Severe stenosis of the proximal left anterior descending artery (LAD) is classified as a high-risk lesion, as it may affect a large part of the left ventricular myocardium. Second-generation drug-eluting stents (DES) have been shown to be more effective and safer when compared to bare-metal or first-generation ones. There are few reports in the literature on the use of these devices for the treatment of isolated lesions in the proximal LAD.
MethodsObservational and prospective study, which included single-vessel patients with de novo lesions in the proximal LAD, electively treated with second-generation DES. In-hospital and late clinical outcomes were evaluated.
ResultsSeventy patients were included, most of them males (70%), with a mean age of 65.4 ± 11.2 years and a high prevalence of diabetes (37%). The most common clinical presentation was stable angina (57.1%) and half of the lesions were type B2 or C. A total of 70 lesions were treated with 71 stents, with 100% angiographic success. The primary endpoint, consisting of cardiac death, nonfatal infarction, or target-vessel revascularization during the 2.5-year clinical follow-up, occurred in 3% of the patients. Cardiac death was 1.5%, and target-lesion revascularization was required in only 1.5% of the patients.
ConclusionsElective treatment with second-generation DES seems to be a safe option in single-vessel patients with de novo lesions in the proximal LAD, with low rates of adverse cardiac events or need for additional revascularization procedure.
A estenose grave do terço proximal da artéria descendente anterior (ADA) é classificada como lesão de alto risco, visto que pode comprometer grande parte do miocárdio ventricular esquerdo. Os stents farmacológicos (SF) de segunda geração têm demonstrado maior eficácia e segurança quando comparados aos não farmacológicos ou aos de primeira geração. São escassos os relatos na literatura do emprego desses dispositivos para o tratamento de lesões isoladas do terço proximal da ADA.
MétodosEstudo observacional e prospectivo, que incluiu pacientes uniarteriais, portadores de lesão de novo no terço proximal da ADA, tratados eletivamente com SF de segunda geração. Avaliamos os desfechos clínicos hospitalares e tardios.
ResultadosForam incluídos 70 pacientes, sendo a maioria do sexo masculino (70%), com média de idades de 65,4 ± 11,2 anos e com alta prevalência de diabetes (37%). O quadro clínico mais frequente foi angina estável (57,1%) e metade das lesões era do tipo B2 ou C. Foram tratadas 70 lesões com 71 stents, com sucesso angiográfico de 100%. O desfecho primário composto por óbito cardíaco, infarto não fatal ou revascularização do vaso alvo no seguimento clínico de 2,5 anos ocorreu em 3% dos pacientes. A mortalidade cardíaca foi de 1,5%, e a revascularização da lesão alvo foi necessária em apenas 1,5% dos pacientes.
ConclusõesEm pacientes uniarteriais com lesões de novo da ADA proximal, o tratamento eletivo com SF de segunda geração parece ser uma opção segura, com baixas taxas de eventos cardíacos adversos ou necessidade de nova revascularização.
Significant stenosis of the proximal left anterior descending artery (LAD) is classified as a high-risk lesion, as it may affect a large part of the left ventricular myocardium, usually having a more unfavorable prognosis than any other territory affected by coronary artery disease.1
Coronary artery bypass grafting (CABG) surgery, using the left internal thoracic artery for the treatment of proximal LAD lesions, has shown excellent results, with a favorable clinical evolution and a high long-term patency rate.2–5 One potential advantage of CABG is the protection of a large arterial territory, compared to only focal treatment obtained with percutaneous coronary intervention (PCI), with benefits in cases of future progression of the coronary artery disease.6
Meta-analyses of randomized trials and registries7,8 of patients with isolated LAD lesions did not report significant differences in rates of mortality, acute myocardial infarction (AMI), or stroke between CABG with left internal thoracic artery and PCI. However, angina recurrence and the need for additional revascularization were, respectively, three and five times higher with the percutaneous treatment in a clinical follow-up of up to 5 years. It is noteworthy that most of these studies used bare-metal stents, considering the greater efficacy of drug-eluting stents (DES) in reducing target-vessel revascularization (TVR).9
The most recent stents, known as second-generation stents, have been shown to be more effective and, especially, safer in the treatment of coronary artery disease,10–13 with scarce reports in the literature on the use of these devices for the treatment of isolated lesions in the proximal LAD.
MethodsObservational and prospective study, carried out from July 2007 to January 2015, in the Complexo Hospitalar da Real e Benemérita Sociedade Portuguesa de Beneficência de São Paulo, in São Paulo (SP), Brazil, using the SAFIRA (SegurançA e eFIcácia dos stents farmacológicos em uma população do mundo ReAl) registry database. The patients were included after having the Informed Consent clarified, read, and signed.
Single-vessel patients with de novo lesions in the proximal LAD, with indication for elective percutaneous revascularization, treated with second-generation DES were selected. Patients with ST-elevation myocardial infarction, non-ST elevation acute coronary syndrome associated with refractory angina, electrical or hemodynamic instability were excluded.14 Other criteria included previous CABG or PCI, and contraindication to the use of antiplatelet or anticoagulant agents predicted by the protocol.
The PCIs were performed according to the usual techniques and following the current guidelines.14 Lesion evaluation was performed by quantitative angiographic analysis after intracoronary nitrate administration. The lesions were classified according to the American College of Cardiology/American Heart Association definition (A, B1, B2, and C).15 Pre- and post-dilation were used at the interventionist's discretion. All patients received unfractionated heparin at the start of the procedure (70 to 100 U/kg), with additional doses being administered if necessary to maintain an activated coagulation time of 250 to 350seconds. Dual antiplatelet therapy was used in all patients, with the administration of acetylsalicylic acid, 100 to 200mg daily, associated with a P2Y12 inhibitor (clopidogrel, prasugrel, or ticagrelor). After the procedure, acetylsalicylic acid was maintained indefinitely at a dose of 100mg per day. The P2Y12 inhibitor of choice was recommended for a minimum period of 1 year.
The primary endpoint was defined as the rate of major adverse cardiac events (MACE), consisting of cardiac death, nonfatal AMI, or TVR guided by ischemia at the clinical follow-up. All deaths were considered cardiac, unless a non-cardiac cause could be clearly established. AMI was characterized as elevation of creatine kinase isoenzyme MB (CKMB) more than three times the reference value, with or without electrocardiographic alterations.
The secondary endpoints included angiographic success rate (defined by residual stenosis < 20% with final Thrombolysis in Myocardial Infarction - TIMI III flow in the absence of thrombi and dissection] and clinical success rate (defined by angiographic success associated with the absence of MACE) during the in-hospital phase. Other secondary endpoints were definitive or probable stent thrombosis, defined according to the Academic Research Consortium classification;16 major bleeding, evaluated by the TIMI criterion (intracranial bleeding, clinical evidence of bleeding associated with a decrease ≥ 5g/dL, or fatal bleeding);17 target lesion revascularization and all-cause mortality.
Patients were followed after hospital discharge by telephone contact, by medical consultation, or through information obtained from the attending physician, with evaluations being carried out at 1, 6, and 12 months and, after that, annually.
Statistical analysisThe categorical variables were expressed as absolute frequency and percentage, whereas the continuous variables were expressed as mean and standard deviation. The event-free survival curve was estimated using the Kaplan-Meier method. Statistical data analysis was performed using the STATA 14 program (STATA Corp, College Station, USA).
ResultsSeventy single-vessel patients with de novo lesions in the proximal LAD submitted to elective PCI with second-generation DES implantation were included from June 2009 to October 2015.
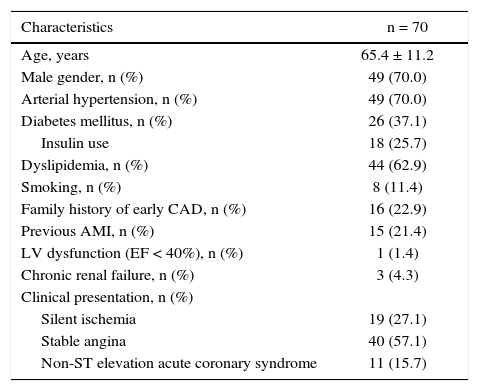
Most patients were males (70%), with a mean age of 65.4 ± 11.2 years and more than one-third (37.1%) were diabetics (Table 1). A minority (1.4%) had moderate or significant ventricular dysfunction and the most frequent clinical presentation was stable angina (57.1%).
Clinical characteristics.
| Characteristics | n = 70 |
|---|---|
| Age, years | 65.4 ± 11.2 |
| Male gender, n (%) | 49 (70.0) |
| Arterial hypertension, n (%) | 49 (70.0) |
| Diabetes mellitus, n (%) | 26 (37.1) |
| Insulin use | 18 (25.7) |
| Dyslipidemia, n (%) | 44 (62.9) |
| Smoking, n (%) | 8 (11.4) |
| Family history of early CAD, n (%) | 16 (22.9) |
| Previous AMI, n (%) | 15 (21.4) |
| LV dysfunction (EF < 40%), n (%) | 1 (1.4) |
| Chronic renal failure, n (%) | 3 (4.3) |
| Clinical presentation, n (%) | |
| Silent ischemia | 19 (27.1) |
| Stable angina | 40 (57.1) |
| Non-ST elevation acute coronary syndrome | 11 (15.7) |
CAD: coronary artery disease; AMI; acute myocardial infarction; LV: left ventricle; EF: ejection fraction.
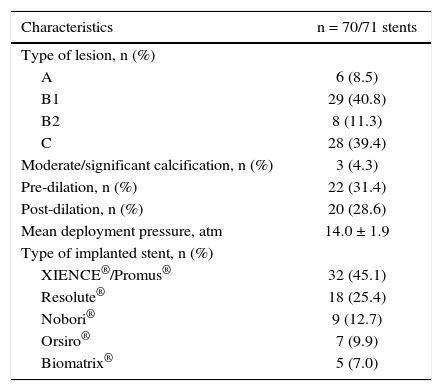
The more complex lesions, types B2 and C, were present in half of the cases. The most often used vascular access was radial (74.3%), followed by femoral access (24.3%); stents with biocompatible polymers were used in approximately 20% of cases (Table 2).
Angiographic and procedural characteristics.
| Characteristics | n = 70/71 stents |
|---|---|
| Type of lesion, n (%) | |
| A | 6 (8.5) |
| B1 | 29 (40.8) |
| B2 | 8 (11.3) |
| C | 28 (39.4) |
| Moderate/significant calcification, n (%) | 3 (4.3) |
| Pre-dilation, n (%) | 22 (31.4) |
| Post-dilation, n (%) | 20 (28.6) |
| Mean deployment pressure, atm | 14.0 ± 1.9 |
| Type of implanted stent, n (%) | |
| XIENCE®/Promus® | 32 (45.1) |
| Resolute® | 18 (25.4) |
| Nobori® | 9 (12.7) |
| Orsiro® | 7 (9.9) |
| Biomatrix® | 5 (7.0) |
The angiographic and clinical success were 100% and 95.7%, respectively. The combined outcome of death, nonfatal AMI, and urgent revascularization occurred in three patients (4.3%), all due to periprocedural non-ST elevation MI. There were no cases of death or need for urgent revascularization during hospitalization. There were no cases of major bleeding.
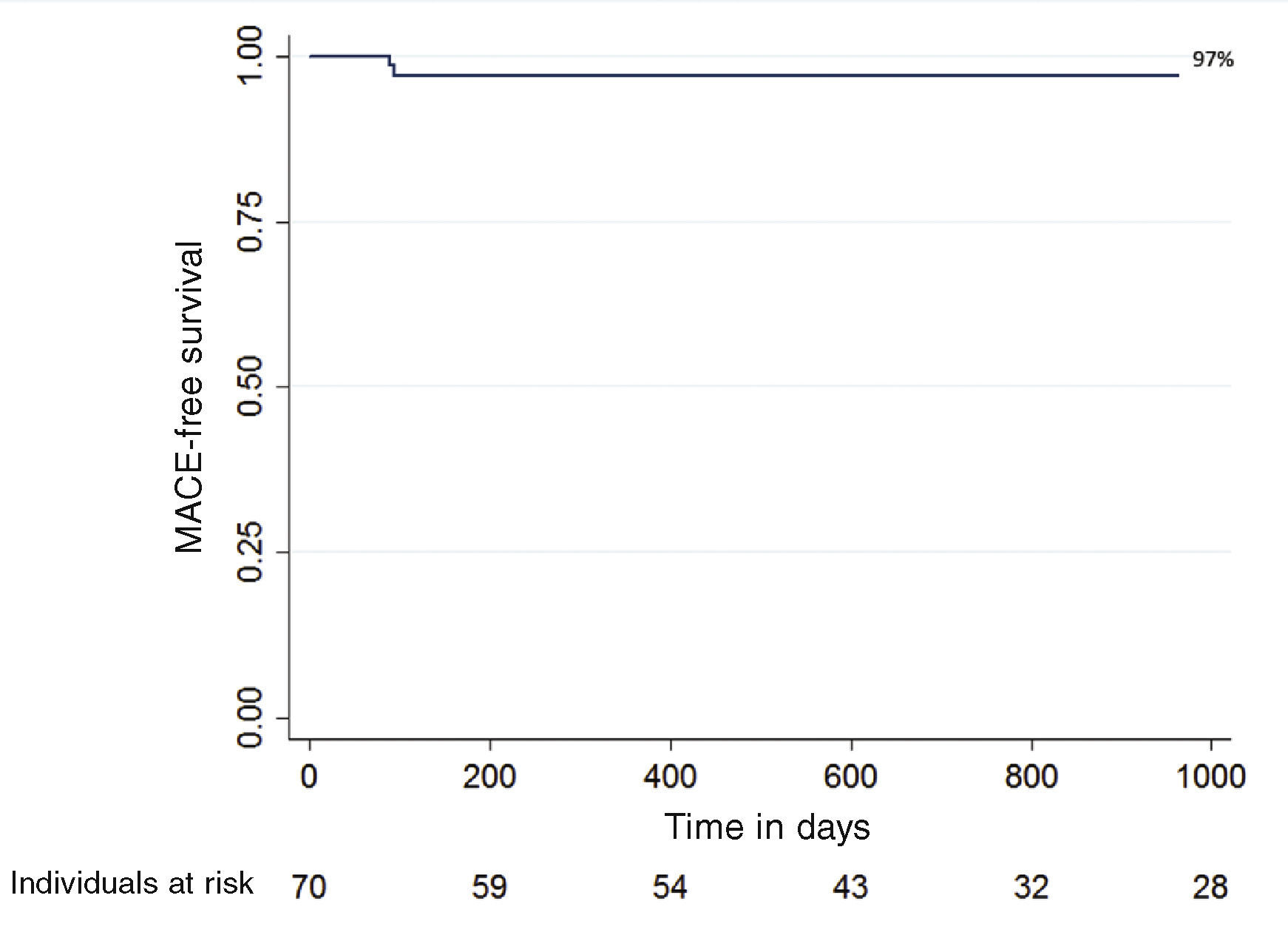
Clinical follow-up was performed in 68 patients (97.1%) during a mean period of 2.5 ± 1.8 years. The combined primary endpoint of cardiac death, nonfatal AMI, or ischemia-driven MI at the clinical follow-up occurred in two patients (3.0%). There were two deaths, one of non-cardiac origin (septic shock), and the other in the immediate postoperative period of the surgical procedure for aortic valve replacement. There were no cases of non-fatal AMI during the follow-up and target-lesion revascularization was necessary in only one patient (1.5%). There were no cases of definite or probable stent thrombosis. The MACE-free probability according to the Kaplan-Meier curve was 97% (Fig. 1).
DiscussionIn the present study, stable patients isolated, proximal, de novo LAD lesions submitted to PCI with second-generation DES had low rates of MACE (3.0%), cardiac mortality (1.5%), and TVR (1.5%) during a 2.5-year clinical follow-up.
The use of DES substantially reduced the need for repeat revascularization when compared to conventional stents. However, as the clinical follow-up data became available, concerns arose regarding the safety of these devices due to an increased incidence of very late thrombosis.18
Second-generation DES were designed to overcome long-term safety issues by employing new coatings with more biocompatible polymers, less toxic antiproliferative drugs, and ultra-thin struts. Compared to the first-generation DES, this technology has provided a significant reduction in the rate of repeated revascularization,19,20 in addition to better safety, with a reduction in the rates of stent thrombosis, AMI, and mortality.21–23
One of the first studies comparing surgical treatment to percutaneous treatment for proximal LAD lesions in stable patients was the MASS (The Medicine, Angioplasty, or Surgery Study) trial,24 which reported a similar rate of death and AMI for balloon angioplasty when compared to CABG. There was, however, a greater need for additional revascularization and more angina in the angioplasty group in a 5-year clinical follow-up.
Two meta-analyses compared the percutaneous treatment with stents CABG using the left internal thoracic artery in patients with proximal LAD lesions. In both, there was no difference in mortality, AMI, or stroke in a follow-up of up to 5 years. However, there was a significant increase in the rate of additional revascularization in the PCI group. It is worth mentioning that most of the studies used bare-metal stents and only one study used first-generation DES.7,8
Some randomized trials and registries compared CABG with PCI using first-generation DES for the treatment of lesions in the proximal LAD. As with bare-metal stents, there was no difference between groups regarding the incidence of MACE, but there was still a greater need for additional revascularization in the DES group.25,26 To the best of the present authors’ knowledge, there are no studies comparing CABG vs. PCI using only second-generation drug-eluting stents in this group of patients.
The present study showed an excellent result in the short and medium terms, with low rates of TVR (1.5%) and cardiac mortality (1.5%). These results are comparable to previously published data regarding surgical revascularization using the left internal thoracic artery, with reported mortality rates between zero and 5.4% and need for additional revascularization in up to 3 years ranging zero to 4.0%.7,8,24
Currently, the indication of PCI for the treatment of proximal LAD lesions is up to seven times more frequent than CABG.26 This fact probably justifies the lack of interest in performing further randomized trials in this population.
The present study used the most modern stents, including stents with biocompatible polymers, and evaluated a real-world population, demonstrating the efficacy and safety of these devices for the treatment of complex lesions in patients with indication for elective revascularization. The results are in accordance with the recommendations of the European guidelines for myocardial revascularization,14 which classify as class IA (treatment is useful and effective with sufficient evidence from multiple randomized trials or meta-analyses) both CABG and PCI for the treatment of proximal LAD lesions with favorable anatomy.
It is worth mentioning the low prevalence of left ventricular dysfunction in the study and the non-inclusion of patients with indication for urgent invasive stratification, and therefore, the data are not applicable to these groups of patients.
Study limitationsThe limitations of this analysis comprised its observational, single-center design, the limited sample size, and the absence of a surgical group for comparison. Another potential limitation was the lack of a longer clinical follow-up, although previous randomized studies with bare-metal stents did not find relevant differences in long-term major clinical outcomes.27
ConclusionsIn single-vessel patients with an indication of elective revascularization in de novo lesions located in the proximal left anterior descending artery, treatment with second-generation drug-eluting stents seems to be a safe option, with low rates of adverse cardiac events and need for additional revascularization. These findings are similar to previously published historical data regarding surgical treatment using the left internal thoracic artery. Randomized studies are needed to confirm these findings.
Funding sourcesNone declared.
Conflicts of interestThe authors declare no conflicts of interest.
Peer review under the responsibility of Sociedade Brasileira de Hemodinâmica e Cardiologia Intervencionista.