Serotonin is highly implicated in the regulation of emotional state and the execution of cognitive tasks, so much so that the serotonin transporter genes (5-HTT, SLC6A4) and the serotonin receptor genes (HTR1A, HTR1B, HTR2A) have become the perfect candidates when studying the effects that these genes and their polymorphic variations have on depression characteristics.
ObjectiveA review of research reports that have studied the effects of variations in the serotonin transporter and receptor genes on different clinical features of depression.
MethodsA search of the Scopus, Web of Science and PubMed databases was conducted using the keywords ("depression" AND "polymorphism").
ConclusionsAccording to the review of 54 articles, the short allele of the 5-HTTLPR polymorphism was found to be the most reported risk factor related to the development of depression and its severity. Variations in the genes studied (SLC6A4, HTR1A, HTR2A) can generate morphological alterations of brain structures.
La serotonina tiene gran implicación en la regulación del estado emocional y la ejecución de tareas cognitivas, de modo que los genes del transportador de serotonina (5-HTT, SLC6A4) y de los receptores de serotonina (HTR1A, HTR1B, HTR2A) se convierten en candidatos adecuados para estudiar los efectos de estos genes y sus variaciones polimórficas en las características de la depresión.
ObjetivoRevisión de reportes de investigación que hayan estudiado los efectos de las variantes de los genes del transportador y de los receptores de serotonina en las diferentes características clínicas de la depresión.
MétodosSe realizó una búsqueda en las bases de datos Scopus, Web of Science y PubMed con las palabras clave “depression”, AND “polymorphism”.
ConclusionesSegún la revisión de 54 artículos, se encontró que el alelo corto del polimorfismo de 5-HTTLPR es el factor de riesgo más reportado en relación con el desarrollo de depresión y su gravedad. Las variantes de los genes estudiados (SLC6A4, HTR1A, HTR1B y HTR2A) pueden generar alteraciones morfológicas de estructuras cerebrales.
Depressive disorders are among the most studied psychiatric illnesses, as their high prevalence, early age of onset, chronic course and disabling nature make them a major public health problem.1 Depression is the main cause of disability worldwide, with more than 300 million people currently affected. This represents an increase of over 18% from 2005 to 2015.2
Depression is characterised by episodes in which negative emotions and thoughts coexist with alterations in cognitive functions, with changes in appetite and libido and sleep disturbances.3 The fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) includes the following symptoms of depression:
- •
Feelings of sadness, hopelessness, worthlessness and guilt.
- •
Low self-esteem.
- •
Negative thoughts focusing on suicide.
- •
Cognitive deficit that affects motivation, selective attention, episodic memory and working memory.4
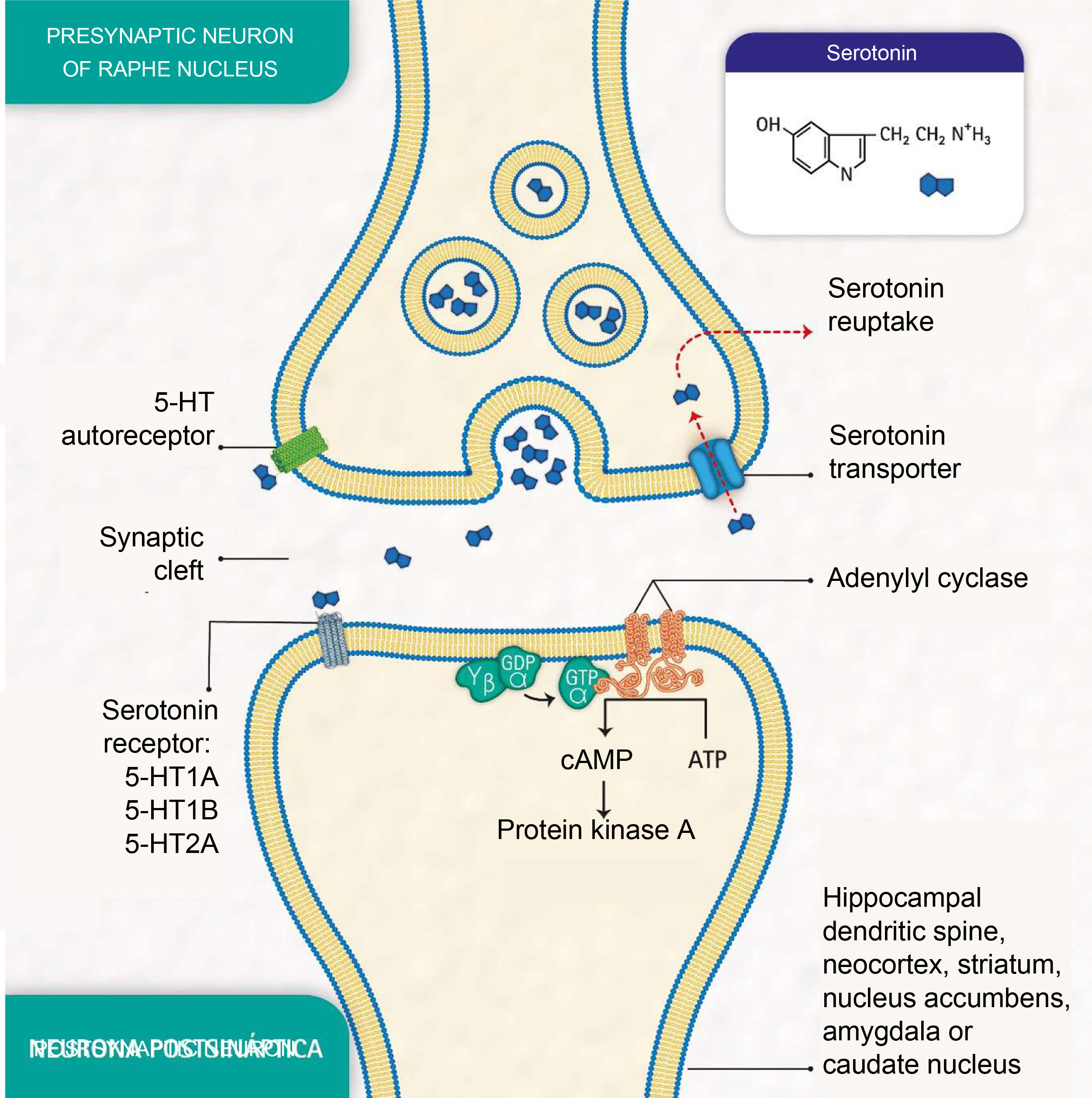
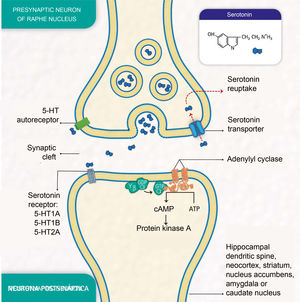
Depressive disorders are also characterised by alterations in synaptic signalling mediated by substances such as serotonin (5-hydroxytryptamine [5-HT]), a neurotransmitter related to the modulation of social behaviour and an individual's emotional response.3 Serotonin is released from presynaptic neurons in the raphe nuclei, which have the function of activating the limbic system. It subsequently binds to postsynaptic serotonin receptors located mainly in limbic areas such as the hippocampus, cortex, dorsal striatum and nucleus accumbens (Fig. 1).
Molecular processes of serotonin biosignalling: serotonin release by the presynaptic neuron; binding of serotonin to one of the receptors on a postsynaptic neuron; activation of protein G, adenylyl cyclase and protein kinase A; formation of cyclic adenosine monophosphate (cAMP); serotonin reuptake by the serotonin transporter in the presynaptic neuron; and serotonin autoreceptor in the presynaptic neuron. (Representation made by the authors and designed by Melissa Zuluaga Hernández).
The serotonin transporter protein (5-HTT) located in presynaptic neurons participates in serotonin reuptake,5 one of the mechanisms for removing the neurotransmitter from the synaptic cleft (Fig. 1). 5-HTT is encoded by the SLC6A4 gene, located on chromosome 17q11.1−17q12.6,7 The serotonin-transporter-linked promoter region (5-HTTLPR) polymorphic region (approximately 1kb upstream of the promoter region) has been reported to mediate the influence of stressful events on human depression.8
The serotonin-1A receptor (5-HT1A), which covers about 1200 pb and whose HTR1A gene is mapped to chromosome 5q11.2–13, is known as a potent regulator of serotonergic neurotransmission9 and autoreceptors located in raphe neurons are reported to play an essential role in the effect of antidepressant medications.10
Additionally, the serotonin-2A receptor (5-HT2A), encoded by the HTR2A gene located at position 13q14-q21, is of particular importance in mood disorders and is associated with the response to antidepressants.11 These receptors are found mainly in the neocortex, the caudate nucleus, the nucleus accumbens and the hippocampus.
Polymorphisms in the genes encoding the above serotonin transporter and receptors generate protein variants with different levels of expression and signal transduction capacity.12 These differences may be important in individual predisposition to developing psychiatric illnesses such as depression13 and in differences in response to drugs.
The results of studies carried out in family nuclei of patients with depression indicate that this disease has a multifactorial origin with a highly complex genetic component, involving a large number of loci,14 each of which contributes in a very small way to the phenotype, making specific genetic variants difficult to identify and leading to conflicting results.15 Moreover, sample sizes of fewer than 100 patients in 48% of the studies reviewed may have further added to the wide variation in the results obtained when polymorphisms in the SLC6A4, HTR1A, HTR1B and HTR2A genes were related to depression. The purpose of this review was therefore to gather scientific information on the effects of genetic variations in the serotonin transporter and receptors on the different clinical characteristics of the disease, or on the risk of suffering from it, in order to gain a better understanding of this relationship and identify topics for future study.
MethodsThe search was carried out in the Scopus, Web of Science and PubMed databases with articles published from 1996 to 2020 at the latest, and it was performed from February to March 2020. We reviewed articles mainly in English with the terms “depression” AND “polymorphism”. The appearance of these terms was restricted to the title and keywords of the article. Research and review articles and book chapters were also considered.
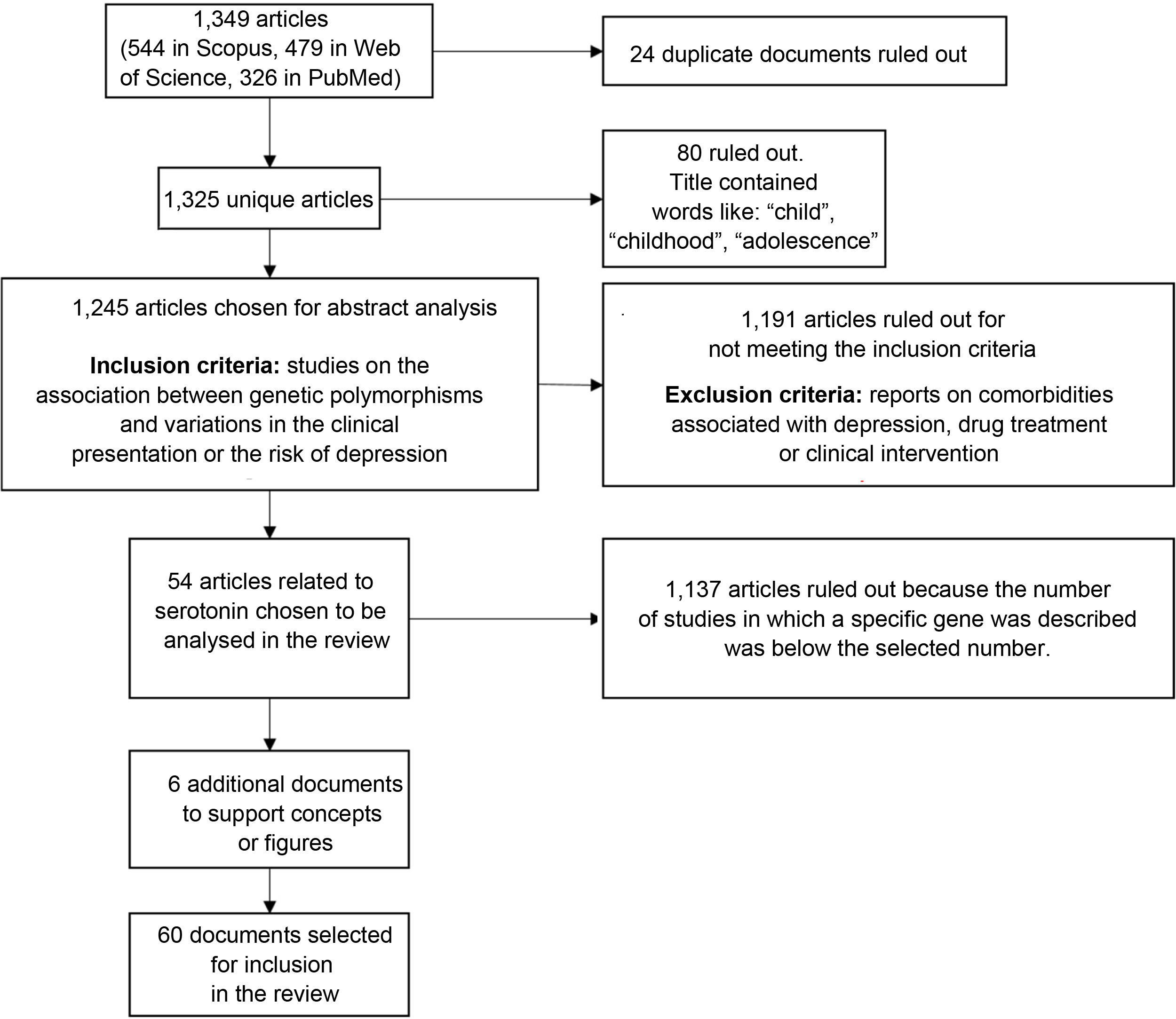
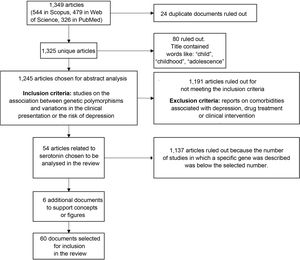
We found a total of 1349 articles (544 in Scopus, 479 in Web of Science and 326 in PubMed); of these, 24 were duplicates. Articles containing words such as "child", "childhood" or "adolescence" in the title were excluded because the population of interest for this review was adults. After applying that filter, 1245 articles were selected for analysis of the abstracts to verify compliance with the inclusion criteria: articles reporting on the association between genetic polymorphisms and variations in the clinical presentation of, or the risk of suffering from, depression. Studies on comorbidities with depression, drug treatment or clinical intervention were also ruled out.
After applying the inclusion and exclusion criteria, we selected the articles with the largest number of studies associated with genes, in particular the genes encoding the serotonin transporter and receptors (41 and 13 articles, respectively), as candidates for inclusion in this review. From these, we selected 54 research articles (Fig. 2).
ResultsDepression is influenced by both a genetic component, which predisposes the individual to the disease, and environmental factors, which affect their behaviour.16,17 An individual's response to stressful events, even minor ones, can be modulated by multiple functional polymorphisms affecting different neurobiological pathways involved in depression, which show stress-dependent effects.8,16
Considering the genetic influence on the predisposition to depression, the results of the information collected in this review are presented in terms of the polymorphisms and implications of a) the serotonin transporter gene (SLC6A4); b) the HTR1A and HTR1B genes of serotonin receptor family 1 (5-HT1); and c) the HTR2A gene of serotonin receptor family 2 (5-HT2).
Genotypic variations associated with SLC6A4Much of the research on the role of serotonin in depression has focused on studying the SLC6A4 gene polymorphism located in the 5-HTTLPR. However, the results of the studies are not consistent.
An example of this can be seen in the results published by certain authors who analysed the polymorphic variations of the 5-HTTLPR. Gonda et al.13 found no relationship between this polymorphism and the risk of suffering depression in a European population sample, while in an American population sample, Contreras et al.17 found that this polymorphism generates a high risk of depression in people exposed to stressful events.
Of all the polymorphisms of this gene, rs25531 is characterised, grouped into “long” (L) or “short” (S) forms. The long allele consists of a 44-base-pair (bp) insertion and the short allele, a deletion of the same size. Humans can have three possible genotypes: a combination of short and long (SL); short and short (SS); or long and long (LL). The presence of these two alleles has been the subject of study as it has been found to affect development of symptoms of depression, dependent on the stressful events the person has experienced.18 However, Willeit et al.19 associated the presence of the S allele with atypical depression and the L allele with melancholic depression.
The 5-HTTLPR genotype is associated with negative information processing styles after exposure to stressful events, which triggers depressive symptoms and sensitivity to stress.20 Tests were conducted on adults to determine whether or not the type of polymorphism in the 5-HTTLPR, traumatic events experienced in childhood and recent negative experiences, all together, affected the possible cognitive endophenotypes of depression (such as attention and the ability to recognise emotions). Results suggested that low-expression 5-HTTLPR alleles (or S alleles) can confer a greater risk of depression as, in the tests carried out, the carriers of these alleles focused their attention on negative information.21 Some authors have reported that people who have the short allele have a greater risk of depression, poorer cognitive performance and increased vulnerability to suffer cognitive impairment in old age compared to people with the long allele.15,22 The frequency of the SS genotype is especially high in subjects with depression with onset before the age of 65 years.23
Kendler et al.24 genotyped a group of male and female twins, and found that homozygotes with a short allele (S) at the SLC6A4 locus were more sensitive to the depressing effects of all stressful events than those with long alleles (L). The influence of the SS genotype was found to be strongly linked to having two or more first-degree relatives with a history of depression.25
Importantly, different studies suggest that it is not necessary to have a short-form homozygous genotype (SS), but that an S allele, even if it is heterozygous (SL), is sufficient to generate a statistically greater risk of depression,14,17,26 in addition to influencing the severity of the disorder.27,28
Another of the variations reported in the effects of the 5-HTTLPR polymorphism on emotional state is patient gender. Rucci et al.,29 for example, found that women with a unipolar disorder and the SS genotype may have higher levels of depression. This is in line with Song et al.,30 who found a higher rate of women diagnosed with depression and the S allele.
Sumner et al.5 reported that people with homozygous S allele (SS) had a better performing autobiographical memory than carriers of L alleles (SL or LL). However, the authors also concluded that the rs25531 polymorphism does not modify the function of the genotype, suggesting that this variation has no effect on a possible diagnosis of depression. That contrasts with the results of the previously mentioned authors.
In relation to the anatomical appearance of the brain, the influence of the 5-HTTLPR polymorphism on the cortical thickness of regions involved in the processing of emotions and cognitive control, as well as on the volumes of different structures in individuals with depression, has been studied by structural magnetic resonance imaging, but no effect of the genotype of this polymorphism was found on the cortical thickness of the regions examined (region of the anterior and posterior cingulate gyrus, frontal-lateral region and parahippocampus). However, larger volumes were found in the thalamus and left putamen of individuals homozygous for the L allele.31
In research using high-resolution magnetic resonance imaging, it was found that patients with the long homozygous genotype (LL) had significantly smaller hippocampal grey matter and white matter than controls with the same genotype.32,33 The authors concluded that the decrease in hippocampus volumes in patients with major depression but not in healthy controls could be related to the development of the central nervous system, modulation by certain effects of the disease, different vulnerabilities to stress or different responses to antidepressant therapy. If the greater reuptake of serotonin in subjects with the LL genotype modulates the course of the disease, hippocampus volumes may be affected as a result of depression or neurotoxic processes related to stress. The LL genotype may cause a different vulnerability to the stress reaction. One hypothesis postulated by other authors is that stress and increased glucocorticoids may contribute to the loss of hippocampal volume through glutamatergic-toxic effects.34 In addition, stress decreases the expression of neurotrophic factor derived from the brain and this has trophic effects on serotonergic neurons in the hippocampus.35
Taylor et al.36 analysed differences in the morphological effects of suffering episodes of depression at different stages of life (early-onset and late-onset depression). The authors found a relationship between diagnosis of the condition and the homozygous LL genotype, specifically in the right hippocampus, which is smaller in subjects with late-onset depression than in those diagnosed at a younger age.
In addition to the studies carried out with magnetic resonance imaging, Cole et al.37 compared healthy people and patients with depression, and found that there were no significant differences in the volume of the hippocampus between S-allele carriers and LL homozygotes. However, in post-mortem brains it has been found that carriers of short alleles have significantly reduced perigenual anterior cingulate cortex (pACC) and amygdala volumes.38 These results are related to the functional connectivity between these two structures and could predict the variation in the measures of temperamental traits related to anxiety and depression.
Costafreda et al.39 analysed the effects of 5-HTTLPR on the connectivity between the different brain structures. They found that the genotype with a short allele in this polymorphism was associated with an increase in the activity of the amygdala and a frontal-limbic connectivity bias. It has been suggested that this polymorphism modulates resting state perfusion in key mood processing structures.40
Mann et al.41 reported that variations in SLC6A4 directly affect the ventral prefrontal cortex, which may be reflected in a generalised deterioration in the serotonergic function involved in the degree of serotonin uptake, significantly lower than that of patients with depression and healthy controls.42
Schneider et al.43 argue that the polymorphic SLC6A4 promoter region, especially its single-nucleotide polymorphism rs25531, is a risk factor for depression; in their study, carriers of risk alleles (SS genotype) had significantly greater activation of the bilateral amygdala when shown images with negative emotional content. The association of the S allele with depression was also described by other authors who found a greater prevalence of this allele in hospitalised patients with depressive episodes.44
In addition to magnetic resonance imaging methods, changes in connectivity have been studied using auditory evoked potentials. Cheng et al.,45 for example, analysed the response to the P200 wave generated in the primary auditory cortex, which is characterised by a high rate of serotonin synthesis, in people with the L allele compared to those homozygous for the S allele. They found that those in the second group had a shorter wave than the other participants, which they believe suggests a relationship between the P200 wave and the SS genotype. This is because depression can alter the response processes to different external stimuli, and the genotype described in the article is strongly related to the severity of the condition.
Other polymorphic regions that have been studied are variable number tandem repeats (VNTR) in intron 2 of the SLC6A4 gene. They are called STin 2. These variations contain a variable number of tandem repeats of 17 bp, which generates three VNTR alleles, STin2.9, STin2.10, and STin 2.12, which contain 9, 10 and 12 copies of the element, respectively. STin2.10 and STin 2.12 have been associated with neurological abnormalities such as affect disorders.6 However, Ogilvie et al.46 found a significant difference between the control group and the depression group, largely explained by the excess of the STin2.9 allele in the unipolar group.
Nevertheless, despite there being great support for the association between the 5-HTTLPR polymorphism and depression, there is a group of authors whose research found no relationship between these two factors, challenging the hypothesis that the variants in that region contribute significantly to the human emotional state.47 Among that group were Baghai et al.,48 who studied the possible effects of partial sleep deprivation in patients with depression and polymorphisms in SLC6A4. However, the effect was only transitory and most patients suffered an increase in depressive symptoms two days after the intervention. Other studies that analysed the genotype with the presence of the short allele in a group of families made up of parents, twins and their siblings found no direct association between 5-HTTLPR and depression,49,50 but it may be associated with specific clinical signs of the disease.51
In addition to the relationship between polymorphisms of the SLC6A4 gene and depression, other areas have been studied, such as the association between depression and cortisol and cholesterol concentrations; to cope with the stress caused by traumatic events, the body needs to generate effective adaptive responses. The most studied end result of this process is the variation in cortisol concentration, with increased values being associated with depression. Therefore, interactions between the cortisol response and the serotonergic system can affect hippocampal volume, specifically in patients with depression and homozygous LL genotype.33
Other studies have also examined the relationship between the allelic variation of SLC6A4 and other risk factors; for example, the concentration of high-density lipoprotein cholesterol (HDL-C). The results suggest that low levels of this type of cholesterol can be a risk factor for suffering depression in old age, but the genotype with the S allele is associated with a greater predisposition.10 The biological hypotheses that explain the relationship between cholesterol concentration and depression maintain that unhealthy lifestyle habits can lead to accumulation of white adipose tissue, which increases the production of cytokines and inflammatory substances. This inflammatory response can, in turn, cause hyperactivation of the hypothalamic-pituitary-adrenal (HPA) axis, which stimulates the release of lipids into the bloodstream, resulting in a reduction in HDL-C, and which is associated with the development of depression.52
Polymorphisms studied in the genes of the HTR1 family (HTR1A and HTR1B)The rs6295 polymorphism of the serotonin 1A receptor (HTR1A) has been studied in depth because it can have functional effects on gene expression. This polymorphism is a common variant at the 1019 site upstream of the basal promoter area; it is therefore also known as polymorphism C(-1019)G.53
The G variant of the C(-1019)G polymorphism has been associated with high expression of 5-HT1A receptors, which can generate a greater risk of depression and a deficient clinical response,9 especially when the individual has suffered stressful events in life.12 Results from the different studies are conflicting, but there are recent findings that support an association between rs6295 and mood disorders such as major depressive disorder and bipolar disorder.13
Another polymorphism studied in this family is G861C, found in the serotonin-1B receptor (HTR1B) gene. This has been associated with the inhibition of the serotonin release process generated in the basal ganglia, mainly because these receptors activate second messengers, which inhibit the activity of adenylate cyclase and manage the release of the neurotransmitter. Huang et al.54 found a strong correlation between this polymorphism and substance abuse (cocaine) in patients diagnosed with major depression. The authors state that they do not know how genetics may affect substance abuse.
Serotonergic neurons project to most parts of the brain and show active postsynaptic heteroreceptors in the cortex, the limbic regions, the hypothalamus and the spinal cord. An increase in the uptake of the neurotransmitter serotonin by the presynaptic transporters could therefore decrease its availability in the extracellular area, thereby affecting postsynaptic uptake and its subsequent distribution to different structures, which can contribute to depression.1
Published HTR2A variantsOf the polymorphisms associated with the serotonin-2A receptor (HTR2A), the two that have been studied the most are the synonymous nucleotide polymorphism c.102C>T (rs6313) and the non-synonymous polymorphism c.1354, p.His452Tyr (rs6313); according to Minov et al.,55 no differential relationship with depression was found in a study comparing adults with depression and healthy controls. The single-nucleotide polymorphism -1438A/G (rs6311), located in the promoter region of HTR2A and studied in a population in north-eastern Thailand, was not found to be associated with depression risk.56 However, we should stress the fundamental role that other variations in this gene, such as T102C(rs6313), can play in the aetiology of depression.
Cao et al.57 and Gonda et al.13 reported that the rs6311 polymorphism seems to be an important susceptibility factor in the aetiology of depression, as it can affect the activation of other genes involved in the disease.
DiscussionMany of the authors included in this review agree that the variation causing the greatest risk of depression is the presence of the short allele in 5-HTTLPR. The discrepancies reported in the results may therefore be due to methodological factors in the studies, as all the populations come from ancestors of different origins, with allele frequencies that can vary and therefore lead to differences in the development of depressive disorder.58
When we compare the S and L alleles, the former is associated with lower serotonin uptake and serotonin transporter transcriptional efficiency,59 which plays an important role in depression.
The interaction between gene and environment in depression is important, as the S allele in the 5-HTTLPR polymorphic region generates a predisposition to depression with greater severity than the L allele, but depression probably does not develop unless the triggering environmental factor, such as experiencing stressful events, also occurs.
The results with imaging techniques, such as magnetic resonance imaging, differ from those where only the genetic component was studied, as the image analysis enabled detection of significant morphological changes related to the LL genotype. These variations involve the decrease in the volume of different structures such as the hippocampus,32,33,36 while larger thalamus and putamen volumes31 were found compared to healthy controls. These techniques have also made it possible to study brain connectivity; greater activation of the bilateral amygdala has been found in response to negative visual stimuli in patients with the SS allele.
The results have shown that the thalamus plays an important role in regulating the expression and experience of emotion. It has also been implicated in the pathophysiology of major depressive disorder.
In relation to the HTR1 gene family, the studies broadly differentiate between the function of the two types of receptor studied; 5-HT1A is related to the risk of suffering from mood disorders,9,10,13 while 5-HT1B has been associated with substance abuse in patients with depression.54 The association with substance abuse links the serotonergic system to addictions, when, from a psychology perspective, it has been generally accepted that such behaviour is related to the dopaminergic pathway, in both positive and negative reinforcement.60 Therefore, it is proposed to evaluate the role of the serotonergic pathway in addictions; the results will allow us to close the gap until now related to this topic and the important influence of both addictions and serotonin in depression.
In terms of HTR2A, like others studied in this review, discordant results are reported. However, an alternative interpretation of the results may be that the modulation of the 5-HT2A receptor is not the primary aetiological mechanism in depressive disorders, but a consequence of the disease process, and may depend on the participation of other genes.
One explanation for the disparity in the results from different studies on polymorphisms of the genes discussed in this review is the phenotypic heterogeneity of the patients included, as the diagnosis is based on each person's symptoms, so in terms of aetiology, a homogeneous sample is not necessarily guaranteed. Connectivity maps and medical images could therefore be important as a diagnostic biomarker of depression, as these can generate a relationship between the morphology and functionality of the different structures involved in the development of the disorder.
Bearing in mind that depression has multifactorial and polygenic origins, according to some authors, the heterogeneity of the results can be attributed to the fact that most genetic studies do not take into account environmental factors such as the effect of stressors. Moreover, other genes, such as GALR2 (galanin receptor type 2), BDNF (brain-derived neurotrophic factor) and P2RX7 (purinergic receptor P2X7) have been shown to be relevant in depression in people exposed to high and moderate levels of stress,13 as the body’s response to stress can be mediated by different pathways and mechanisms. It is therefore important to broaden the scope of studies that relate these genes with those studied in this review, as it is essential to expand the body of evidence concerning their influence on the development of depression.
For future research, we need to make use of different technologies to integrally relate morphological or functional characteristics with the genotypic and behavioural characteristics. This could narrow the gap between the different results obtained, in addition to endorsing different image analysis or connectivity techniques as quantitative diagnostic aids.
ConclusionsThe polymorphism in the SLC6A4 gene moderates the sensitivity of individuals to the depressive effects of stressful events to a large extent, as greater sensitivity to the impact of lived events is seen in individuals with the SS homozygous genotype.
Specific disease processes related to the serotonergic system can increase susceptibility to morphological changes in brain regions. On the one hand, the short allele (S) is related to a decrease in the volume of structures such as the amygdala and the perigenual anterior cingulate cortex while, on the other, the presence of the long allele (L) leads to a decrease in the volume of the hypothalamus and an increase in the volume of structures such as the thalamus and the putamen. The presence of the short allele in the 5-HTTLPR is a potential contributing factor to the risk of more severe depression.
Conflicts of interestThe authors have no conflicts of interest to declare.
We would like to thank the Colombian Ministry of Science, Technology and Innovation for their support in conducting this research during Call for Proposal 736 of 2018 for Young Researchers in Health. The project through which this study was produced receives support from the Universidad Autónoma de Manizales [Autonomous University of Manizales] (code: 736-2018).