SARS-CoV-2, the virus responsible for the current pandemic, predominantly affects the respiratory tract, and a growing number of publications report the predisposition of patients with COVID-19 to develop thrombotic phenomena.
ObjectiveTo determine the prevalence of pulmonary embolism in patients with COVID-19; to determine the possible relationship between the severity of pulmonary involvement and d-dimer levels; to analyze the location of pulmonary embolisms in patients with COVID-19 and to compare it with the location in patients without COVID-19.
MethodsThis retrospective study analyzed all CT angiograms of the pulmonary arteries done in patients with suspected pulmonary embolisms between March 15 and April 30, 2020 and compared them with studies done in the same period one year earlier.
ResultsWe included 492 pulmonary CT angiograms (342 (69.9%) in patients with COVID-19 and 147 (30.1%) in patients without COVID-19). The prevalence of pulmonary embolisms was higher in patients with COVID-19 (26% vs. 16.3% in patients without COVID-19, p = 0.0197; relative risk = 1.6). The prevalence of pulmonary embolisms in the same period in 2019 was 13.2%, similar to that of the group of COVID-19-negative patients in 2020 (p = 0.43). There were no significant differences in d-dimer levels or the location of pulmonary embolisms between the two groups. CT showed moderate or severe pulmonary involvement in 78.7% of the patients with COVID-19.
ConclusionsPatients with COVID-19 have an increased prevalence of pulmonary embolisms (26%), and most (78.7%) have moderate or severe lung involvement on CT studies. The location of pulmonary embolisms and the degree of elevation of d-dimer levels does not differ between patients with COVID-19 and those without.
El coronavirus SARS-CoV-2, responsable de la pandemia actual, afecta preferentemente al tracto respiratorio, con un número creciente de publicaciones sobre su predisposición a fenómenos trombóticos.
ObjetivoConocer la prevalencia de tromboembolismo pulmonar (TEP) en pacientes con COVID-19; determinar su posible relación con la gravedad de la enfermedad pulmonar y los niveles de dímeros-D, y analizar la localización del TEP en pacientes con COVID-19 comparándolos con los negativos.
MétodoEstudio retrospectivo de todas las angio-TC de arterias pulmonares por sospecha de TEP del 15 de marzo al 30 de abril de 2020. Se compara con las angio-TC realizadas durante el mismo periodo en 2019.
ResultadosSe incluyeron 492 angio-TC pulmonares, 342 (69,9%) de pacientes con COVID-19 y 147 (30,1%) de pacientes sin infección. La prevalencia de TEP fue del 26% en el grupo COVID-19 positivo y del 16,3% en el negativo (p = 00,197), con un riesgo relativo de 1,6 veces. La prevalencia de TEP en el mismo período del año 2019 fue del 13,2%, similar a la del grupo COVID-19 negativo del año 2020 (p = 043). No hubo diferencias significativas en el nivel de dímeros D ni en la localización del TEP entre ambos grupos. El 78,7% de los pacientes con COVID-19 con TEP mostraron una extensión de la afectación pulmonar moderada o grave en la tomografía computarizada.
ConclusionesLos pacientes con COVID-19 tienen una prevalencia aumentada de TEP (26%) y la mayoría (78,7%) presentan una extensión moderada o grave de afectación pulmonar en la tomografía computarizada. No hay diferencias significativas en la localización del material embólico ni en el grado de elevación de dímeros D respecto a los pacientes sin COVID-19.
Severe acute respiratory syndrome caused by the coronavirus 2 (SARS-CoV-2) has triggered an unprecedented economic and health crisis, forcing the World Health Organization to declare a global public health emergency on 11 March 2020.1 It represents the biggest medical challenge in decades. As of the date of submission of this manuscript, there were 236,259 patients diagnosed with this infection in Spain, 123,182 of whom required hospitalisation. Of these, 11,364 required admission to intensive care units. The estimated fatality rate for the pandemic in Spain is 11.5%.2
In most patients with COVID-19, the disease manifests with respiratory symptoms, fever, dry cough, dyspnoea, and myalgia. Between 17% and 29% of patients suffer from respiratory distress and require ventilatory support.3 Other clinical characteristics in these patients are systemic inflammatory conditions, endothelial dysfunction, hypercoagulable states and multiple organ failure.4
COVID-19 is diagnosed microbiologically, usually by identifying SARS-CoV-2 with a reverse transcription-polymerase chain reaction (RT-PCR) test. This test is generally performed on nasopharyngeal or respiratory section samples. Imaging tests play an important role in the diagnosis and management of the disease. Conventional or portable chest X-ray is the first-line imaging method, and in many cases the only imaging method, given its widespread availability. Computed tomography (CT) of the chest is more sensitive than chest X-ray. It can assess both lung involvement and complications, as well as provide alternative diagnoses.5 CT is used in certain complications that chest X-ray does not duly assess (e.g. bacterial superinfection with pleural effusion to rule out pleural collections/empyema), when a PCR test is not available for patients who are strongly suspected of having COVID-19 but have a normal chest X-ray, and in patients who follow a poor clinical course, especially those with suspected pulmonary thromboembolism (PTE).
More and more references in the literature are reporting the presence of PTE in the context of COVID-196–8 with elevated biomarkers such as d-dimers.9
The primary objective of this article is to determine the prevalence of acute PTE in patients diagnosed with COVID-19 at a tertiary hospital and compare it to that of PTE in patients without COVID-19. The secondary objectives including analysing the relationship between the severity of lung involvement and the presence of PTE, as well as the relationship to blood d-dimer levels in patients with PTE, comparing patients with COVID-19 to patients without COVID-19.
Material and methodsThe study was conducted in accordance with the ethical guidelines of the Declaration of Helsinki. None of the authors had any conflicts of interest.
PatientsThis retrospective study enrolled all consecutive adult patients with suspected acute PTE who underwent CT angiography of the pulmonary arteries between 15 March and 30 April 2020 at a tertiary university hospital during the SARS-CoV-2 pandemic. The indication for pulmonary CT angiography was based on medical criteria. The main reasons for ordering this imaging test were clinical deterioration with development or worsening of dyspnoea, desaturation, chest pain and elevated d-dimer levels.
Demographic, clinical and laboratory characteristics were obtained in an anonymised fashion from the patient's electronic medical record.
They were classified as:
- 1
Patients with COVID-19, those with positive PCR results or, failing that, those with consistent symptoms (respiratory symptoms, fever, dry cough, dyspnoea and myalgia) and lung parenchyma lesions characteristic of COVID-19 infection on a chest CT (ground-glass opacities or alveolar consolidations with a bilateral and peripheral distribution).10
- 2
COVID-19-negative patients were considered to be those without positive PCR results and without clinical or radiological (chest CT) findings raising suspicion of COVID-19.
Although COVID-19 is diagnosed microbiologically, in addition to patients with positive PCR results (patients with confirmed COVID-19), those with signs and symptoms as well as chest CT findings typical of COVID-19 who had negative PCR results or did not undergo PCR testing (probable COVID-19 patients) were included in the COVID-19 group. This was because the sensitivity of PCR is relatively low (60–70%), with a reported rate of false negatives of 30% in nasopharyngeal samples11, varying by time elapsed since exposure to SARS-CoV-2, with a rate of 67–100% in the presymptomatic period and 66% around day 21 from the onset of symptoms.12 On the other hand, it has been reported that chest CT has a sensitivity exceeding 90% for the diagnosis of COVID-1913 and can show typical characteristics of the disease even when PCR is negative or the patient is asymptomatic.14 This is especially useful in patients with strong clinical suspicion, either due to suggestive symptoms or history of exposure, in periods of high disease prevalence. Our study was conducted at a time of peak prevalence of the pandemic, in a period of community transmission.
In patients with COVID-19 and PTE, the time elapsed from the onset of the infectious signs and symptoms to the embolic event was recorded.
D-dimer levels were collected in patients with PTE and corrected according to the patient's age.15,16
Finally, CT angiography of pulmonary arteries ordered due to suspected acute PTE in the same period in 2019 was retrospectively analysed to determine the prevalence thereof in the same setting in a non-epidemic environment.
CT angiography of pulmonary arteriesAll CT angiography scans of pulmonary arteries were performed using 64- or 16-detector scanners (Phillips Brilliance, Phillips Medical System, Eindhoven, Netherlands). The acquisition parameters were as follows: 120 kVp, 50−350 mA, with dose modulation and collimation of 64 × ̴0.625 mm or 16 × 0.75 mm, rotation time 0.8 s, with a thickness of 2 mm and a reconstruction interval of 1 mm. Image reconstruction was performed using a soft-tissue algorithm.
The examination was performed with the patient in supine decubitus, in apnoea or with gentle breathing depending on the patient's clinical situation, and coverage from the thoracic outlet to the diaphragm.
A total of 50−90 ml of "Ioexol" non-ionic iodinated intravenous contrast (Omnipaque 350; GE Health Ireland) were administered at an injection rate of 4 mL/s using an injector (Medrad, Stellant, Pittsburgh, Pennsylvania, United States). To achieve optimal intravascular opacification, an automated region of interest (ROI) detector was placed in the pulmonary artery trunk at the level of the junction with the aorta, and a threshold of 150 Hounsfield units (HU) was established.
Imaging analysisAll studies were evaluated by a third- or fourth-year diagnostic imaging resident and supervised by at least one radiologist from the A&E department or the chest division with at least 15 years of experience. Discrepancies were resolved by consensus between two of the more experienced radiologists.
All CT angiography scans of pulmonary arteries were evaluated from a perspective of image quality. A high-quality study was considered to be one that showed good opacification of the pulmonary arterial tree and enabled proper visualisation of the vascular anatomy at least down to a segmental level. A low-quality study was considered to be one with images not optimal for suitable assessment of the anatomy of the pulmonary arterial tree. The causes of insufficient image quality are: presence of movement artefacts (respiratory movement artefacts due to lack of tolerance to apnoea); insufficient intravascular opacification; and presence of noise, mainly in obese patients.
Imaging analysis was performed at diagnostic workstations (Philips Workstation), based on an effective slice thickness of 1 mm, using image post-processing techniques. A thin slice thickness was used to assess the lumen of the pulmonary arteries from their origin, avoiding overlap with the pulmonary veins. Images were evaluated with a lung window (1500, -500 HU), with a mediastinal window (350, 50 HU) and with an angiographic window (700, 100 HU). Image post-processing was performed using maximum intensity projection (MIP) and multiplanar reconstruction (MPR) techniques.
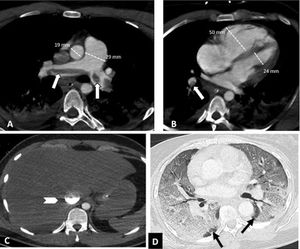
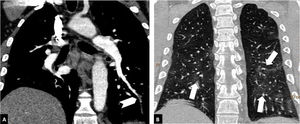
Depending on the location of the most proximal filling defect, thromboembolic involvement was classified as: a) proximal, if the most proximal thrombus was located in the trunk of the pulmonary artery or in the main pulmonary arteries (Fig. 1); b) medial, if the material was located in the proximal lobar or segmental arteries (Fig. 2); or c) distal, if the involvement was distal segmental or subsegmental (Fig. 3).17
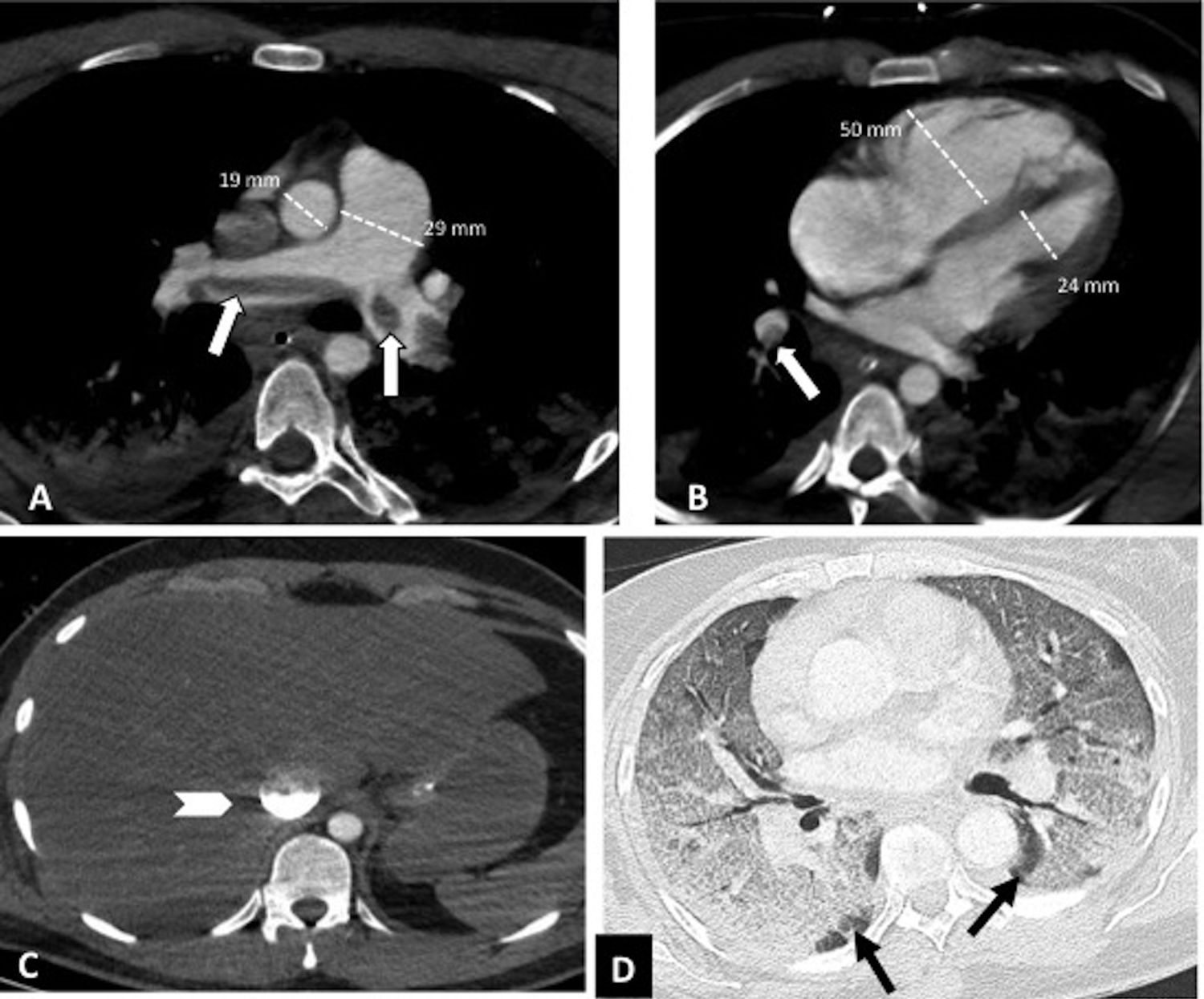
Central pulmonary thromboembolism with signs of right heart overload in a patient with severe COVID-19. Chest CT angiography. A-C) Knuckle thrombus at the bifurcation of the pulmonary arteries and in the lobar artery of the right lower lobe (white arrows). Signs of right heart overload: pulmonary artery/aorta ratio >1, enlargement of the right heart chambers with straightening of the intraventricular septum and reflux of intravenous contrast into the inferior vena cava (arrow head). D) Severe parenchymal involvement by COVID-19. Ground-glass pattern involving more than 60% of the lung parenchyma. The black arrows point to the intact lung parenchyma in both lower lobes.
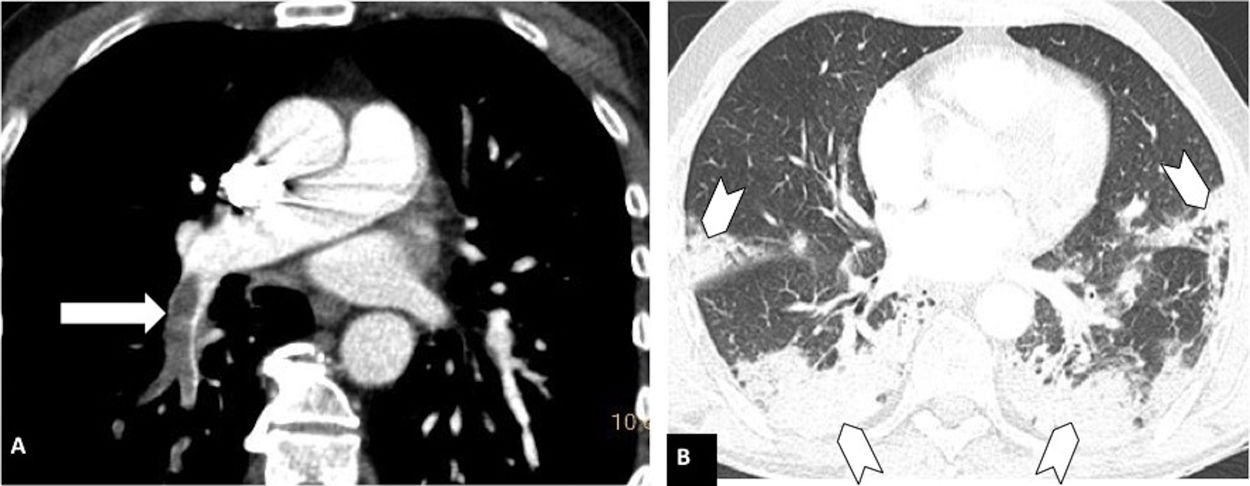
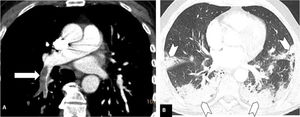
Pulmonary thromboembolism in a medial location in a patient with moderate parenchymal involvement by COVID-19. Chest CT angiography. A) Filling defect in the right lower lobar artery (white arrow). B) Parenchymal involvement with peripheral consolidations in the posterior region of both lower lobes, the medial lobe and the lingula (arrow heads), involving 30-60% of the lung parenchyma.
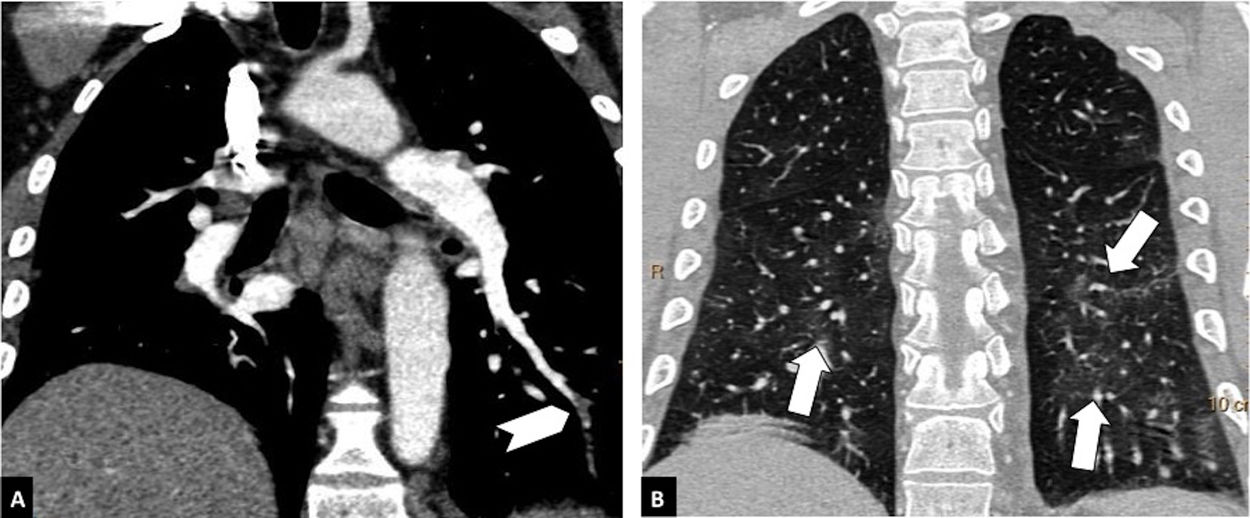
Distal pulmonary thromboembolism in a patient with mild parenchymal involvement by COVID-19. Chest CT angiography. A) Filling defect in a basal distal segmental artery of the left lower lobe (white arrow head). B) Ground-glass parenchymal involvement in both lower lobes (arrows), affecting less than 30% of the lung parenchyma.
The distribution of thromboembolic material was recorded as left, right or bilateral. The presence of vascular signs potentially indicative of right heart overload (dilation of the right heart chambers, straightening of the interventricular septum and reflux of contrast into the inferior vena cava)18 (Fig. 1) and the presence or absence of pleural or pericardial effusion were also assessed. The right heart chambers were measured on a 4-chamber plane, and right ventricular dilation was considered to be present when the right ventricular chamber was larger than the left — that is, when the right/left ventricle ratio exceeded 1.18 Straightening of the interventricular septum was considered to be present with the loss of the normal right convexity thereof.18
Pulmonary involvement on the chest CT in patients with COVID-19 was classified by dominant pattern into four groups: a) normal; b) predominance of ground-glass opacities; c) predominance of consolidations; and d) predominance of consolidations with architectural distortion. In accordance with the Fleischner Society's Glossary of Terms, ground-glass opacity was defined as increased pulmonary attenuation that does not obscure the blood vessels or airway, and consolidation was defined as pulmonary opacity that obscures the blood vessels and airway walls.19 Architectural distortion was considered to be a combination of interlobular septal thickening, traction bronchiectasis, loss of volume, pleural thickening, reticulation and subpleural bands. These findings are reported mainly in late-stage disease20 and may or may not be associated with consolidations.
Severity of involvement was classified according to extent of lesions in the lung parenchyma as: a) normal: no involvement; b) mild: <30% involvement of the lung parenchyma (Fig. 3); c) moderate: 30–60% involvement (Fig. 2); or d) severe: >60% involvement of the lung parenchyma (Fig. 1).20–24
Statistical analysisStatistical analysis was performed with SPSS software (IBM, Inc.). The unpaired Student's t test was used to compare continuous variables with a normal distribution, and the χ2 test was used to compare nominal variables. Statistical significance was set at p < 0.05.
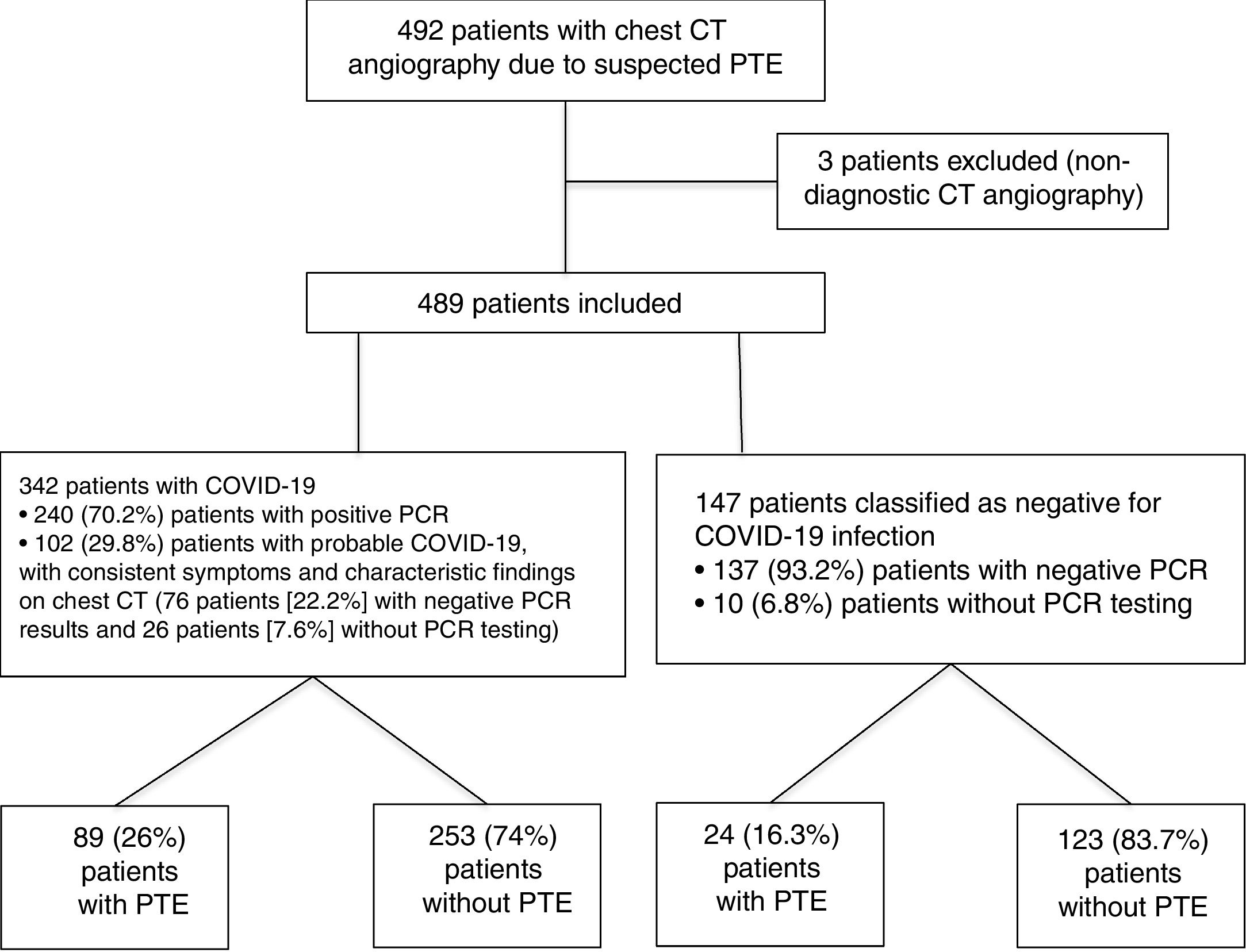
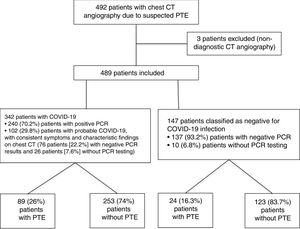
ResultsA total of 492 CT angiography scans of pulmonary arteries were performed due to suspected PTE in a 1.5-month period during the COVID-19 pandemic, from 15 March to 30 April 2020. Three of them were excluded because they were not valid for diagnosis (two due to suboptimal vascular opacification and the third due to significant movement artefacts). Of the total of 489 CT angiography scans included in the study, 342 (69.9%) corresponded to patients diagnosed with COVID-19, and 147 (30.1%) corresponded to patients without COVID-19. Within the COVID-19 group, 240 (70.2%) patients were classified as confirmed COVID-19 patients, with positive PCR results, and 102 (29.8%) patients were classified as probable COVID-19 patients, of which 76 (22.2%) had negative PCR results and 26 (7.6%) had not undergone PCR testing. Within the COVID-19-negative group, 137 (93.2%) patients had negative PCR results and 10 (6.8%) had not undergone PCR testing. The flow chart of the patients included is shown in Fig. 4.
Acute PTE was seen on pulmonary CT angiography in 89 of the 342 patients with COVID-19 had (26%, 95% confidence interval [CI], 21.7−30.1%), and 24 of the 147 patients without COVID-19 (16.3%, 95% CI, 11.2−23.1%). This difference was statistically significant (p = 0.0197). Of the 89 patients with PTE in the COVID-19 group, PCR was positive in 68 (76.4%) patients, negative in 17 patients and not performed in 4 (4.5%) patients. PCR was negative in the 24 patients with PTE in the COVID-19-negative group.
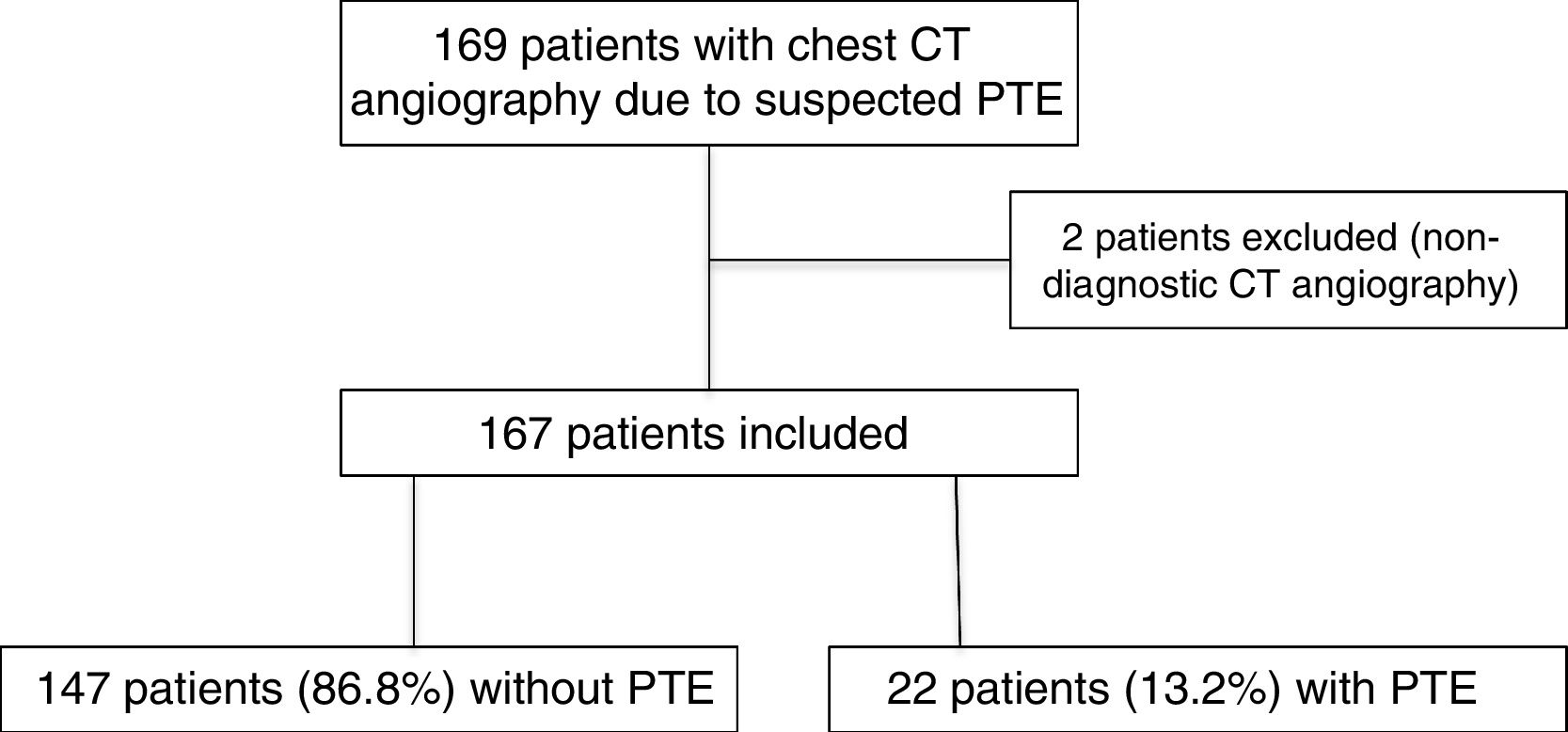
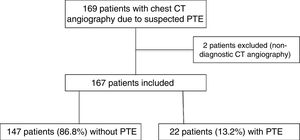
In 2019, 169 CT angiography scans of pulmonary arteries were performed due to clinical suspicion of PTE in the same period. Two of these were excluded because they were inconclusive for diagnosis due to insufficient vascular opacification (Fig. 5). Among the 167 CT angiography scans included, 22 patients (13.2%, 95% CI, 9–19%) showed PTE. The difference in prevalence of PTE in 2019 (13.2%) and in the COVID-19-negative group in 2020 (16.3%) did not attain statistical significance (p = 0.43).
In the 2020 patients, PTE was more common in males, both in the COVID-19 group (65.2%) and in the COVID-19-negative group (58.3%) (p = 0.54). The patients in the two groups had a similar mean age (62.4 ± 16 years versus 67.2 ± 17.9 years, respectively, p = 0.21) (Table 1).
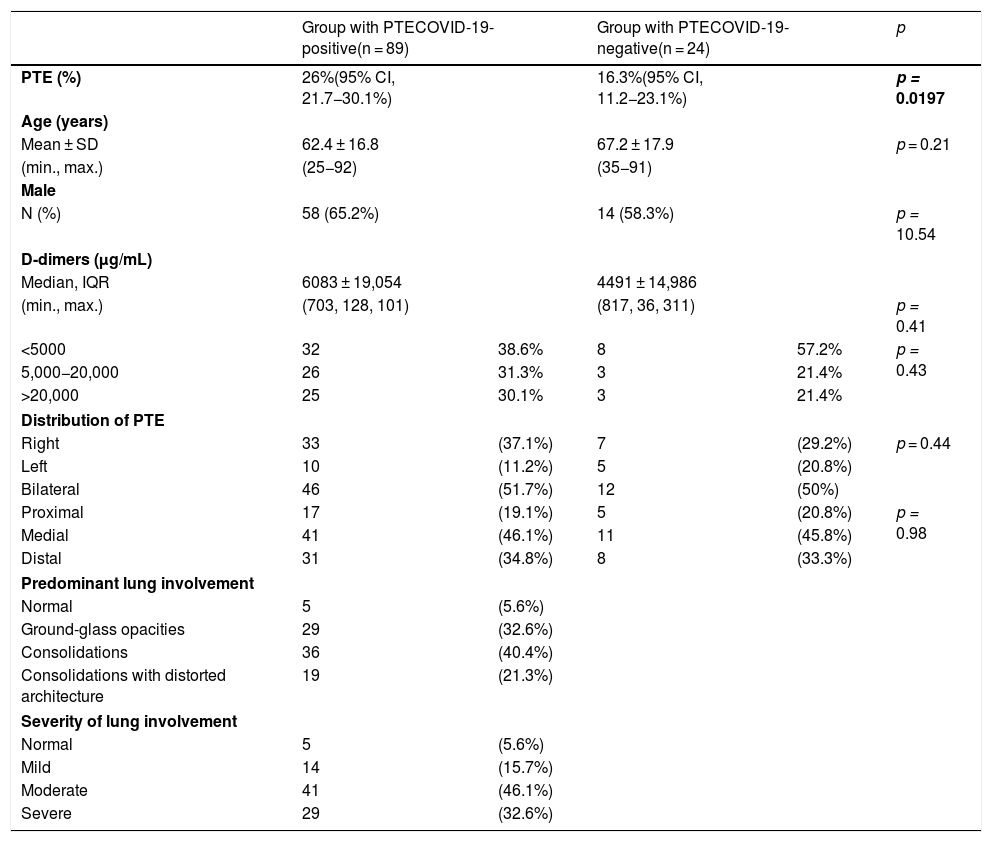
Characteristics of patients with acute pulmonary thromboembolism on pulmonary CT angiography by COVID-19-positive or COVID-19-negative group.
| Group with PTECOVID-19-positive(n = 89) | Group with PTECOVID-19-negative(n = 24) | p | |||
|---|---|---|---|---|---|
| PTE (%) | 26%(95% CI, 21.7−30.1%) | 16.3%(95% CI, 11.2−23.1%) | p = 0.0197 | ||
| Age (years) | |||||
| Mean ± SD | 62.4 ± 16.8 | 67.2 ± 17.9 | p = 0.21 | ||
| (min., max.) | (25−92) | (35−91) | |||
| Male | |||||
| N (%) | 58 (65.2%) | 14 (58.3%) | p = 10.54 | ||
| D-dimers (μg/mL) | |||||
| Median, IQR | 6083 ± 19,054 | 4491 ± 14,986 | |||
| (min., max.) | (703, 128, 101) | (817, 36, 311) | p = 0.41 | ||
| <5000 | 32 | 38.6% | 8 | 57.2% | p = 0.43 |
| 5,000−20,000 | 26 | 31.3% | 3 | 21.4% | |
| >20,000 | 25 | 30.1% | 3 | 21.4% | |
| Distribution of PTE | |||||
| Right | 33 | (37.1%) | 7 | (29.2%) | p = 0.44 |
| Left | 10 | (11.2%) | 5 | (20.8%) | |
| Bilateral | 46 | (51.7%) | 12 | (50%) | |
| Proximal | 17 | (19.1%) | 5 | (20.8%) | p = 0.98 |
| Medial | 41 | (46.1%) | 11 | (45.8%) | |
| Distal | 31 | (34.8%) | 8 | (33.3%) | |
| Predominant lung involvement | |||||
| Normal | 5 | (5.6%) | |||
| Ground-glass opacities | 29 | (32.6%) | |||
| Consolidations | 36 | (40.4%) | |||
| Consolidations with distorted architecture | 19 | (21.3%) | |||
| Severity of lung involvement | |||||
| Normal | 5 | (5.6%) | |||
| Mild | 14 | (15.7%) | |||
| Moderate | 41 | (46.1%) | |||
| Severe | 29 | (32.6%) | |||
CI: confidence interval; IQR: interquartile range; max.: maximum value; min.: minimum value; SD: standard deviation.
Pulmonary emboli in COVID-19 patients showed a bilateral distribution in 46 (51.7%) patients, a right-sided distribution in 33 (37.1%) patients and a left-sided distribution in 10 (11.2%) patients. They affected the proximal pulmonary arterial tree in 17 (19.1%) patients, the medial pulmonary arterial tree in 41 (46.1%) patients and the distal pulmonary arterial tree in 31 (34.8%) patients. The distribution and location of the pulmonary emboli showed no significant differences compared to the COVID-19-negative group (Table 1).
Eleven patients (9.7%) showed signs of right heart overload on CT: 4 patients with confirmed COVID-19 (5.9%), 2 patients with probable COVID-19 (9.5%) and 5 patients from the group without COVID-19 (20.8%), though these differences did not attain statistical significance (p = 0.26). The distribution was bilateral in 9 (81.8%) patients and affected the proximal pulmonary arterial tree in 7 (63.6%) patients, the medial pulmonary arterial tree in 3 (27.3%) patients and the distal pulmonary arterial tree in 1 (9.1%) patient. The extent of lung involvement in the COVID-19 group was severe in 1 patient, moderate in 3 patients and mild in 2 patients.
All PTE patients in both groups showed elevated d-dimer levels (median 6083 μg/mL in the group of patients with COVID-19 and 4491 μg/mL in the group without COVID-19 infection). However, the difference in elevation was not significant (p = 0.41) (Table 1).
The mean time elapsed from the onset of symptoms to the appearance of PTE was 19.2 ± 11.2 days in patients with COVID-19.
Parenchymal involvement in COVID-19 patients with PTE was classified as normal in 5 (5.6%) patients, with a predominance of ground-glass opacities in 29 (32.6%) patients, a predominance of alveolar consolidations in 36 (40.4%) patients, and featuring consolidations with architectural distortion in 19 (21.3%) patients. Severity by extent of lung involvement was classified as normal in 5 (5.6%) patients, mild in 14 (15.7%) patients, moderate in 41 (46.1%) patients and severe in 29 (32.6%) patients.
No statistically significant differences were found by distribution of PTE between the proximal, medial or distal pulmonary arterial tree and the extent of COVID-19 involvement (normal, mild, moderate or severe) (p = 0.78), or with the type of parenchymal involvement (normal, ground-glass opacities, consolidations or consolidations with architectural distortion) (p = 0.06).
DiscussionOur study confirms the high prevalence of acute pulmonary embolism in patients with COVID-19 (26%) (95% CI, 21.7−30.1%), in accordance with the published scientific literature.4,6,25 The prevalence of PTE in patients with COVID-19 is higher than that in patients without COVID-19 (26% versus 16%), which confirms the data published in other series.4,26
Multiple scientific references have reported that patients with COVID-19 are in an inflammatory status in which, among other mechanisms, activation of coagulation and situations of endothelial dysfunction are reported, leading to an increased risk of thromboembolic events4,27, to which are added other risk factors such as bed confinement, mechanical ventilation and intensive care unit (ICU) admission.4,6,8,27–29 Probably in relation to the presence of these known risk factors, during the study period which coincided with the period of greatest intensity of the pandemic, the demand for CT angiography of pulmonary arteries virtually tripled compared to the same period in the previous year (492 versus 169 CT angiography scans).
All patients enrolled in our study showed elevated d-dimer levels corrected for age (median of 6083 μg/mL in the group of COVID-19 patients and 4491 μg/mL in the group of non−COVID-19 patients), though the difference was not significant (p = 0.41). Some authors have suggested that elevated d-dimer levels may be a prognostic marker of the course of the disease, linking it to activation of coagulation in response to an inflammatory response syndrome in patients with COVID-19 or even as a direct result of SARS-CoV-2 itself.4,7,27,30
On average, PTE was diagnosed 19 days after the onset of symptoms. According to various authors, in patients with COVID-19 whose condition worsens, this is usually seen from the second week of the onset of symptoms27 and typically manifests as distress, sepsis or septic shock.
Notably, 94% of patients with COVID-19 and PTE showed lung involvement on a chest CT scan. Of these, 32.6% had ground-glass opacities, 40.4% had consolidations and 21.3% had consolidations and distorted lung architecture. Put differently, 61.7% had consolidations, reported as a fundamental finding in the peak phase of the disease, from 9 to 13 days after the onset of symptoms.20–24 Meanwhile, most patients with COVID-19 and PTE (78.7%) presented a moderate to severe extent of lung involvement on CT — that is, involvement of at least 30% of the lung parenchyma. However, other authors have found no significant differences between the presence of PTE and the extent of lung involvement.31
All of the above underscores the importance of radiological assessment in the management of patients with COVID-19, both to assess the extent of pulmonary involvement and to rule out additional complications, such as PTE, or even other diseases.6,30,32,33 Some authors have suggested that pulmonary CT angiography should be indicated in all patients with COVID-19 with elevated d-dimers.31
Our study has limitations stemming from its retrospective design, the inclusion of patients with probable COVID-19 in the same group as patients with COVID-19 confirmed with positive PCR results, and the heterogeneity of the population enrolled, which included A&E, hospitalised and ICU patients. A detailed study of these groups could clarify whether any of them is at higher risk. Moreover, the percentage of lung parenchyma involvement was calculated in an approximate and subjective way, which could limit its reproducibility. There are also difficulties in assessing the distal pulmonary arterial tree, especially in patients with greater parenchymal involvement, who are usually the most severely ill, with greater respiratory failure and, therefore, more respiratory movement artefacts. Finally, we would like to mention that we did not compare the extent of lung parenchyma involvement or other semiological characteristics of lung involvement on CT in patients with SARS-CoV-2 infection with or without PTE.
ConclusionThe prevalence of PTE is higher in patients with COVID-19. This higher prevalence may be related to the severity of the extent of lung parenchyma involvement. However, no significant differences were found in the location of embolic material or in the degree of elevation of d-dimers in patients with PTE and COVID-19 infection compared to patients with PTE without COVID-19. Further studies are required to determine which factors are associated with PTE in patients with COVID-19 and to better define indications for pulmonary CT angiography.
AuthorshipResponsible for study integrity: EMC, TYRO, LIS.
Study conception: EMC, TYRO, MPN, LIS.
Study design: EMC, TYRO, MPN, SBN, LIS.
Data collection: EMC, TYRO, CCCRG, LIS.
Data analysis and interpretation: EMC, TYRO, MPN, SBN, CCCGR, LIS.
Statistical processing: EMC, TYRO, CCCGR, LIS.
Literature search: EMC, TYRO, SBN, LIS.
Drafting of the manuscript: EMC, TYRO, MPN, LIS.
Critical review of the manuscript with intellectually significant contributions: EMC, TYRO, MPN, SBN, CCCGR, LIS.
Approval of the final version: EMC, TYRO, MPN, SBN, CCCGR, LIS.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Martínez Chamorro E, Revilla Ostolaza TY, Pérez Núñez M, Borruel Nacenta S, Cruz-Conde Rodríguez-Guerra C, Ibáñez Sanz L. Tromboembolismo pulmonar en pacientes con COVID-19: estudio de prevalencia en un hospital terciario. Radiología. 2021;63:13–21.