconcussive blows; pCTE: probable chronic traumatic encephalopathy; PPCS: prolonged postconcussive symptoms; RHI: repetitive head injury; smTBI: single mild traumatic brain injury.
Source: Mayer et al.20
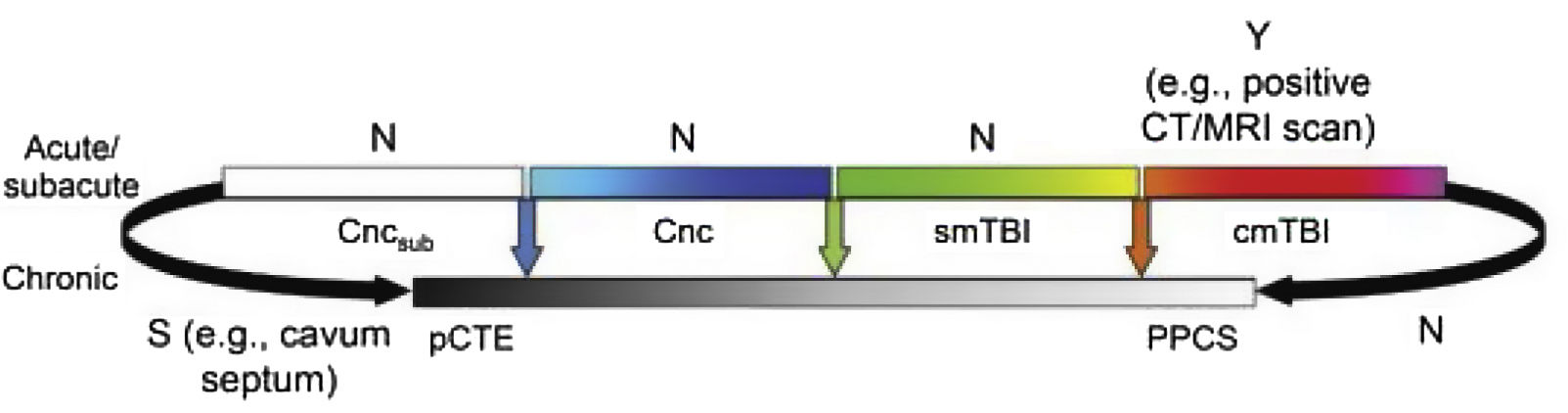
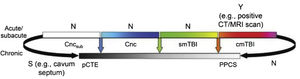
Fig. 2 presents the spectrum of mild TBI from the acute to the chronic phase. The colour code used is the same as that of Fig. 1. Furthermore, it presents each entity as a distinct diagnosis rather than combining them, and defines each with a specific, objective biomarker (represented by vertical arrows), which currently only exists for cmTBI. The availability of evidence for in vivo biomarkers is indicated with Y for yes, S for some, or N for none.
Ideal diagnostic nosology of mild traumatic brain injury. cmTBI: complicated mild traumatic brain injury; Cnc: concussion; Cncsub: subconcussive blows; CT: computed tomography; MRI: magnetic resonance imaging; N: none; pCTE: probable chronic traumatic encephalopathy; PPCS: prolonged postconcussive symptoms; S: some; smTBI: single mild traumatic brain injury; Y: yes.
Source: Mayer et al.20
In general terms, the management protocol for individuals presenting concussion always starts with physical and cognitive rest until symptoms disappear. The Post-Concussion Symptom Scale (PCSS) is applied from the first day after the injury (Table 1 in Supplementary Material). After 48 hours, or if symptoms do not resolve, the protocol establishes a process for patients to return to physical activity (Table 2 in Supplementary Material).21 No standardised parameters have been established for therapeutic exercise.22 Vestibular rehabilitation is a novel treatment for vestibular balance disorders, although evidence supporting its effectiveness remains limited.23–25 Furthermore, physical rest is considered the first-line treatment in these patients, despite the lack of evidence on its effects.26–28
Significance of the studyConcussion and mild TBI are frequently regarded as synonyms for the mildest form of TBI after a head impact.29 In view of the controversies around these concepts, we will use both terms interchangeably.
Few epidemiological data are available, although researchers from the United States have previously informed about the magnitude of this health problem for healthcare systems and the scientific community.30
Adolescents and young adults are at greater risk of mild TBI (with the increase in sports practice playing an important role), and their recovery is more prolonged since they are still maturing from a physiological viewpoint.31 Furthermore, emergency care of these patients presents limitations, with symptoms persisting in one-third of cases.32
The purpose of this review is to analyse and compare the effects of different interventions (therapeutic exercise, vestibular rehabilitation, and physical rest) on the symptoms of concussion in adolescents and young adults.
Material and methodsDesignThis study followed the PICO (patient, intervention, comparison, outcome) strategy (Table 1) to construct the research question and select the best available scientific evidence.33,34
Research question: What are the benefits of therapeutic exercise on post-concussion symptoms in adolescents and young adults, as compared to physical rest and vestibular rehabilitation?
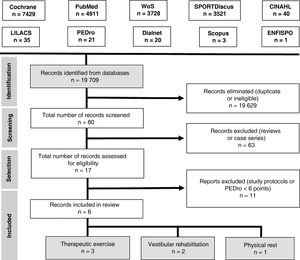
Search strategyAn electronic search was performed on the following databases: Cochrane Library, PubMed, Web of Science, SPORTDiscus, CINAHL, LILACS, PEDro, Dialnet, Scopus, and ENFISPO.
We used the following search strategy with keywords included in the Health Sciences Descriptors (DeCS) vocabulary and the Medical Subject Headings (MeSH) thesaurus:
(brain concussion OR mild traumatic brain injury) AND (adolescent OR young adult) AND (exercise therapy OR rest OR vestibular rehabilitation). We only included clinical trials and reviews. We searched for articles published up to 12 December 2017.
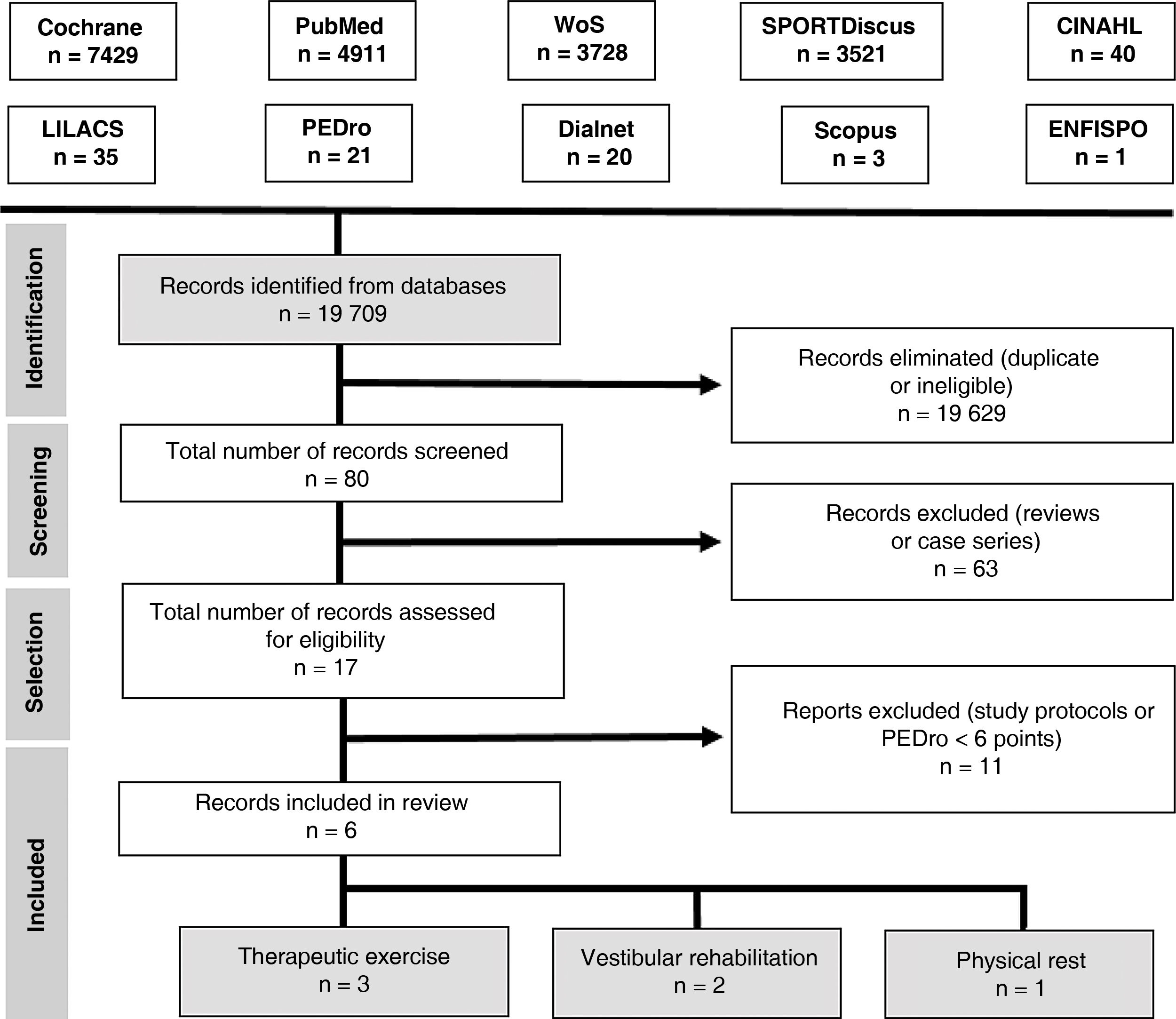
Our search yielded 6 articles. Fig. 3 presents the PRISMA flow diagram.35
Study selectionWe included randomised clinical trials published in English or Spanish between 1 January 2010 and 1 October 2018 and including adolescents and young adults (ages 13–25 years) with concussion or mild TBI. The methodological quality of the studies was evaluated with the PEDro scale; only studies achieving a minimum score of 6 points were included in our review.
We excluded all articles analysing interventions other than therapeutic exercise, vestibular rehabilitation, and physical rest, as well as those only including patients aged over 25 years.
Synthesis of resultsTherapeutic exerciseIn the study by Kurowski et al.,36 the intervention group completed a home aerobic cycling programme, cycling for 80% of the duration that exacerbated symptoms, whereas the control group completed a full-body stretching programme that rotated on a 2-week basis. Caregivers were asked to gather data on post-concussion symptoms using the Post-Concussion Symptom Inventory (PCSI).
Self-reported post-concussion symptoms (Table 2) decreased significantly (F = 4.11; P = .044) in the cycling group as compared to the stretching group; however, differences in caregiver-reported PCSI ratings between groups were not significant (F = 0.17; P = .68).
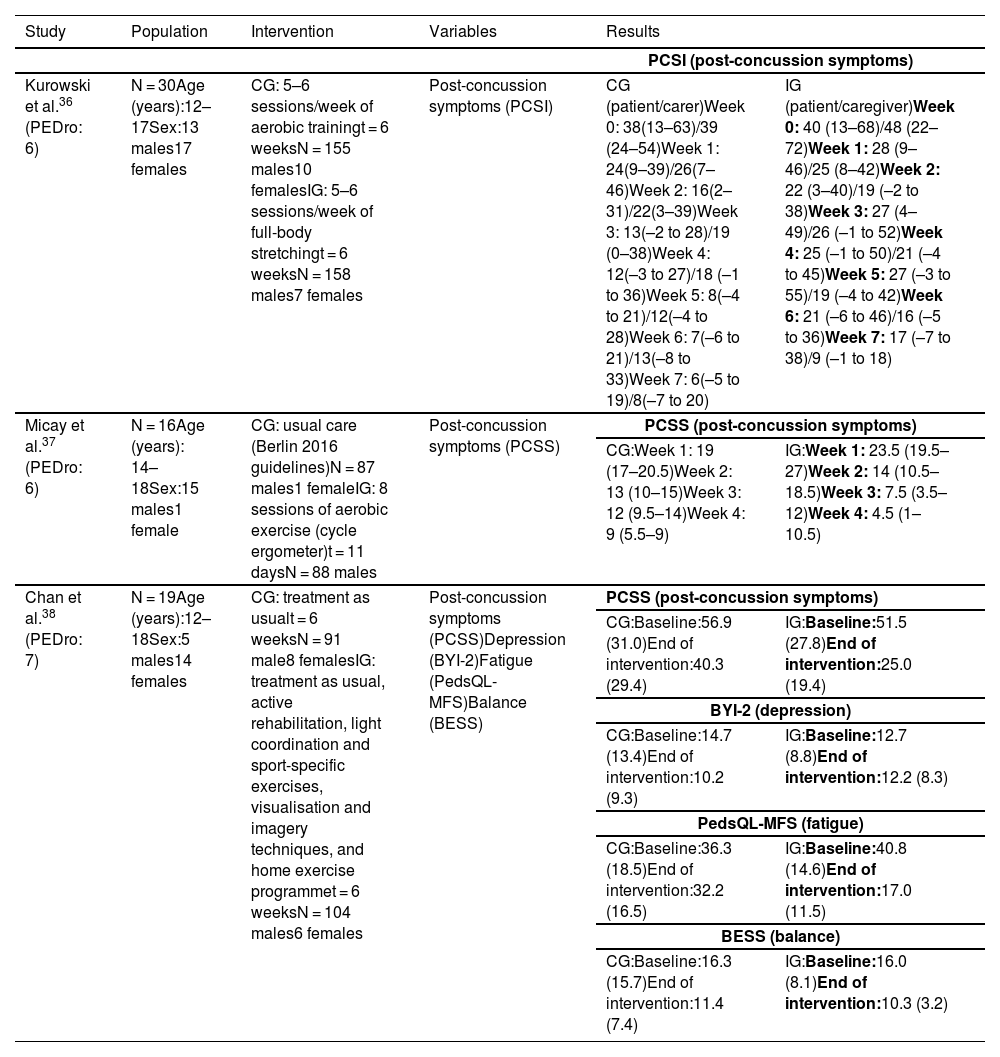
Summary of the results of studies into therapeutic exercise.
| Study | Population | Intervention | Variables | Results | |
|---|---|---|---|---|---|
| PCSI (post-concussion symptoms) | |||||
| Kurowski et al.36 (PEDro: 6) | N = 30Age (years):12–17Sex:13 males17 females | CG: 5–6 sessions/week of aerobic trainingt = 6 weeksN = 155 males10 femalesIG: 5–6 sessions/week of full-body stretchingt = 6 weeksN = 158 males7 females | Post-concussion symptoms (PCSI) | CG (patient/carer)Week 0: 38(13–63)/39 (24–54)Week 1: 24(9–39)/26(7–46)Week 2: 16(2–31)/22(3–39)Week 3: 13(–2 to 28)/19 (0–38)Week 4: 12(–3 to 27)/18 (–1 to 36)Week 5: 8(–4 to 21)/12(–4 to 28)Week 6: 7(–6 to 21)/13(–8 to 33)Week 7: 6(–5 to 19)/8(–7 to 20) | IG (patient/caregiver)Week 0: 40 (13–68)/48 (22–72)Week 1: 28 (9–46)/25 (8–42)Week 2: 22 (3–40)/19 (–2 to 38)Week 3: 27 (4–49)/26 (–1 to 52)Week 4: 25 (–1 to 50)/21 (–4 to 45)Week 5: 27 (–3 to 55)/19 (–4 to 42)Week 6: 21 (–6 to 46)/16 (–5 to 36)Week 7: 17 (–7 to 38)/9 (–1 to 18) |
| Micay et al.37 (PEDro: 6) | N = 16Age (years): 14–18Sex:15 males1 female | CG: usual care (Berlin 2016 guidelines)N = 87 males1 femaleIG: 8 sessions of aerobic exercise (cycle ergometer)t = 11 daysN = 88 males | Post-concussion symptoms (PCSS) | PCSS (post-concussion symptoms) | |
| CG:Week 1: 19 (17–20.5)Week 2: 13 (10–15)Week 3: 12 (9.5–14)Week 4: 9 (5.5–9) | IG:Week 1: 23.5 (19.5–27)Week 2: 14 (10.5–18.5)Week 3: 7.5 (3.5–12)Week 4: 4.5 (1–10.5) | ||||
| Chan et al.38 (PEDro: 7) | N = 19Age (years):12–18Sex:5 males14 females | CG: treatment as usualt = 6 weeksN = 91 male8 femalesIG: treatment as usual, active rehabilitation, light coordination and sport-specific exercises, visualisation and imagery techniques, and home exercise programmet = 6 weeksN = 104 males6 females | Post-concussion symptoms (PCSS)Depression (BYI-2)Fatigue (PedsQL-MFS)Balance (BESS) | PCSS (post-concussion symptoms) | |
| CG:Baseline:56.9 (31.0)End of intervention:40.3 (29.4) | IG:Baseline:51.5 (27.8)End of intervention:25.0 (19.4) | ||||
| BYI-2 (depression) | |||||
| CG:Baseline:14.7 (13.4)End of intervention:10.2 (9.3) | IG:Baseline:12.7 (8.8)End of intervention:12.2 (8.3) | ||||
| PedsQL-MFS (fatigue) | |||||
| CG:Baseline:36.3 (18.5)End of intervention:32.2 (16.5) | IG:Baseline:40.8 (14.6)End of intervention:17.0 (11.5) | ||||
| BESS (balance) | |||||
| CG:Baseline:16.3 (15.7)End of intervention:11.4 (7.4) | IG:Baseline:16.0 (8.1)End of intervention:10.3 (3.2) | ||||
BESS: Balance Error Scoring System; BYI-2: Beck Youth Inventories, Second Edition; CG: control group; IG: intervention group; PCSI: Post-Concussion Symptom Inventory; PCSS: Post-Concussion Symptom Scale; PedsQL-MFS: Pediatric Quality of Life Multidimensional Fatigue Scale.
In the study by Micay et al.,37 the control group followed an intervention focusing on progressive increases in intensity and duration on a cycle ergometer, according to the Berlin consensus statement (stage 1: no exercise; stage 2: light aerobic exercise; stage 3: sport-specific exercise; stage 4: non-contact training drills; stage 5: full contact practice; and stage 6: return to sport). The intervention group completed 8 sessions of aerobic exercise on a cycle ergometer over the course of 11 days (2 days of exercise followed by one day of rest). The first session lasted 10 minutes, at an intensity of 50% of the participant’s age-predicted maximal heart rate, whereas the following 7 sessions lasted 20 minutes, with intensity increasing by 5% of the individual’s age-predicted maximal heart rate per session. Post-concussion symptoms improved significantly in the intervention group (t[7] = 7.8; P < .01) as compared to controls (t[6] = 2.5; P < .05) (Table 2).
In the study by Chan et al.,38 all participants received a session of occupational therapy and attended a consultation with a physician and a physiatrist before randomisation. The intervention group completed an aerobic training programme with a stationary bicycle at 60% of maximal aerobic capacity for 15 minutes, 10 minutes of sport-specific coordination exercises, and visualisation and imagery techniques, as well as a home exercise programme.
Post-concussion symptoms improved significantly in the intervention group as compared to the control group.
The variables depression, fatigue, and balance were evaluated with the Beck Youth Inventories Second Edition, the Pediatric Quality of Life Multidimensional Fatigue Scale, and the Balance Error Scoring System, respectively; results were gathered for exploratory and descriptive purposes, showing a minimal change in the intervention group (Table 2).
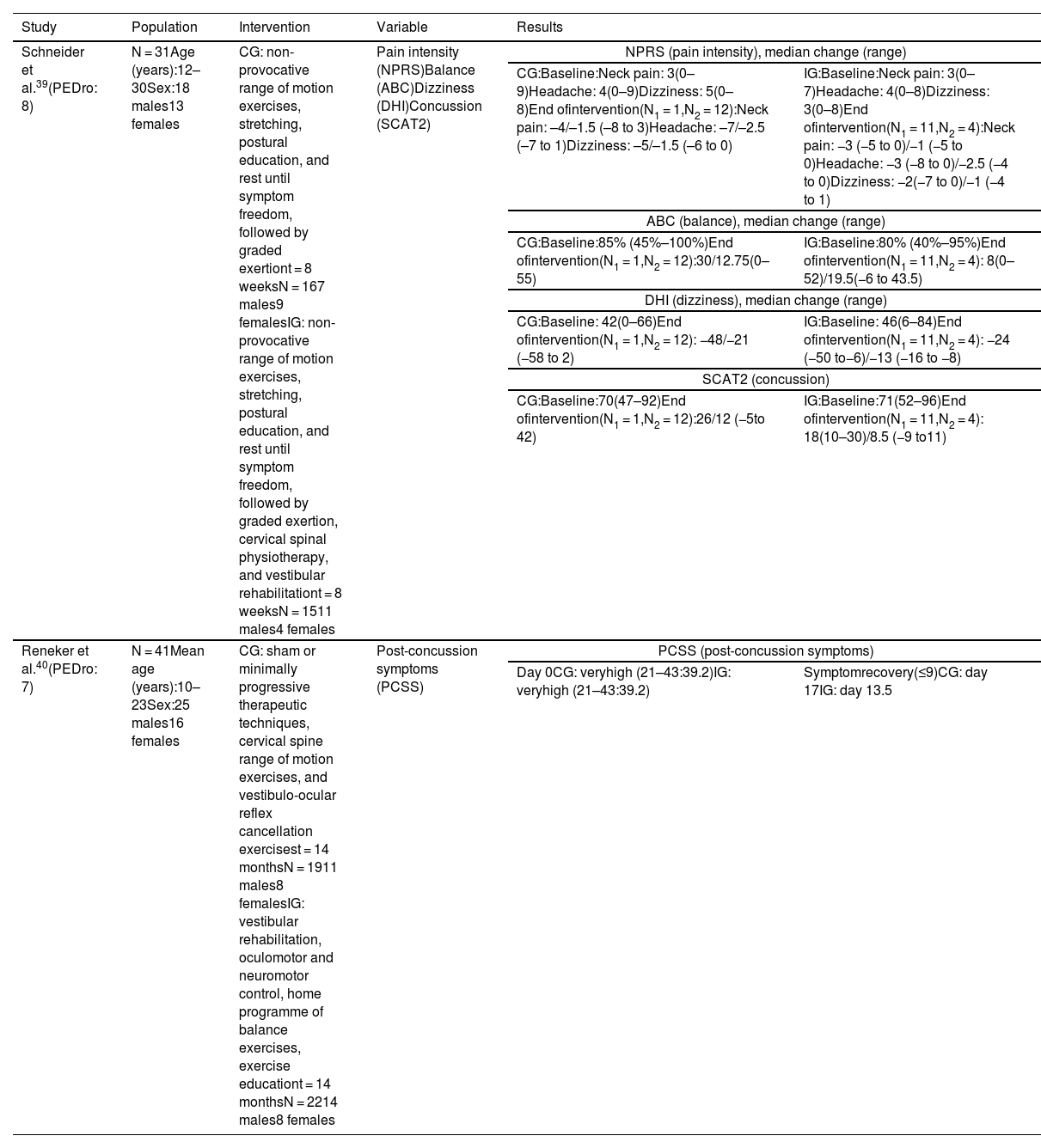
Vestibular rehabilitationIn the study by Schneider et al.,39 the control and intervention groups performed non-provocative range of motion exercises, stretching, and postural education, and were instructed to rest until symptom freedom, followed by a protocol of graded exertion. The intervention group also underwent cervical spine physiotherapy based on manual therapy for the cervical and thoracic spine (joint mobilisation techniques), cervical neuromotor retraining exercises (craniovertebral flexor and extensor retraining), and sensorimotor retraining exercises, as well as vestibular rehabilitation based on an individualised programme of habituation, gaze stabilisation, adaptation exercises, standing and dynamic balance exercises, and canalith repositioning manoeuvres.
The intervention group showed improvements in all variables (Table 3) as compared to the control group (χ2 = 13.08; P < .001).
Summary of results reported by studies on vestibular rehabilitation.
| Study | Population | Intervention | Variable | Results | |
|---|---|---|---|---|---|
| Schneider et al.39(PEDro: 8) | N = 31Age (years):12–30Sex:18 males13 females | CG: non-provocative range of motion exercises, stretching, postural education, and rest until symptom freedom, followed by graded exertiont = 8 weeksN = 167 males9 femalesIG: non-provocative range of motion exercises, stretching, postural education, and rest until symptom freedom, followed by graded exertion, cervical spinal physiotherapy, and vestibular rehabilitationt = 8 weeksN = 1511 males4 females | Pain intensity (NPRS)Balance (ABC)Dizziness (DHI)Concussion (SCAT2) | NPRS (pain intensity), median change (range) | |
| CG:Baseline:Neck pain: 3(0–9)Headache: 4(0–9)Dizziness: 5(0–8)End ofintervention(N1 = 1,N2 = 12):Neck pain: –4/–1.5 (–8 to 3)Headache: –7/–2.5 (–7 to 1)Dizziness: –5/–1.5 (–6 to 0) | IG:Baseline:Neck pain: 3(0–7)Headache: 4(0–8)Dizziness: 3(0–8)End ofintervention(N1 = 11,N2 = 4):Neck pain: −3 (−5 to 0)/−1 (−5 to 0)Headache: −3 (−8 to 0)/−2.5 (−4 to 0)Dizziness: −2(−7 to 0)/−1 (−4 to 1) | ||||
| ABC (balance), median change (range) | |||||
| CG:Baseline:85% (45%–100%)End ofintervention(N1 = 1,N2 = 12):30/12.75(0–55) | IG:Baseline:80% (40%–95%)End ofintervention(N1 = 11,N2 = 4): 8(0–52)/19.5(−6 to 43.5) | ||||
| DHI (dizziness), median change (range) | |||||
| CG:Baseline: 42(0–66)End ofintervention(N1 = 1,N2 = 12): −48/−21 (−58 to 2) | IG:Baseline: 46(6–84)End ofintervention(N1 = 11,N2 = 4): −24 (−50 to−6)/−13 (−16 to −8) | ||||
| SCAT2 (concussion) | |||||
| CG:Baseline:70(47–92)End ofintervention(N1 = 1,N2 = 12):26/12 (−5to 42) | IG:Baseline:71(52–96)End ofintervention(N1 = 11,N2 = 4): 18(10–30)/8.5 (−9 to11) | ||||
| Reneker et al.40(PEDro: 7) | N = 41Mean age (years):10–23Sex:25 males16 females | CG: sham or minimally progressive therapeutic techniques, cervical spine range of motion exercises, and vestibulo-ocular reflex cancellation exercisest = 14 monthsN = 1911 males8 femalesIG: vestibular rehabilitation, oculomotor and neuromotor control, home programme of balance exercises, exercise educationt = 14 monthsN = 2214 males8 females | Post-concussion symptoms (PCSS) | PCSS (post-concussion symptoms) | |
| Day 0CG: veryhigh (21–43:39.2)IG: veryhigh (21–43:39.2) | Symptomrecovery(≤9)CG: day 17IG: day 13.5 | ||||
ABC: Activities-specific Balance Confidence scale; CG: control group; DHI: Dizziness Handicap Inventory; IG: intervention group; N1: discharged; N2: not discharged; NPRS: Numeric Pain Rating Scale; SCAT2: Sport Concussion Assessment Tool 2.
In the study by Reneker et al.,40 the control group completed interventions ranging from sham, subtherapeutic, and non-progressive therapeutic techniques to minimally progressive therapeutic techniques, as well as cervical isometric exercises, gentle cervical spine range of motion exercises, and vestibulo-ocular reflex cancellation exercises, whereas the intervention group completed a vestibular rehabilitation programme (including habituation and adaptation techniques), oculomotor and neuromotor control (including proprioceptive and kinaesthetic awareness), and balance exercises to be performed at home, as well as exercise education. Individuals with cervical dysfunction were treated with soft tissue release, mobilisations, and/or thrust manipulations.
The intervention group showed a significantly shorter symptomatic recovery time than the control group (log-rank = 0.13) (Table 3).
In these studies, post-concussion symptoms were analysed with the Sport Concussion Assessment Tool 2 (which includes a list of symptoms that is identical to that of the PCSS)39 and the PCSS,40 which detected improvements in these symptoms. Schneider et al.39 assess pain, dizziness, and balance with the Numeric Pain Rating Scale, the Dizziness Handicap Inventory, and the Activities-specific Balance Confidence Scale, reporting improvements in all 3 variables in the intervention group.
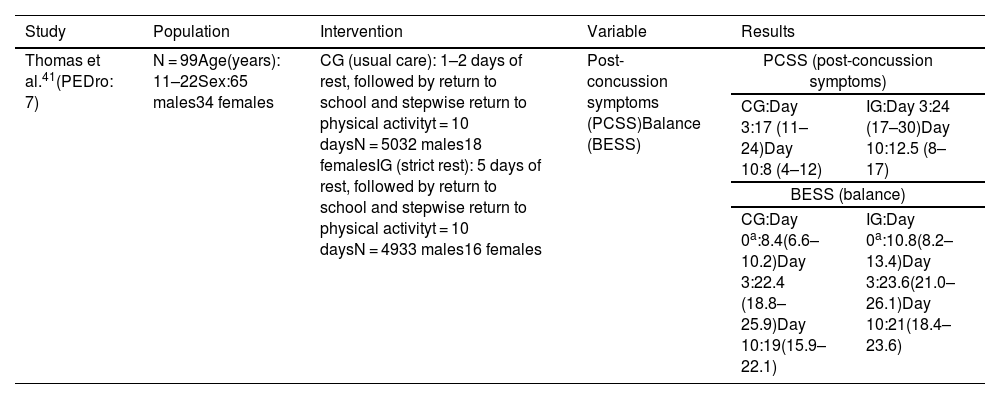
Physical restIn the study by Thomas et al.,41 participants were randomly allocated to strict rest for 5 days (intervention group) or 2 days (control group). Patients in both groups were instructed to follow the ACE-Emergency Department care plan, according to which they had to rest for a set period before stepwise return to school and physical exercise. Return to exercise started with 10–15 minutes of aerobic training on a cycle ergometer or running to increase heart rate, followed by sport-specific non-contact activities, non-contact progression exercises, contact exercises, and finally return to sport. The authors found no significant differences in post-concussion symptoms or balance between groups (Table 4).
Summary of results reported by studies into physical rest for patients with post-concussion symptoms.
| Study | Population | Intervention | Variable | Results | |
|---|---|---|---|---|---|
| Thomas et al.41(PEDro: 7) | N = 99Age(years): 11–22Sex:65 males34 females | CG (usual care): 1–2 days of rest, followed by return to school and stepwise return to physical activityt = 10 daysN = 5032 males18 femalesIG (strict rest): 5 days of rest, followed by return to school and stepwise return to physical activityt = 10 daysN = 4933 males16 females | Post-concussion symptoms (PCSS)Balance (BESS) | PCSS (post-concussion symptoms) | |
| CG:Day 3:17 (11–24)Day 10:8 (4–12) | IG:Day 3:24 (17–30)Day 10:12.5 (8–17) | ||||
| BESS (balance) | |||||
| CG:Day 0a:8.4(6.6–10.2)Day 3:22.4 (18.8–25.9)Day 10:19(15.9–22.1) | IG:Day 0a:10.8(8.2–13.4)Day 3:23.6(21.0–26.1)Day 10:21(18.4–23.6) | ||||
BESS: Balance Error Scoring System; CG: control group; IG: intervention group; PCSS: Post-Concussion Symptom Scale.
According to some studies, early intervention with rest, therapeutic exercise, and vestibular rehabilitation improves post-concussion symptoms in adolescents and young adults.36–40
Regarding therapeutic exercise, the studies by Kurowski et al.,36 Micay et al.,37 and Chan et al.38 report significant improvements in post-concussion symptoms in patients completing an aerobic exercise protocol, despite differences in intensity (50%–80% of maximal aerobic capacity). However, the intergroup differences observed in the study by Micay et al.37 may have been due to the small size of the sample and the small number of female patients included.
In line with these results, other studies36–38 have shown that aerobic training at 80% of maximal aerobic capacity significantly reduces post-concussion symptoms as compared to the control intervention.
Leddy et al.42 found a correlation between a low heart rate threshold and poorer prognosis (ie, longer recovery time). In another study,43 20 minutes of sub-threshold aerobic exercise was found to decrease post-concussion symptoms and prevent long recovery times (> 30 days) as compared to stretching (control intervention).
However, Maerlander et al.44 found that high levels of exertion are associated with longer recovery times. In contrast, moderate physical exertion may be beneficial, although more specific guidance is needed on several parameters. In any case, that study included a small sample, as well as a small number of men.
Yuan et al.45 reported the benefits of aerobic training as compared to stretching, which they attribute to the increase in global efficiency and the decrease in normalised characteristic path length. Kurowski et al.36 propose vestibular rehabilitation as a complementary treatment for individuals with vestibular and oculomotor symptoms, as these patients are less likely to benefit from the aerobic exercise intervention studied.
Schneider et al.39 and Reneker et al.40 provide solid evidence that vestibular rehabilitation considerably decreases post-concussion symptoms associated with vestibular system alterations as compared to controls, after 8 weeks of treatment. Likewise, Kleffelgaard et al.46 reported the short-term benefits (2–3 months) of vestibular rehabilitation on post-concussion symptoms. However, it should be noted that the study included patients aged 16–60 years with mild-to-moderate TBI.
The studies by Schneider et al.39 and Reneker et al.40 included complementary interventions in addition to vestibular rehabilitation. Schneider et al.39 underscore the positive effects of combining vestibular rehabilitation with exercise.
Thomas et al.47 did not observe differences in post-concussion symptoms as a function of the duration of physical rest prescribed. Other studies report similar results. Sufrinko et al.48 observed no improvement in post-concussion symptoms within 10 days of the lesion, and Varner et al.49 found no improvements at weeks 2 and 4. These findings may be due to short follow-up times (7–30 days).48,49 These studies show that long rest periods are associated with increased symptom reporting and lower mental activity.47
Therapeutic exercise and vestibular rehabilitation have been shown to be the most effective approaches, with the studies included in this review reporting significant improvements.
ConclusionTherapeutic exercise in general, and aerobic training in particular, improves post-concussion symptoms and quality of life in adolescents and young adults. Improvements are more marked when therapeutic exercise is combined with vestibular rehabilitation. Prolonged rest may result in longer symptom duration.
Physiotherapists play an essential role in supervising patients during therapeutic exercise and vestibular rehabilitation, ensuring that the intervention is performed correctly and establishing the most appropriate parameters according to the patient’s age and severity, in coordination with a multidisciplinary team.
In the light of the above, there is a need to standardise interventions in terms of intensity, number of sessions, and quantity of contents, adapting them to the needs of children and adolescents to increase treatment adherence.
FundingThis study has received no specific funding from any public, commercial, or non-profit organisation.
Conflicts of interestThe authors have no conflicts of interest to declare.