The MGuardTM stent, which has a microscopic polymer mesh coating, is intended to reduce the distal embolization of fragments during percutaneous coronary intervention (PCI) in saphenous vein grafts (SVG). This study evaluated the early and late clinical outcomes of patients undergoing PCI in SVG with MGuardTM stents vs. drug-eluting stents (DES).
MethodsObservational, retrospective study conducted at two tertiary centers, involving a cohort of patients with SVG lesions submitted to elective or emergency treatment with MGuardTM stents or DES.
ResultsA total of 271 patients were included, of whom 220 were treated with DES. The MGuardTM group had a higher proportion of women (25.5% vs. 10.5%; p = 0.01), with a mean age of 65.0 ± 13.9 years vs. 69.0 ± 9.6 years (p = 0.06). The DES group more frequently used distal protection filter (5.8% vs. 10.0%; p = 0.001). Angiographic success was attained in most cases (96.2% vs. 98.0%; p = 0.22). The MGuardTM group had lower rates of early major adverse cardiovascular events (MACE) than the DES group (1.9% vs. 13.6%; p = 0.01), due solely to the lower incidence of periprocedural infarction. However, the MACE rate at 1 year was higher in the MGuardTM group (14.3% vs. 4.4%; p = 0.01) at the expense of a higher rate of target lesion revascularization (7.1% vs. 1.3%; p = 0.048).
ConclusionsThe use of the MGuardTM stent resulted in a reduction of events during hospitalization; however, in the long term the DES were superior in reducing major outcomes, mainly the need for target lesion revascularization.
O stent MGuardTM, revestido por malha polimérica microscópica, tem a finalidade de reduzir a embolização distal de fragmentos durante a intervenção coronária percutânea (ICP) em enxertos de veia safena (EVS). Avaliamos os desfechos clínicos precoces e tardios de pacientes submetidos à ICP de EVS com stents MGuard™ vs. stents farmacológicos (SF).
MétodosEstudo observacional, retrospectivo, realizado em dois centros terciários, envolvendo uma coorte de pacientes com lesões em EVS, tratados de forma eletiva ou de emergência com stents MGuard™ ou SF.
ResultadosForam incluídos 271 pacientes, sendo 220 tratados com SF. O Grupo MGuard™ apresentou maior proporção de mulheres (25,5% vs. 10,5%; p=0,01), com média de idades de 65,0 ± 13,9 anos vs. 69,0 ± 9,6 anos (p = 0,06). O Grupo SF utilizou com maior frequência filtro de proteção distal (5,8% vs. 10,0%; p = 0,001). Sucesso angiográfico foi obtido na maioria dos casos (96,2% vs. 98,0%; p = 0,22). O Grupo MGuard™ teve menores taxas de eventos cardiovasculares adversos maiores (ECAM) na fase hospitalar que o Grupo SF (1,9% vs. 13,6%; p = 0,01) devido exclusivamente à menor incidência de infarto periprocedimento. Entretanto, a taxa de ECAM em 1 ano foi maior no grupo MGuard™ (14,3% vs. 4,4%; p = 0,01) à custa de maior taxa de revascularização da lesão alvo (7,1% vs. 1,3%; p = 0,048).
ConclusõesA utilização de stent MGuard™ resultou em redução de eventos na fase hospitalar, porém, no longo prazo, os SF foram superiores em reduzir desfechos maiores, sobretudo a necessidade de revascularização da lesão alvo.
The interventional treatment of saphenous vein grafts (SVG) remains a challenge, even in the modern practice of interventional cardiology, due to higher rates of acute complications and late clinical outcomes, compared to intervention in native coronary arteries.1
Percutaneous coronary intervention (PCI) in this scenario has unique technical challenges, due to the presence of friable lesion material prone to distal embolization during the procedure, resulting in greater risk of complications, such as the no-reflow phenomenon and periprocedural acute myocardial infarction (AMI).2,3
Although drug-eluting stents (DES) have promoted the reduction of restenosis rates in virtually all clinical and angiographic scenarios, furthering the results of PCI, there are still doubts about the effectiveness and late safety of these devices when used to treat lesions in SVG.4,5
In this scenario, the use of the MGuardTM stent (InspireMD, Tel-Aviv, Israel), coated with a microscopic ultrathin mesh and aiming to reduce distal embolization of fragments and thus, the rates of acute and late complications post-PCI, has been highlighted.6–8
This analysis aimed to evaluate the early and late clinical outcomes of PCI with MGuardTM stenting vs. DES in SVG.
MethodsStudy design and target populationThis was an observational retrospective study, carried out at two tertiary centers, involving a cohort of patients with lesions in SVG treated with MGuardTM stent or DES.
The population of this analysis included patients undergoing elective or emergency PCI of SVG, in the routine of the invasive cardiology services of the centers involved, between 2007 and 2012. Patients in the study were selected from the local database; data collection was performed by reviewing medical charts, reports, and angiograms, as well as by telephone contact.
Study devicesThe MGuardTM stent is a metallic 316L stainless steel laser-cut platform, with strut thickness of around 80 to 95 uM, covered by a double net of microscopic polyethylene terephthalate fibers, which are adhered to the metallic struts. During stent implantation, the net stretches and slides over the expanding stent struts, creating pores ≤ 200 microns in diameter. MGuardTM stents are available in diameters ranging from 2.5 to 4.0mm and extensions of 12 to 39mm. Details about this stent have been previously published.3,6–8
Several drug-eluting stents were used in the DES group, both first and second generation stents, especially the sirolimus, zotarolimus, everolimus, and paclitaxel-eluting stents.
ProcedureIn general, interventions were carried out according to the current guidelines.9 The decision to perform pre- and/or post-dilation was made at the interventionist's discretion, as well as the use of a distal protection filter and glycoprotein IIb/IIIa inhibitors.
The dual antiplatelet pre-treatment procedure consisted of an aspirin (100 to 300mg) and clopidogrel (300 to 600mg) loading dose. After the intervention, acetylsalicylic acid 100mg daily was prescribed indefinitely and clopidogrel 75mg daily was maintained for 12 months. During the PCI, antithrombin therapy consisted of unfractionated heparin at a dose of 100 IU/kg (or 70 IU/kg, in the case of glycoprotein IIb/IIIa inhibitor use), aiming to achieve activated clotting time > 250seconds (or between 200 and 250seconds in the case of glycoprotein IIb/IIIa inhibitor use).
Angiographic analysisQualitative and quantitative angiographic analyses were performed offline by an experienced interventionist, following a predefined protocol. In general, pre- and post-procedure coronary angiographies were performed after intracoronary nitroglycerin administration (50 to 200μg) in at least two corresponding orthogonal projections, which were stored in the DICOM digital format.
Coronary flow was determined according to the criteria of the Thrombolysis in Myocardial Infarction (TIMI) study.
The quantitative coronary angiography (QCA) analysis was performed using a dedicated computer program with semi-automatic lumen edge detection (QAngio XA, Medis Medical Imaging System, Leiden, The Netherlands).
Clinical follow-up and definitionsThe aim of the current analysis was to evaluate the rate of major adverse cardiovascular events (MACE), defined as the occurrence of death, nonfatal myocardial infarction, and target lesion revascularization (TLR), both in the in-hospital and late follow-up (1 year).
Nonfatal AMI was defined as an elevation greater than three-fold in the reference laboratory values of creatine kinase MB isoenzyme (CK-MB) levels. Target lesion revascularization was defined as a new revascularization by PCI or surgical graft in a previously treated segment. Angiographic success was defined as the successful implantation of an MGuardTM stent in the target lesion.
Statistical analysisContinuous variables are shown as mean ± standard deviation and compared using Student's t-test. Categorical variables are reported as absolute numbers and percentages and compared using the Chi-squared test or Fisher's exact test, as appropriate, with p < 0.05 being considered statistically significant. Analyses were performed using the Statistical Package for Social Sciences (SPSS) program, version 16.0 (SPSS Inc., Chicago, USA).
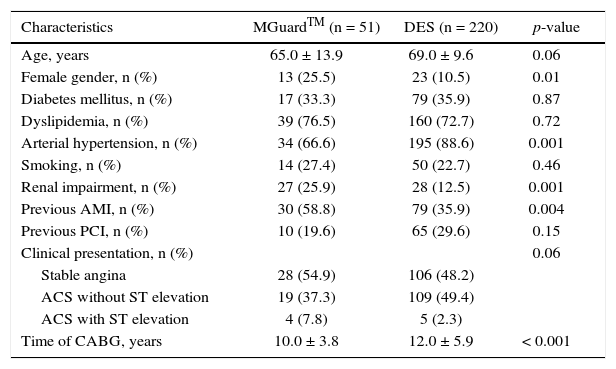
ResultsA total of 271 patients were analyzed, with 220 in the DES Group. The mean age of patients was 65.0 ± 13.9 years in the MGuardTM group and 69.0 ± 9.6 years in the DES group (p = 0.06). The MGuardTM group had a higher proportion of female patients (25.5% vs. 10.5%; p = 0.01), previous myocardial infarction (58.8% vs. 35.9%; p = 0.003) and renal impairment (25.9% vs. 12.5%; p = 0.001), and a lower proportion of hypertensive patients (66.6% vs. 88.6%; p = 0.001). The time since coronary artery bypass graft was shorter in the MGuardTM group (10.0 ± 3.8 years vs. 12.0 ± 5.9 years; p < 0.001). The clinical characteristics of patients are shown in Table 1.
Basal clinical characteristics.
| Characteristics | MGuardTM (n = 51) | DES (n = 220) | p-value |
|---|---|---|---|
| Age, years | 65.0 ± 13.9 | 69.0 ± 9.6 | 0.06 |
| Female gender, n (%) | 13 (25.5) | 23 (10.5) | 0.01 |
| Diabetes mellitus, n (%) | 17 (33.3) | 79 (35.9) | 0.87 |
| Dyslipidemia, n (%) | 39 (76.5) | 160 (72.7) | 0.72 |
| Arterial hypertension, n (%) | 34 (66.6) | 195 (88.6) | 0.001 |
| Smoking, n (%) | 14 (27.4) | 50 (22.7) | 0.46 |
| Renal impairment, n (%) | 27 (25.9) | 28 (12.5) | 0.001 |
| Previous AMI, n (%) | 30 (58.8) | 79 (35.9) | 0.004 |
| Previous PCI, n (%) | 10 (19.6) | 65 (29.6) | 0.15 |
| Clinical presentation, n (%) | 0.06 | ||
| Stable angina | 28 (54.9) | 106 (48.2) | |
| ACS without ST elevation | 19 (37.3) | 109 (49.4) | |
| ACS with ST elevation | 4 (7.8) | 5 (2.3) | |
| Time of CABG, years | 10.0 ± 3.8 | 12.0 ± 5.9 | < 0.001 |
DES: drug-eluting stent; AMI: acute myocardial infarction; PCI: percutaneous coronary intervention; ACS: acute coronary syndrome; CABG: coronary artery bypass grafting.
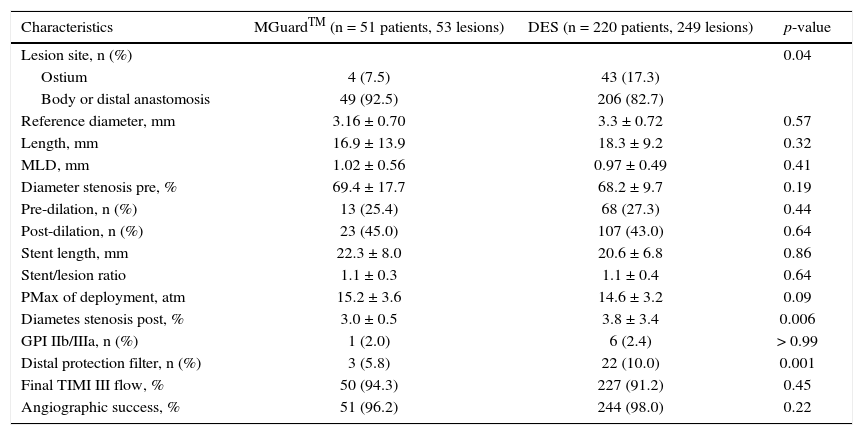
Angiographic and procedural characteristics are shown in Table 2. Most of the treated lesions were located in the body or distal anastomosis of the graft, particularly in the MGuardTM group. Minimal luminal diameter, lesion length, and diameter stenosis were similar between groups. The DES group used distal protection filter more often when compared to the MGuardTM group (5.8% vs. 10.0%; p = 0.001), but the use of glycoprotein IIb/IIIa inhibitors showed no difference between groups (2.0% vs. 2.7%; p > 0.99). Angiographic success was attained in most cases in both groups (96.2% vs. 98%; p = 0.22).
Angiographic and procedural characteristics.
| Characteristics | MGuardTM (n = 51 patients, 53 lesions) | DES (n = 220 patients, 249 lesions) | p-value |
|---|---|---|---|
| Lesion site, n (%) | 0.04 | ||
| Ostium | 4 (7.5) | 43 (17.3) | |
| Body or distal anastomosis | 49 (92.5) | 206 (82.7) | |
| Reference diameter, mm | 3.16 ± 0.70 | 3.3 ± 0.72 | 0.57 |
| Length, mm | 16.9 ± 13.9 | 18.3 ± 9.2 | 0.32 |
| MLD, mm | 1.02 ± 0.56 | 0.97 ± 0.49 | 0.41 |
| Diameter stenosis pre, % | 69.4 ± 17.7 | 68.2 ± 9.7 | 0.19 |
| Pre-dilation, n (%) | 13 (25.4) | 68 (27.3) | 0.44 |
| Post-dilation, n (%) | 23 (45.0) | 107 (43.0) | 0.64 |
| Stent length, mm | 22.3 ± 8.0 | 20.6 ± 6.8 | 0.86 |
| Stent/lesion ratio | 1.1 ± 0.3 | 1.1 ± 0.4 | 0.64 |
| PMax of deployment, atm | 15.2 ± 3.6 | 14.6 ± 3.2 | 0.09 |
| Diametes stenosis post, % | 3.0 ± 0.5 | 3.8 ± 3.4 | 0.006 |
| GPI IIb/IIIa, n (%) | 1 (2.0) | 6 (2.4) | > 0.99 |
| Distal protection filter, n (%) | 3 (5.8) | 22 (10.0) | 0.001 |
| Final TIMI III flow, % | 50 (94.3) | 227 (91.2) | 0.45 |
| Angiographic success, % | 51 (96.2) | 244 (98.0) | 0.22 |
DES: drug-eluting stents; MLD: minimal luminal diameter; PMax: maximum pressure; GPI IIb/IIIa: glycoprotein IIb/IIIa inhibitors; TIMI: Thrombolysis in Myocardial Infarction.
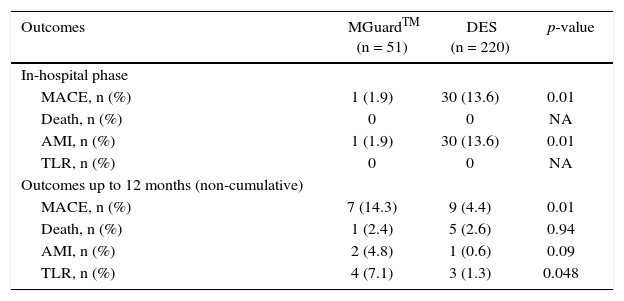
The MGuardTM group had better in-hospital clinical outcomes (MACE: 2.0% vs. 13.6%; p = 0.01; odds ratio – OR: 7.89; 95% confidence interval – 95% CI: 1.05-59.3) due exclusively to a lower incidence of periprocedural myocardial infarction (2.0% vs. 13.6%; p = 0.014), and showed no difference regarding mortality rates and TLR (Table 3).
In-hospital and late clinical outcomes.
| Outcomes | MGuardTM (n = 51) | DES (n = 220) | p-value |
|---|---|---|---|
| In-hospital phase | |||
| MACE, n (%) | 1 (1.9) | 30 (13.6) | 0.01 |
| Death, n (%) | 0 | 0 | NA |
| AMI, n (%) | 1 (1.9) | 30 (13.6) | 0.01 |
| TLR, n (%) | 0 | 0 | NA |
| Outcomes up to 12 months (non-cumulative) | |||
| MACE, n (%) | 7 (14.3) | 9 (4.4) | 0.01 |
| Death, n (%) | 1 (2.4) | 5 (2.6) | 0.94 |
| AMI, n (%) | 2 (4.8) | 1 (0.6) | 0.09 |
| TLR, n (%) | 4 (7.1) | 3 (1.3) | 0.048 |
DES: drug-eluting stent; MACE: major adverse cardiovascular events; AMI: acute myocardial infarction; TLR: target-lesion revascularization; NA: not applicable.
However, contrary to the initial in-hospital outcomes, the MACE rate at 1 year of follow-up was statistically higher in the MGuardTM group (14.3% vs. 4.4%, p = 0.01; OR: 0.24; 95 CI: 0.07-0.76). At the analysis of these late clinical outcomes, there was no difference in the incidence of death (2.4% vs. 2.6%; p = 0.94) and AMI (4.8% vs. 0.6%; p = 0.09), but there was a higher TLR rate in the MGuardTM group (7.1% vs. 1.3%; p = 0.048).
DiscussionThe main finding of the present study is the fact that the MGuardTM stent has demonstrated its effectiveness in the immediate phase of PCI, with a reduction in periprocedural AMI. On the other hand, as it is not an antiproliferative drug-eluting device, its efficacy in the medium term was reduced when compared to DES, which are now considered the gold standard for restenosis prevention after PCI.
The performance of the MGuardTM device has been tested in several studies. Grube et al. published a prospective study of 41 patients evaluating the MGuardTM stent, and PCI in saphenous vein grafts represented 56% of the procedures. In that study, no patient received glycoprotein IIb/IIIa inhibitors or distal embolic protection device. The angiographic and procedure success rates were 100% and 95%, respectively. After 6 months of follow-up, 19.5% of patients were submitted to target vessel revascularization. At the later follow-up of these patients (12 months to 27 months), only one additional target vessel revascularization was observed.10
Costa Jr. et al., in an angiographic study protocol at 6 months after MGuardTM stent implantation in 30 patients, found a mean age of patients of 63 years, with 38% of diabetics. None of the patients used distal embolic protection devices. The late luminal loss was 1.0 ± 0.4mm and the percentage of in-stent volume obstruction was 28.5 ± 15.6%.11
Guimarães et al. tested the MGuardTM device in a retrospective analysis of 65 patients, of whom 68% of the interventions were in SVG. The angiographic success rate was 91.8% and, in the late follow-up (2.6 ± 1.4 years), the TLR rate was 12.3%.7
The findings of these studies are similar to the present one, in which angiographic success was attained in 98% of procedures and the rate of repeat revascularization 12 months after the MGuardTM device implant was 7.1%.
Regarding the performance of the DES when compared to conventional stents, the RRISC (Reduction of Restenosis in Saphenous Vein Grafts with Cypher Sirolimus-Eluting Stent) randomized trial selected 75 patients (96 lesions in saphenous vein grafts) to receive the CypherTM DES or bare-metal stents. In this analysis, the use of DES was associated with 37% reduction in the risk of binary restenosis (11.3% vs. 30.6%; p < 0.024), with similar rates of death and AMI in the short-term follow-up.12
Regarding the present analysis, in-hospital AMI rates were favorable for MGuardTM when compared to the DES group (2.0% vs. 13.6%; p = 0.01), a finding that may be explained by the fact that the device is coated with an ultrathin microscopic net, which may have been able to reduce distal embolization and the resulting complications. As for the rate of AMI in the long-term follow-up (4.8% vs. 0.6%; p = 0.09), the lack of statistical significance may have resulted from the number of patients included in the study.
Esteves et al. published a comparative, nonrandomized study of PCI in saphenous vein grafts with 38 patients (16 in the MGuardTM group and 22 in the group with conventional stenting with distal embolic protection filter), demonstrating similar MACE rates between the groups.6
Assali et al. published the results of the comparison between 68 patients treated with DES and 43 patients who received bare-metal stents for lesions in SVG. At the 2-year follow-up, the use of bare-metal stents was associated with a marked reduction in event-free survival (58.1% in the bare-metal stent group vs. 79.4% in the DES group; p = 0.02), essentially due to a decrease in the TLR rate (14.7% vs. 32.6%; p = 0.03).13
In another randomized study, Brilakis et al. compared TaxusTM DES with bare-metal stents in PCI of SVG (39 patients in the bare-metal stent group and 41 patients in the DES group). In the follow-up (mean of 1.5 years), the use of DES was associated with significant reduction in MACE rate (28% vs. 5%; relative risk – RR = 0.38; 95% CI: 0.15 to 0.74; p < 0.003), as well as a reduction in TLR (46% vs. 22%; RR = 0.65; 95% CI: 0.42-0.96; p < 0.03), with no difference in infarction and mortality rates.14
Two recent meta-analyses including more than 5,000 patients found better outcomes with use of DES compared to the use of bare-metal stents, mainly at the expense of lower rates of TLR.15,16
The present study is in line with those described above, with higher MACE rates at 12 months in the MGuardTM group (non-drug-eluting device) compared to the DES group, at the expense mainly of a higher incidence of TLR (7.1% vs. 1.3%; p = 0.048). Considering the higher event rates in the treatment of venous grafts compared to intervention in native coronary arteries, and the mean age of patients (≥ 65 years in both groups), it is observed that the rates of MACE and, mainly, of TLR in the present study can be considered acceptable.
Study limitationsThis is a retrospective, nonrandomized study, whose main limitations are the small number of patients and a heterogeneous population. A multicenter, randomized trial with a longer follow-up is necessary to evaluate the efficacy of the MGuardTM device in the long term compared to DES.
ConclusionsThe use of the dedicated MGuardTM stent resulted in a reduction of clinical events during hospitalization. However, in the long term, drug-eluting stents were superior in reducing major adverse outcomes, especially regarding the need for target lesion revascularization.
The results suggest that the next generation of MGuardTM stents, with the use of anti-proliferative drugs, may combine the best of both devices.
Funding sourcesNone declared.
Conflicts of interestThe authors declare no conflicts of interest.
Peer review under the responsibility of Sociedade Brasileira de Hemodinâmica e Cardiologia Intervencionista.