Eosinophilic granulomatosis with polyangiitis (EGPA) is a type of systemic necrotising vasculitis affecting small- and medium-sized vessels, which belongs to the spectrum of anti-neutrophil cytoplasmic antibody (ANCA)–associated vasculitis. Disease progression presents 3 phases, which may or may not be sequential: an allergic phase (asthma, rhinitis, and nasal polyposis), an eosinophilic phase (eosinophilia in peripheral blood or lung or gastrointestinal tissues), and a vasculitic phase, which may involve multiple organs, including the peripheral and, rarely, the central nervous systems (CNS). In 1990, the American College of Rheumatology created a set of diagnostic criteria (asthma, eosinophilia > 10% in peripheral blood, mono- or polyneuropathy, non-fixed pulmonary infiltrates, paranasal sinus abnormalities, and extravascular eosinophilia), with diagnosis being established in patients meeting 4 of the 6 criteria; these criteria have sensitivity of 85% and specificity of 99.7%.1 Treatment includes corticosteroids and such immunosuppressants as cyclophosphamide in refractory cases. Current series suggest a clear improvement in survival rates, increasing from 70% to 90% at 5 years.2 Cardiac involvement is the main cause of death in patients with EGPA, followed by brain haemorrhage.
We report the case of a 54-year-old man diagnosed with EGPA due to history of severe cortico-dependent asthma, paranasal polyposis, eosinophilic dermatitis, peripheral eosinophilia, and recently diagnosed axonal sensory polyneuropathy. He was treated exclusively with prednisone, dosed at 5 mg/day.
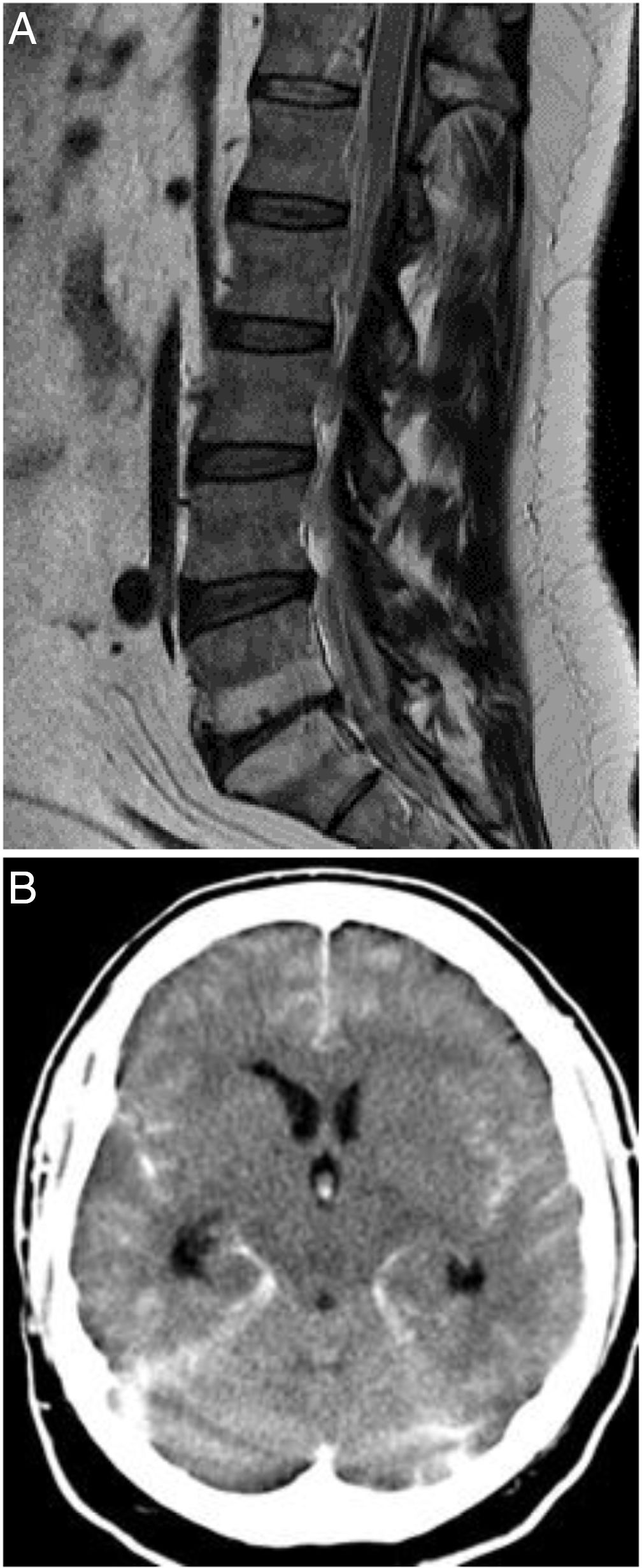
The patient was assessed at the emergency department due to a 48-h history of spontaneous, sudden-onset low back pain, developing into progressive weakness of the left leg, paraesthesia in the left thigh, pollakiuria, and constipation. Physical examination identified no fever, and arterial blood pressure was 157/111 mm Hg. Neurological examination revealed proximal weakness (3/5) in the left leg, hypoaesthesia at the L1 level, exaggerated left patellar and Achilles reflexes, and left Babinski sign. At baseline, laboratory results (glycaemia, ions, liver and kidney function) and coagulation were normal. A complete blood count revealed leukocytosis (20 000 cells/μL; normal range: < 10 200) and eosinophilia (1300 cells/μL [14.8%]; normal range, 500 [< 5% of total]). A chest radiography displayed no abnormalities. A thoracolumbar MRI study (Fig. 1A) identified epidural haemorrhages at the T8-L1 and S1-S2 levels; emergency T12-L1 laminectomy and evacuation of the haemorrhages were performed. A spinal cord arteriography performed 24 hours after the procedure revealed normal findings.
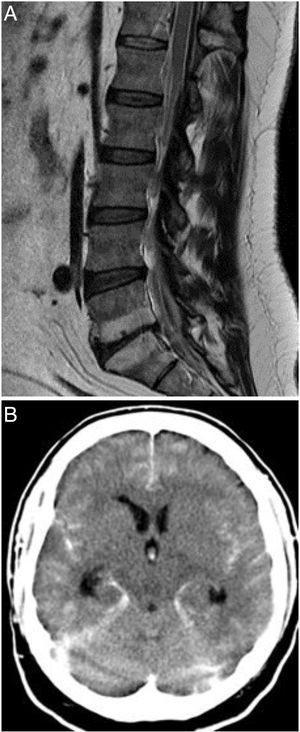
A) T1-weighted MRI study of the spine (sagittal plane), showing epidural haemorrhage of the thoracolumbar spine. Loculi with greater posterior blood pooling are observed at the T12-L1 and S1-S2 levels. The morphology of the spinal cord is normal. B) Non-contrast head CT scan showing perimesencephalic, intraventricular, and cerebellar subarachnoid haemorrhage (Fisher grade IV).
On the third day after admission, the patient presented a sudden decrease in the level of consciousness; a head CT scan (Fig. 1B) detected a spontaneous subarachnoid haemorrhage (SAH), classed as grade IV on the Fisher scale. A subsequent CT angiography identified no relevant alterations. A brain arteriography performed 36 hours later yielded normal findings. The patient died on day 6 after admission.
Our patient meets diagnostic criteria for EGPA of 3 years of progression; given the recent diagnosis of polyneuropathy, it may be classified as being in the vasculitic phase.3
CNS involvement is reported in 6%-10% of cases of EGPA,4 with ischaemic stroke and brain haemorrhages being particularly common; multiple strokes may occur, and stroke may be the first sign of the disease.4–12 Spinal haemorrhages are much rarer.13,14
The largest series of haemorrhagic complications involving the CNS in patients with EGPA is probably that reported by Sabio et al.3 Of 28 patients diagnosed with EGPA, 14 (50%) presented spontaneous SAH, 13 (46%) presented intraparenchymal haemorrhage, 5 (17.9%) presented intraventricular haemorrhage, and 3 (10.7%) presented spinal haematomas. Ross et al.14 report 6 cases of EGPA with spinal complications, including 4 with subdural or subarachnoid haemorrhage.
Our patient initially presented a spontaneous spinal epidural haematoma (SSEH), as observed in the neuroimaging study and confirmed during surgery, and subsequently a spontaneous SAH.
It has been suggested that spontaneous SAH in the context of EGPA may be explained both by vasculitis, with disruption of the internal elastic lamina, and by rupture of the post-stenotic dilatation caused by granuloma formation in small vessels. The cause of SSEH is undetermined in 40% of cases. Some authors propose that it may be caused by venous plexus involvement, whereas others consider it to originate in the epidural arteries.
In our patient, the fact that SSEH occurred in the context of EGPA, almost simultaneously with spontaneous SAH, suggests vasculitis as the most probable cause.15 To our knowledge, this is the first case of SSEH in a patient with EGPA. The simultaneous presentation of spontaneous SAH in the brain is also unusual.
FundingThis study has received no specific funding from any public, commercial, or non-profit organisation.
ConsentConsent was not obtained from the patient due to his death. The final author certifies the effort made to search for family contacts and the complete anonymisation of patient data.
We would like to thank Dr J.M. Sabio of the internal medicine department at Hospital Virgen de las Nieves, Granada (Spain), for his series of patients with EGPA and CNS complications.
Please cite this article as: Lázaro Romero A, Carilla Sanromán A, Horna Cañete L, Serrano Ponz M. Hematoma epidural espinal espontáneo y hemorragia subaracnoidea no aneurismática en paciente con granulomatosis eosinofílica con poliangitis. Neurología. 2021;36:723–725.