Special Issue: Reumatología en época COVID
More infoKawasaki disease (KD) is an acute vasculitis with multisystem involvement. Recently, the increasing incidence of a condition that closely resembles KD in many cases, named multisystem inflammatory syndrome in children (MIS-C), has set off alarms amid the current worldwide coronavirus disease-19 (COVID-19) pandemic. Hence, the aim is to conduct a systematic review of the literature about KD in Colombia and contrast it with COVID-19-related MIS-C.
Materials and methodsA search was carried out in both international and Latin American electronic databases for publications concerning patients with KD in the Colombian population. Records were then screened by titles and/or abstracts, assessed for eligibility, and reviewed. Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines were followed. The search included studies reporting MIS-C associated with COVID-19, and compared these patients with our findings of KD in Colombia.
ResultsOut of 36 publications retrieved, 17 were included, representing 120 individuals. Male to female ratio was 1.6, and most patients (90.4%) were aged 5 years or less. Among the main features of KD, fever was the most frequent (96.2% of the patients), while cervical lymphadenopathy was present in only 40.6%. Intravenous immunoglobulin was administered in 91.4% cases and 6.2% were resistant. Cardiac involvement was found in around 30%, and 20% had coronary artery lesions. Comparison between MIS-C associated with COVID-19 and KD in Colombia indicates that patients affected by MIS-C were older (72.2% of MIS-C patients>5 years), had higher rates of cardiac involvement, and required critical care more often.
ConclusionsOur findings of KD in Colombia are consistent with the available descriptions of KD in the scientific literature. Given the increasing rate of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection in Colombia and Latin America, our study raises awareness about MIS-C in pediatric patients with COVID-19 and its relationship with KD.
La enfermedad de Kawasaki (EK) es una vasculitis aguda con compromiso multisistémico. Recientemente, la incidencia creciente de una condición que se asemeja en forma considerable a la EK en muchos casos, denominada síndrome inflamatorio multisistémico (SIMS) en niños, ha encendido las alarmas en medio de la actual pandemia mundial de la enfermedad COVID-19. Por consiguiente, nos propusimos realizar una revisión sistemática de la literatura acerca de la EK en Colombia y contrastarla con el SIMS relacionado con COVID-19 en niños.
Materiales y métodosBuscamos publicaciones respecto a pacientes con EK en población colombiana, en bases de datos electrónicas tanto internacionales como latinoamericanas. Los registros hallados fueron tamizados por títulos o resúmenes, evaluados para elegibilidad y revisados. Se siguieron las guías Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA). Posteriormente, buscamos estudios que reportaran SIMS temporalmente asociado con COVID-19 en niños y comparamos estos pacientes con nuestros hallazgos de EK en Colombia.
ResultadosDe 36 publicaciones encontradas se incluyeron 17, las cuales representaron 120 individuos. La razón hombre a mujer fue de 1,6 y la mayoría de los pacientes (90,4%) tenía 5 años o menos. Entre las principales características de EK, la fiebre fue la más frecuente (96,2%), mientras que la linfadenopatía cervical estuvo presente solo en el 40,6%. La inmunoglobulina intravenosa se administró en el 91,4% de los casos y 6,2% presentaron resistencia. Se encontró compromiso cardiaco en alrededor del 30% de los pacientes, en tanto que el 20% tuvo lesiones de arterias coronarias. La comparación entre las características clínicas de la EK y el SIMS asociado a COVID-19 mostró que los individuos afectados por el SIMS eran mayores (72,2% con SIMS tenían más de cinco años), tuvieron mayores índices de compromiso cardiaco y requirieron cuidado crítico con mayor frecuencia.
ConclusionesNuestros hallazgos de EK en Colombia son consistentes con las descripciones disponibles de esta enfermedad en la literatura científica. Debido al aumento de infección por SARS-CoV-2 en Colombia y Latinoamérica, nuestro estudio busca crear conciencia sobre el SIMS en pacientes pediátricos con COVID-19 y su relación con la EK.
Kawasaki disease (KD) is an acute, self-limited, systemic vasculitis, which primarily involves small and medium-sized vessels.1 It affects mostly males between 6 months and 5 years old, being the most common medium-sized-vessel vasculitis.2,3 Several theories have been proposed regarding the etiology of this condition, including pathogens, toxins, and autoimmune or autoinflammatory processes.4,5 Even though the current evidence points toward an infectious agent accompanied by a dysregulated immune response, a univocal agreement about the exact cause or causes of KD is lacking so far.2 Therefore, KD is nowadays considered to be a vasculitis of unknown origin, with a predilection for coronary arteries.6 Although acute manifestations usually resolve promptly upon adequate treatment, long-term cardiovascular sequelae, mainly coronary artery lesions (CALs), may develop in some patients. In fact, KD is the leading cause of acquired heart disease among children in developed countries.7 Nonetheless, if KD is identified and treated early, the risk of coronary artery aneurysms (CAAs) declines from 25% to 4%.8
In 1967, six years after seeing the first child presenting with a particular constellation of clinical manifestations, Japanese pediatrician Tomisaku Kawasaki described 50 patients that experienced an ‘acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes’.9,10 Subsequently, this syndrome was named mucocutaneous lymph node syndrome, and cases were reported outside Japan.11,12 Some years after that, KD was defined as equivalent to infantile periarteritis nodosa.13
Currently, the coronavirus-19 disease (COVID-19), caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) virus, has become a global public health issue. Interestingly, a link between SARS-CoV-2 infection and KD has been suggested following an increase of cases of KD, Kawasaki-like disease, and other inflammatory syndromes in multiple countries struck by the COVID-19.14–18 This condition has been termed multisystem inflammatory syndrome in children (MIS-C) temporally associated with COVID-19, among other names, and has posed several questions about the nature and the implications of this relationship.19 Since KD is considered not a very frequent condition, and the COVID-19 pandemic has been associated with MIS-C, we aimed to review the status of KD in Colombia and contrast the results with the clinical characteristics of MIS-C linked to COVID-19 in order to raise awareness of this association.
Materials and methodsInformation sources and search strategyA systematic review of the literature was done up to July 18th, 2020. The search was performed both in international and Latin American electronic databases. For this purpose, we searched using the terms: (“Kawasaki disease” OR “Kawasaki's disease” OR “Kawasaki syndrome” OR “Kawasaki's syndrome” OR “Kawasaki vasculitis” OR “Kawasaki's vasculitis” OR “mucocutaneous lymph node syndrome”) AND “Colombia”, or their Spanish language counterparts, in PubMed, LILACS, Embase and Web of Science. A more stringent search approach was also done in LILACS but yielded the same results (see Additional file 1). To identify potential additional studies for inclusion, we also hand-searched through other sources, including gray literature, and manually looked up the references of the articles found by the search strategy initially described above. No date, language, or population limits were applied.
Eligibility criteriaArticles included had to have been conducted in Colombian population and to describe methods implemented to study, diagnose, treat, or rehabilitate patients suffering from KD at any age. In addition to this, studies reporting the clinical outcomes of patients with KD were also eligible. Case reports, case series, cross-sectional, case–control, cohort (either prospective or retrospective), or clinical trial studies were suitable for inclusion. Studies that were not carried in the population of interest, or that did not present relevant outcomes for the population of interest separately to those for other populations were excluded. We also ruled out records whose full text was not available to us, as well as systematic or narrative reviews and meta-analyses.
Study selection and data collection processFirst, a primary reviewer screened all titles and/or abstracts of the publications found. Following this, eligibility assessment was performed. Retrieved articles were rejected if eligibility criteria were not met, and two secondary reviewers were consulted in cases in which eligibility criteria were unclear. After this, a single researcher collected the information regarding population, study design, clinical characteristics, treatment, and outcomes of all included studies; thereafter, a second reviewer verified the extracted information. Any discrepancies or missing information were resolved by consensus.
Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines were followed.20
Comparison with MIS-C associated with COVID-19In order to find articles reporting cases of MIS-C related to COVID-19, we searched on PubMed on July 18th, 2020 using the following search string: (SARS-CoV-2 OR COVID-19) AND (multisystem inflammatory syndrome in children OR MIS-C OR pediatric multisystem inflammatory syndrome OR PIMS) and selected the articles corresponding to MIS-C related to COVID-19. No date, language, or population limits were applied. Some references found through hand-searching were also included. We then contrasted our results of KD in Colombia with the information on these studies. Statistical analysis was performed using χ2 test and p-value<0.05 was considered significant.
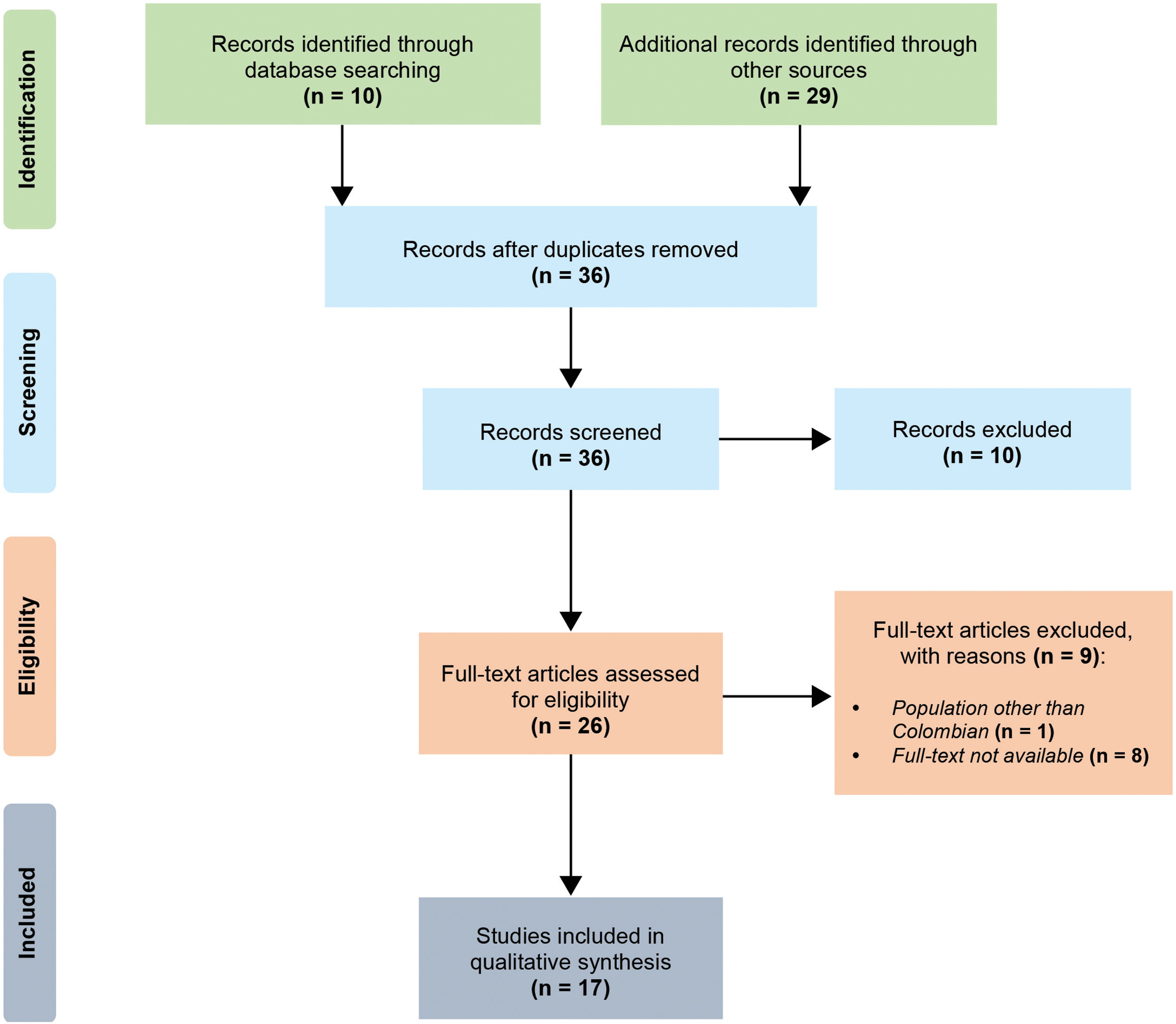
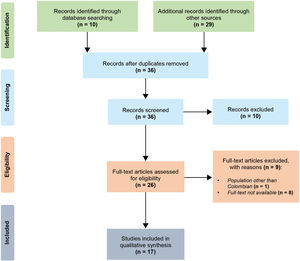
ResultsSearch and study selection resultsA total of ten articles were identified through database searching, and 29 additional records from other resources were found. After duplicates removal, the titles and/or abstracts of 36 articles were screened. In the screening phase, ten articles were excluded. Twenty-six full-text articles were assessed for eligibility.
A study by Momenah et al.21 was excluded because it was not about Colombian population. Likewise, eight articles whose titles contained the search terms were also excluded because it was not possible to find out the full texts.
Ultimately, 17 publications were included in the qualitative synthesis for analysis (Fig. 1). Table 1 summarizes the main features of these studies.22–38
Selection process. We followed the PRISMA guidelines for reporting in systematic reviews and meta-analyses.20
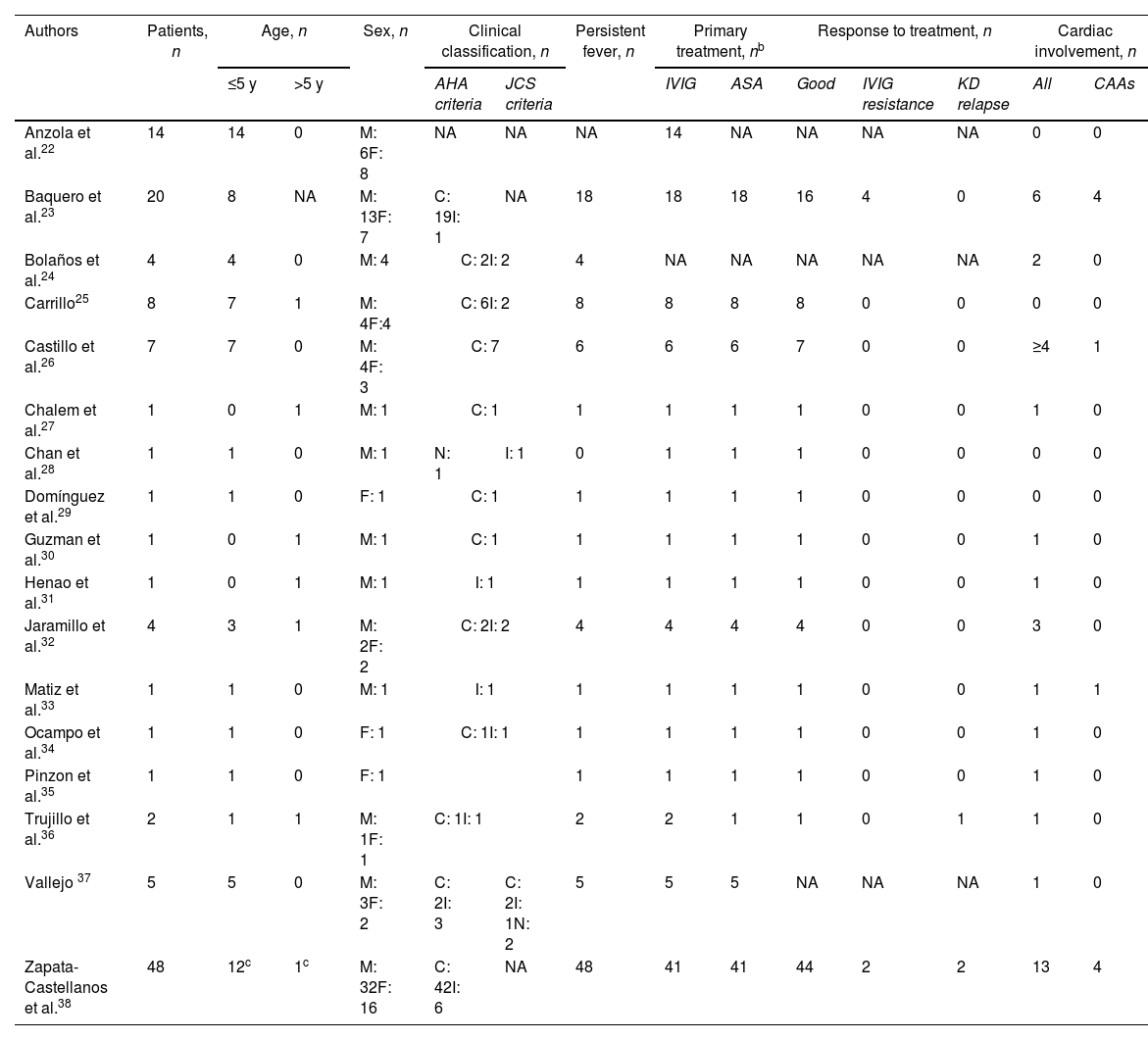
Description of KD cases in Colombia.a
| Authors | Patients, n | Age, n | Sex, n | Clinical classification, n | Persistent fever, n | Primary treatment, nb | Response to treatment, n | Cardiac involvement, n | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤5 y | >5 y | AHA criteria | JCS criteria | IVIG | ASA | Good | IVIG resistance | KD relapse | All | CAAs | ||||
| Anzola et al.22 | 14 | 14 | 0 | M: 6F: 8 | NA | NA | NA | 14 | NA | NA | NA | NA | 0 | 0 |
| Baquero et al.23 | 20 | 8 | NA | M: 13F: 7 | C: 19I: 1 | NA | 18 | 18 | 18 | 16 | 4 | 0 | 6 | 4 |
| Bolaños et al.24 | 4 | 4 | 0 | M: 4 | C: 2I: 2 | 4 | NA | NA | NA | NA | NA | 2 | 0 | |
| Carrillo25 | 8 | 7 | 1 | M: 4F:4 | C: 6I: 2 | 8 | 8 | 8 | 8 | 0 | 0 | 0 | 0 | |
| Castillo et al.26 | 7 | 7 | 0 | M: 4F: 3 | C: 7 | 6 | 6 | 6 | 7 | 0 | 0 | ≥4 | 1 | |
| Chalem et al.27 | 1 | 0 | 1 | M: 1 | C: 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | |
| Chan et al.28 | 1 | 1 | 0 | M: 1 | N: 1 | I: 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Domínguez et al.29 | 1 | 1 | 0 | F: 1 | C: 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | |
| Guzman et al.30 | 1 | 0 | 1 | M: 1 | C: 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | |
| Henao et al.31 | 1 | 0 | 1 | M: 1 | I: 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | |
| Jaramillo et al.32 | 4 | 3 | 1 | M: 2F: 2 | C: 2I: 2 | 4 | 4 | 4 | 4 | 0 | 0 | 3 | 0 | |
| Matiz et al.33 | 1 | 1 | 0 | M: 1 | I: 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | |
| Ocampo et al.34 | 1 | 1 | 0 | F: 1 | C: 1I: 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | |
| Pinzon et al.35 | 1 | 1 | 0 | F: 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | ||
| Trujillo et al.36 | 2 | 1 | 1 | M: 1F: 1 | C: 1I: 1 | 2 | 2 | 1 | 1 | 0 | 1 | 1 | 0 | |
| Vallejo 37 | 5 | 5 | 0 | M: 3F: 2 | C: 2I: 3 | C: 2I: 1N: 2 | 5 | 5 | 5 | NA | NA | NA | 1 | 0 |
| Zapata-Castellanos et al.38 | 48 | 12c | 1c | M: 32F: 16 | C: 42I: 6 | NA | 48 | 41 | 41 | 44 | 2 | 2 | 13 | 4 |
AHA, American Heart Association; ASA, acetylsalicylic acid; C, complete Kawasaki disease; CAAs, coronary artery aneurysms; F, female; I, incomplete Kawasaki disease; IVIG, intravenous immunoglobulin; JCS, Japanese Circulation Society; KD, Kawasaki disease; M, male; NA, not applicable or not available.
In the included studies, a total of 120 Colombian patients affected by KD were reported. 74 were males, with a male to female ratio of 1.6:1. Almost all of our patients (99.2%) were children or adolescents (<18 years). Among the 73 patients whose exact age was reported, 66 were ≤5 years. A single case was reported in an adult male aged 36 years.
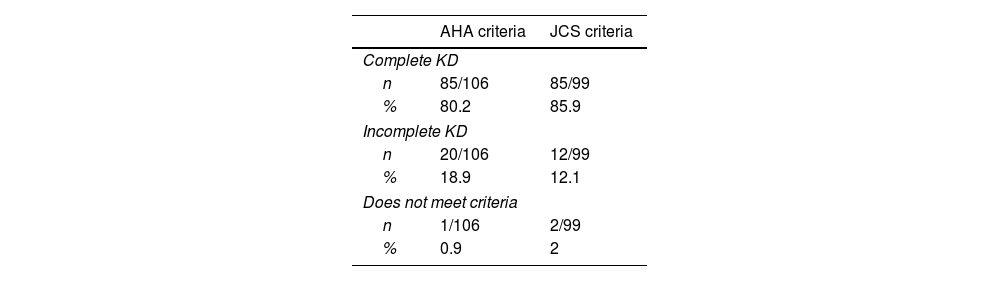
Clinical classification and findingsFrom the reported cases, information about clinical presentation was available for 106 of them. The studies included were reviewed in the light of the American Heart Association (AHA)8 and the Japanese Circulation Society (JCS) criteria.39 All patients met at least one of these criteria sets for either complete or incomplete KD. Data regarding AHA and JCS criteria fulfillment are presented in Table 2.
Fulfillment of AHA diagnostic criteria vs JCS diagnostic criteria among KD cases in Colombia.
| AHA criteria | JCS criteria | |
|---|---|---|
| Complete KD | ||
| n | 85/106 | 85/99 |
| % | 80.2 | 85.9 |
| Incomplete KD | ||
| n | 20/106 | 12/99 |
| % | 18.9 | 12.1 |
| Does not meet criteria | ||
| n | 1/106 | 2/99 |
| % | 0.9 | 2 |
Note: all patients met at least one set of diagnostic criteria. AHA American Heart Association, JCS Japanese Circulation Society, KD Kawasaki disease.
Concerning the principal symptoms of KD in these 106 patients, 102 of them presented with prolonged fever, 67–69 had peripheral extremity involvement (i.e., edema or desquamation of hands or feet), 74–76 had oral or pharyngeal mucosal changes (i.e., erythema, cracking of lips and/or strawberry tongue), 43 had cervical lymphadenopathy, 67 had conjunctival injection, and 78 had rash. From 58 patients, leukopenia was observed in 2 of them, lymphopenia in 3, thrombocytosis in 17, high C reactive protein (CRP) in 16, high erythrocyte sedimentation rate (ESR) in 14, abnormal liver tests in 7, hyponatremia in 2 and hypoalbuminemia in 2. Of these patients, pre- vs. post-treatment changes in platelets, CRP and ESR was reported in 20 of them. More data about other medical findings, including laboratory and imaging findings, are presented in Additional file 2.
Treatment and clinical responseOut of the 120 patients, the therapeutic approach was described for 116 of them. Among those, intravenous immunoglobulin (IVIG) was administered to 106 usually at doses of 2g/kg, while 91 received acetylsalicylic acid (ASA). Eighteen patients were treated with antibiotics during the course of the illness (one of them also with an antiviral drug), 3 with paracetamol and/or metamizole, 2 with antihistamines, 4 with systemic corticosteroids (either as primary or secondary treatment), and 1 with inhaled short-acting β-agonist and corticosteroids. One patient was also prescribed a topical ophthalmic drug because of secondary anterior uveitis. Besides this, the adult patient required intensive care unit (ICU) management with mechanic ventilation and renal replacement therapy.
Clinical response to treatment was reported in 97 cases. The majority of these (88, i.e., 90.7%) had a good clinical response, with resolution of fever and amelioration or full disappearance of the other complaints. IVIG resistance, defined as persistence or recrudescence of fever at least 36h after the completion of the IVIG infusion,8 was observed in 6 patients (6.2%). On the other hand, 3 of them (3.1%) presented a relapse of the disease after 48h of defervescence. IVIG resistant patients and those with relapses received additional doses of IVIG, were administered systemic corticosteroids, or both. Further treatment for these patients also included ASA in four of them.
Cardiac involvement and sequelaeCardiac involvement was evidenced either clinically, by electrocardiography, or by cardiac imaging, in 36–38 patients. At least 48 individuals had an echocardiogram performed, and 21 or more showed abnormal results. Valvular heart disease was evidenced in 9 cases (regurgitation in all of them). Pericardial effusion or pericarditis was observed in 8 cases as well. Ejection fraction was reported as 25% in one patient, and below 70% in 2. Importantly, one had a previous history of a restrictive interventricular communication but also showed pericardial effusion in an echocardiogram performed during the workup. Two follow-up echocardiograms of this patient were reported as normal. CALs, including CAAs, were diagnosed in 24 cases. Ten patients had evidence of CAAs. No deaths were reported in the reviewed articles.
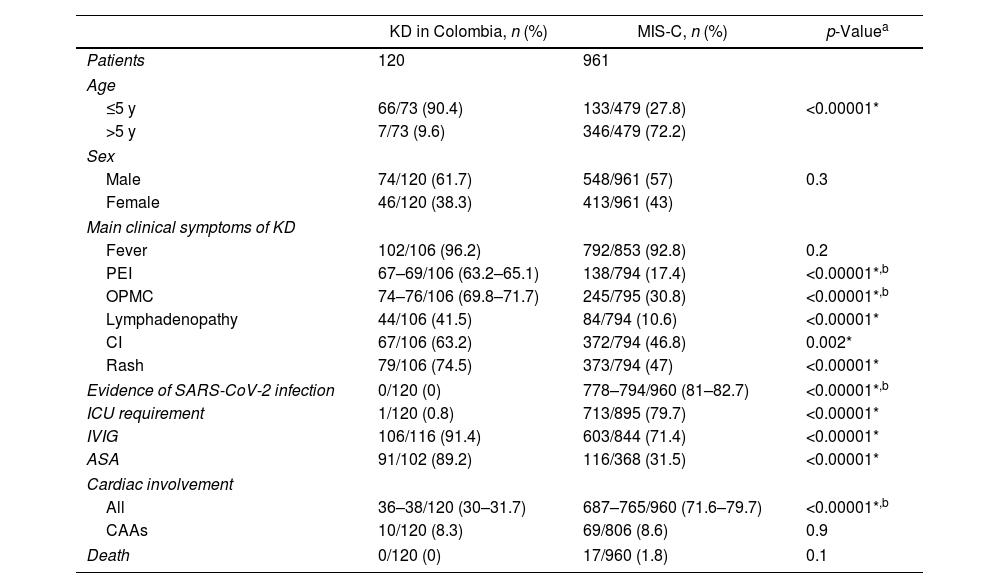
KD in Colombia vs. MIS-C associated with COVID-19We collected 45 articles respecting MIS-C linked to COVID-19 and compared the patients reported there with those included in our review of KD in Colombia. Of note, all the latter were diagnosed with KD before the COVID-19 epidemic. MIS-C group comprehended 961 patients, three of them being adults (0.3%). Taking into account that the cases of KD in Colombia also included an adult cases, these three adult cases were not excluded. Information about age, sex, presence of main symptoms of KD, evidence of SARS-CoV-2 infection, ICU requirement, use of IVIG and ASA, cardiac involvement, and mortality for both groups is shown in Table 3.14–18,40–79
Comparative analysis between KD in Colombia and reported MIS-C cases globally.
| KD in Colombia, n (%) | MIS-C, n (%) | p-Valuea | |
|---|---|---|---|
| Patients | 120 | 961 | |
| Age | |||
| ≤5 y | 66/73 (90.4) | 133/479 (27.8) | <0.00001* |
| >5 y | 7/73 (9.6) | 346/479 (72.2) | |
| Sex | |||
| Male | 74/120 (61.7) | 548/961 (57) | 0.3 |
| Female | 46/120 (38.3) | 413/961 (43) | |
| Main clinical symptoms of KD | |||
| Fever | 102/106 (96.2) | 792/853 (92.8) | 0.2 |
| PEI | 67–69/106 (63.2–65.1) | 138/794 (17.4) | <0.00001*,b |
| OPMC | 74–76/106 (69.8–71.7) | 245/795 (30.8) | <0.00001*,b |
| Lymphadenopathy | 44/106 (41.5) | 84/794 (10.6) | <0.00001* |
| CI | 67/106 (63.2) | 372/794 (46.8) | 0.002* |
| Rash | 79/106 (74.5) | 373/794 (47) | <0.00001* |
| Evidence of SARS-CoV-2 infection | 0/120 (0) | 778–794/960 (81–82.7) | <0.00001*,b |
| ICU requirement | 1/120 (0.8) | 713/895 (79.7) | <0.00001* |
| IVIG | 106/116 (91.4) | 603/844 (71.4) | <0.00001* |
| ASA | 91/102 (89.2) | 116/368 (31.5) | <0.00001* |
| Cardiac involvement | |||
| All | 36–38/120 (30–31.7) | 687–765/960 (71.6–79.7) | <0.00001*,b |
| CAAs | 10/120 (8.3) | 69/806 (8.6) | 0.9 |
| Death | 0/120 (0) | 17/960 (1.8) | 0.1 |
ASA, acetylsalicylic acid; CAAs, coronary artery aneurysms; CI, conjunctival injection; ICU, intensive care unit; IVIG, intravenous immunoglobulin; MIS-C, multisystem inflammatory syndrome in children; OPMC, oral and pharyngeal mucosal changes; PEI, peripheral extremities involvement; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2.
KD occurs mainly in children and adolescents, and 85% of the patients are ≤5 years old.80 Therefore, the diagnosis of KD in adults is exceedingly rare, and very few cases have been reported worldwide.30 Furthermore, this condition is more frequent among male individuals, with a male to female ratio around 1.5–1.2 Consistent with these data, in the patients included in this study, 90.4% were aged 5 years or less, and most of them were male, with a male to female ratio of 1.6–1. However, in the articles reviewed, a case of a 36-years-old adult diagnosed with KD was reported.30
There are two main sets of diagnostic criteria used for KD, proposed by the AHA and the JCS separately.8,39 Despite being very similar to each other, the main difference between them is the fever being or not a requisite for the diagnosis. According to our analysis, the fulfillment of AHA and JCS criteria is very similar through the cases reported (Table 2).
In the AHA criteria, persistent fever (usually persisting≥5 days) is a sine qua non characteristic for the diagnosis of complete (or classic) KD and must be accompanied by at least four out of five main clinical findings.8 Conversely, the JCS criteria consider fever as one of six principal symptoms, and diagnosis is made when at least 5 of them are present.39 Differential diagnosis is key in KD, given that other illnesses must be ruled out to reach a correct diagnosis of KD.
Besides fever, changes of the oral cavity and lips are the commonest clinical feature in KD (96.5%), followed by polymorphous rash (96%), non-purulent bilateral conjunctivitis (89%), changes in the extremities (75.6%), and cervical lymphadenopathy (62.7%).81 In our patients, fever was present in 96.2% of them, while cervical lymphadenopathy was the least frequent of the main symptoms, found in 40.6% of the cases.
Moreover, some patients that do not meet criteria to diagnose complete KD are thus evaluated for incomplete KD. AHA guidelines include an algorithm for the management of these patients according to C reactive protein values, other laboratory findings, and echocardiogram results.8Table 2 shows how many patients were classified as incomplete KD according to each set of criteria. Special attention should be paid to the incomplete forms of the disease, which may be observed early during the course of the illness (i.e., the first days), as well as the forme frustre of this syndrome.28,31,34,35
The mainstay of the treatment for KD consists of IVIG (2g/kg) within 10 days of the onset of symptoms, and ASA.4 The administration of IVIG must be done slowly, and premedication with antihistamines is often encouraged.4 Most patients resolve following this therapy; however, around 15% of the patients show IVIG resistance, requiring further doses of IVIG and/or use of systemic corticosteroids.2,4 The use of medications other than IVIG as adjunctive primary treatment, such as systemic corticosteroids, infliximab, or cyclosporin, has been studied, with mixed clinical results.4 Accordingly, most of the patients reviewed in this study received IVIG (91.4%) and ASA (89.2%) as part of their initial treatment. Systemic corticosteroids were used as primary treatment in two patients and two were treated with antihistamines, although not as premedication for IVIG in neither of both cases. None of the reviewed patients received infliximab or cyclosporin. IVIG resistance was present in 6.2% of the people and was treated with further IVIG doses and/or systemic corticosteroids.
The development of CALs, particularly CAAs, is the main determinant for the prognosis, long-term morbidity, and mortality of patients with KD. Thanks to the introduction of IVIG for the treatment of this syndrome, the rates of CAAs have dropped from 25% in untreated children to around 1 to 3% in children who received this medication.4 Fortunately, mortality in KD is very low.4,39 In this group of patients, altered coronary arteries were found in 20% of the cases, and CAAs were diagnosed in 8.3% (41.7% of the total CALs). Furthermore, cardiac involvement, in general, was identified in approximately 30% of the patients. There were no deaths reported. Our results in Colombian patients are similar to the data reported by the REKAMLATINA network, mainly those concerning sex distribution, clinical presentation, treatment, and CAAs prevalence.82
Finally, motivated by the recent rise in MIS-C cases and their association with COVID-19, we decided to compare the Colombian literature on KD included in this review with patients reported as suffering from MIS-C or related syndromes associated with COVID-19 (hereafter referred to only as MIS-C). It is well known that KD and MIS-C, despite sharing many similarities (not only clinically but also on a pathogenesis level), have important differences.83,84 Notwithstanding, given the undeniable resemblance in many cases and the paramount clinical importance, we made this contrast of KD in Colombia with global MIS-C cases since local ones are not available in the peer-reviewed literature at the time of writing. Of the MIS-C patients, at least 81% had laboratory evidence consistent with SARS-CoV-2 infection. Accordant with available descriptions,85 we found that 72.2% of MIS-C cases were in children older than 5 years, which is substantially different than our KD in Colombia findings. Furthermore, while KD has been associated with East Asian origin, MIS-C tends to affect more children of African American, Caribbean and Hispanic ancestry.85 This is of special interest to us given the triethnic origin of our population; withal, lack of consistency in reporting ethnic origin or ancestry among Colombian KD cases precluded further comparisons.
As previously described,85 the proportions of principal clinical findings of KD were lower in MIS-C patients compared to those included in our review, with statistically significant differences (except for fever). Correspondingly, as opposed to Colombian patients, IVIG was used less in the MIS-C group. These findings can be explained by the fact that not all the patients diagnosed with MIS-C had a presentation leading to KD diagnosis.
Besides traditionally measured acute phase reactants such as CRP and ESR, serum ferritin is very often elevated in MIS-C patients.84 Ferritin is a significant marker of M1 macrophage activation.86 Thus, it plays a key role in the workup of patients with suspected MIS-C.87 Nonetheless, we were not able to compare ferritin levels and other inflammatory parameters between global MIS-C versus Colombian KD patients because those variables are not systematically assessed in KD and were not included in Colombian reports.
Interestingly, we found a much greater rate of cardiac involvement in MIS-C patients than that found by us in Colombian KD patients. Likewise, more MIS-C patients required ICU management because of shock or other severe presentations. These results are consistent with the existing literature about this syndrome.19,88 Despite mortality being higher in the MIS-C group (1.8%), this difference was not statistically significant.
Nevertheless, some limitations should be noted. First, we collected a limited sample of patients, which may be in part due to the relative infrequency and/or underdiagnosis of the condition studied; but it is also because we could not retrieve the full text of all the articles reporting KD cases in Colombian population. The heterogeneous reporting of clinical characteristics and other attributes, along with the changing definition of MIS-C, may have hindered our analyses. We could not fully exclude a potential overlap within both groups. It is of note that we did not intend to perform a systematic review or a detailed description of the MIS-C cases here included, but to make a comparison between this cases and Colombian KD cases ascertained through a systematic review.
Conclusion and perspectivesKD is an idiopathic medium-sized-vessel vasculitis with multisystemic involvement that causes damage predominantly in extraparenchymal arteries, mostly coronary arteries. This syndrome is almost exclusive of pediatric patients but can seldom be found in adults. Even though the diagnosis is challenging, the timely administration of adequate treatment is a game-changer in the long-term outcomes of the disease. In this study, we found that KD Colombian patients exhibit similar characteristics with the international literature.
On the other hand, the rather recent outbreak of COVID-19 all over the world has brought forward several problems, including an association with an increase of cases of MIS-C, many of them presenting with features consistent with KD. This syndrome linked to COVID-19 seems to affect older children more, have higher rates of cardiac involvement, and entail more severe presentations requiring ICU management than the regular KD patients. Future descriptions of MIS-C cases related to COVID-19 in Colombia will broaden our understanding of how this entity behaves in our populations and will clarify the parallel between this condition and KD in our country. Consequently, given the high prevalence of SARS-CoV-2 infection, pediatric clinicians must maintain a high level of suspicion for the development of features of MIS-C in their patients. A guidance statement does not recommend immunomodulatory therapy for the majority of pediatric patients, who typically develop mild or moderate COVID-19. For children with severe or critical illness, the use of immunomodulatory agents may be beneficial.89
Follow up of these patients will allow us to know their real outcomes and sequelae. Also, the study of MIS-C offers a unique opportunity to explore the relationship between infection and autoimmune/autoinflammatory conditions such as KD.90
Authors’ contributionsJMA conceived the study, contributed to the literature search, was the secondary reviewer for eligibility criteria, and drafted the article. KLC did the literature search, carried out the data analysis and drafted the manuscript. YR contributed to the literature search and helped to draft the manuscript. JFS contributed to the literature search. MRJ contributed to the literature search and helped to draft the manuscript. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Availability of data and materialsData generated or analyzed during this study are included in this published article (and its supplementary information files). Further datasets are available from the corresponding author on reasonable request.
FundingThis work was supported by Universidad del Rosario, Bogota, Colombia (ABN-011).
Conflict of interestsThe authors declare that they have no competing interests.