The physiological mechanism in systemic lupus erythematosus is not yet fully elucidated. It is currently known that it is a multifactorial autoimmune disease which includes genetic, environmental and immune factors. Over the years it has been reported that cytokines play a predominant role during the course of the disease. In this review some findings reported in recent years were reviewed and the most important cytokines in SLE were considered; and we analyzed how the levels of each cytokine have been found in patients and how each of them can contribute to the proposed mechanisms and the relationship with the disease, as well as their possible effects on the triggering and control of systemic lupus erythematosus. The aimed of this article is to provide a focused review of the current knowledge of cytokines in SLE.
Los mecanismos fisiopatológicos en el lupus eritematoso sistémico (LES) aún no están completamente elucidados. Actualmente se sabe que es una enfermedad autoinmune multifactorial que comprende factores genéticos, ambientales e inmunes. A lo largo de los años se ha reportado que las citocinas tienen un papel preponderante durante el curso de la enfermedad. En esta revisión, algunos hallazgos reportados durante los últimos años fueron revisados para algunas de las citocinas más importantes descritas en el lupus, se analizó cómo se han encontrado los niveles de cada citocina en los pacientes y cómo cada una de ellas puede contribuir a los mecanismos propuestos, también se abordó su relación con la enfermedad, así como sus posibles efectos en el desencadenamiento y control del LES. El propósito de este artículo es brindar una revisión focalizada en el conocimiento actual de las citocinas en el LES.
Systemic lupus erythematosus (SLE) is an autoimmune disease that affects around 5 million people worldwide.1 Like several other autoimmune diseases, SLE affects women more frequently than men, mainly women in fertile age between 15 and 44 years old, with female to male ratios exceeding 9:1.2 Despite years of study, this sex predilection and its consequences remain incompletely understood.
In Colombia, between 2012 and 2016, 431,834 cases of SLE were registered, for a prevalence of 91.9 per 100,000 inhabitants. The prevalence in women was 161.3 per 100,000 population, and in men it was 20.9 per 100,000 population, for a female: male ratio of 7.9: 1. The departments of Colombia with the highest prevalence were in their order Bogotá DC, Antioquia and Valle del Cauca.
In the city of Bogotá, 13,747 patients with this pathology were registered, being the city with the highest prevalence followed by the Antioquia region with 9893 affected.3 Cytokines play an important role during the course of the disease.4 In this review, some of the most important cytokines findings during the last years in SLE were addressed, it was analyzed how each cytokine can contribute to the proposed mechanisms, and its relationship with the disease, as well as its possible effects on the triggering and control of SLE. The aimed of this article is to provide a focused review of the current knowledge of cytokines in SLE.
MethodsOriginal and review articles were selected published in English or Spanish from 2009 to 2020 in MEDLINE/Pubmed and Science Direct (Elsevier). The following search terms were used ((Systemic lupus erythematosus [Title]) AND (Cytokines [Mesh] OR chemokines)) AND (Pathology [Mesh] OR pathophysiology)). The articles were then selected regarding the measured level of the cytokine or chemokine and the potential role in the pathophysiology mechanism proposed to SLE.
ResultsIn the following part, we present for each cytokines studied, a brief description of the general role of each cytokine, then how the level of the cytokine is found in SLE patients in comparison with healthy controls and finally, the possible implications in the SLE mechanism in the pathophysiology.
IFN-αIFN-α is a cytokine that belongs to the family of type I interferons, which are glycoproteins known for their ability to interfere with viral infections. This cytokine is mainly produced by plasmacytoid dendritic cells in response to exogenous stimuli, such as bacterial and viral pathogens, as well as to endogenous stimuli, such as nucleic acids, and immune complexes containing nucleic acids that are recognized by Toll-like receptors (TLR).4 The multiple effects of IFN-α include activation of dendritic cells; promotion of the proliferation, survival and differentiation of monocytes into dendritic cells, and differentiation of B cells into plasma cells. In addition, it has been described that it improves the activity of natural killer cells enhancing the production of cytokines, degranulation and cytotoxic activity of these cells.5 It also stimulates the Th1 pathway but is not sufficient to promote the differentiation.6 IFN-γ as a Th1 hallmark cytokine, preventing apoptosis of activated cytotoxic T cells; and suppresses regulatory T cells.4,7
IFN-α has been found increased in patients with active disease, compared to those with mild or moderate disease and healthy controls, and a positive correlation has been described between IFN-α levels and the SLEDAI (Systemic Lupus Erithematosus Disease Activity Index) suggesting that the presence of higher levels of IFN-α increase the activity in SLE patients. Studies carried out by Abdel et al., showed that IFN-α levels are increased in the group of patients with highly active disease or severe disease (measured by SLEDAI index≥12), compared to the groups that had mild or moderate disease; in this study it was also pointed out that there was a positive correlation between the serum levels of this cytokine and SLEDAI in patients with lupus nephritis.8 Similar results were reported by Liu et al., who also found an overproduction of IFN-α in SLE compared to healthy controls and patients with rheumatoid arthritis (RA), and how it helped the production of IL-6 by transitional B lymphocytes of patients (Btr), promoting their survival. Btr is a subset of lymphocytes described as important link between immature B cells in bone marrow and mature B cells in periphery that play a regulatory role and are functional impairment in autoimmune diseases.9,10 A positive correlation has also been described between IFN-α levels and the presence of immune complexes in the serum of patients, as well as deposits of immune complexes in kidney sections from SLE patients. The increased IFN-α can inhibit the production of c-reactive protein (CRP) through enhancer binding proteins such as (C/EBPs) β and δ and STAT-3, leading to an increase in available autoantigens, this is because it has been reported that CRP participates in the opsonization and clearance of apoptotic cells.11–13 This cytokine is one of the most strongly implicated in the pathogenesis of SLE; elevated serum levels of this cytokine have been correlated with clinical manifestations such as fever, rash, and lymphopenia.14 One of the mechanisms in which it contributes to disease development is the promotion in the maturation of antigen-presenting cells and their respective molecules necessary for antigen presentation such as MHC-I, MHC-II, and stimulating molecules.15 IFN-α can increase the expression of autoantigens such as Ro52, a well-established autoantigen, which is translocated into the nucleus promoting apoptosis and generating cell fragments16; autoantibodies capable of forming interferogenic immune complexes together with RNA-type autoantigens were also detected in cerebrospinal fluid, indicating that this cytokine also compromises the central nervous system,17 likewise the glomerular and synovial tissues, in which the accumulation of IFN-α-producing plasmacytoid dendritic cells has been reported promoting nephritis. Additionally, it can also be found in skin lesions a continuous release IFN-α leading to skin damage such as malar rash.18,19 Finally, it has been associated with increased autoantibody production, defective clearance of apoptotic cells (inefficient remotion of these cells) and the promotion of T-cell-dependent inflammation.20Table 1 shows the levels of this cytokine found in the studies respect to the control groups.
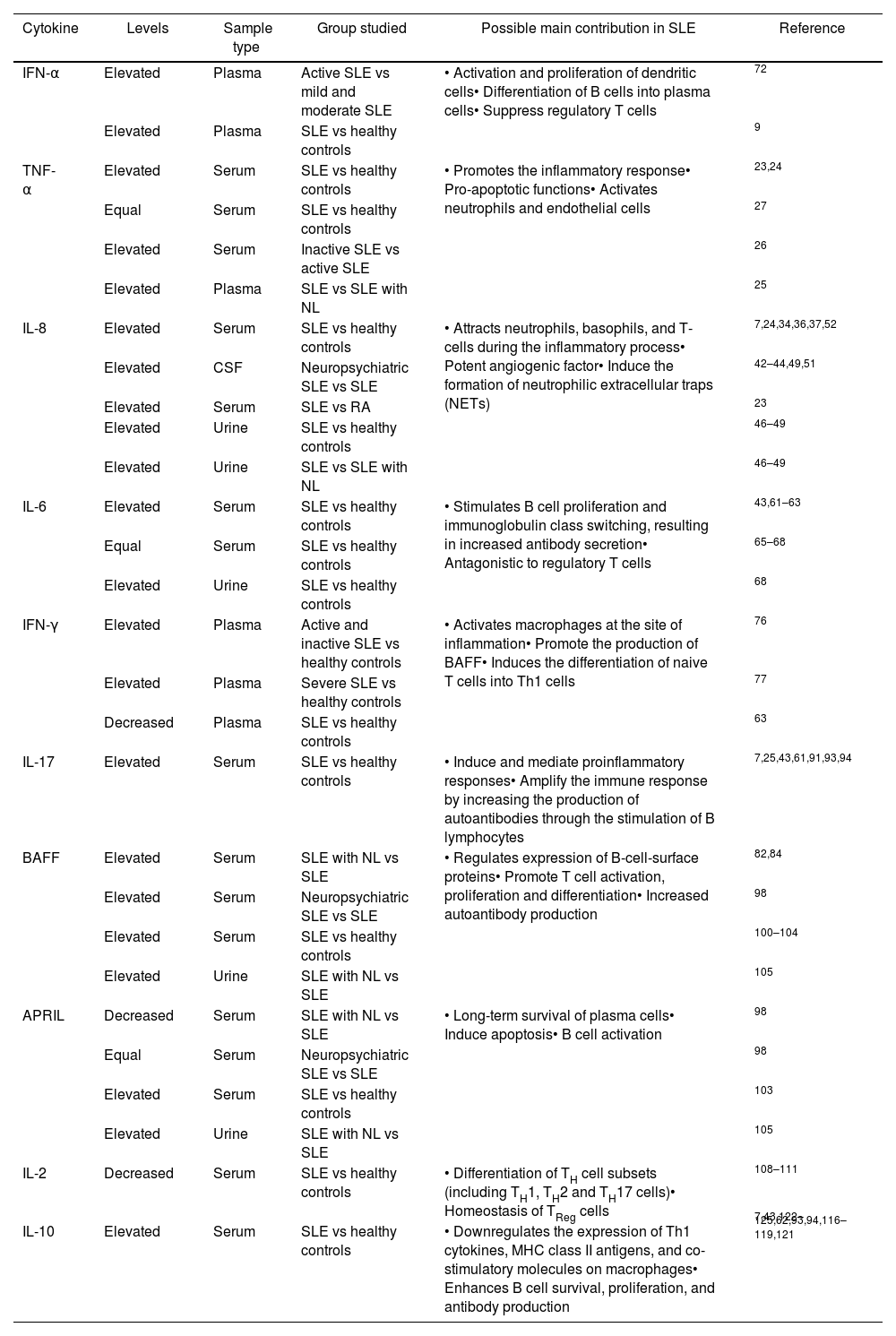
Cytokines in SLE.
| Cytokine | Levels | Sample type | Group studied | Possible main contribution in SLE | Reference |
|---|---|---|---|---|---|
| IFN-α | Elevated | Plasma | Active SLE vs mild and moderate SLE | • Activation and proliferation of dendritic cells• Differentiation of B cells into plasma cells• Suppress regulatory T cells | 72 |
| Elevated | Plasma | SLE vs healthy controls | 9 | ||
| TNF-α | Elevated | Serum | SLE vs healthy controls | • Promotes the inflammatory response• Pro-apoptotic functions• Activates neutrophils and endothelial cells | 23,24 |
| Equal | Serum | SLE vs healthy controls | 27 | ||
| Elevated | Serum | Inactive SLE vs active SLE | 26 | ||
| Elevated | Plasma | SLE vs SLE with NL | 25 | ||
| IL-8 | Elevated | Serum | SLE vs healthy controls | • Attracts neutrophils, basophils, and T-cells during the inflammatory process• Potent angiogenic factor• Induce the formation of neutrophilic extracellular traps (NETs) | 7,24,34,36,37,52 |
| Elevated | CSF | Neuropsychiatric SLE vs SLE | 42–44,49,51 | ||
| Elevated | Serum | SLE vs RA | 23 | ||
| Elevated | Urine | SLE vs healthy controls | 46–49 | ||
| Elevated | Urine | SLE vs SLE with NL | 46–49 | ||
| IL-6 | Elevated | Serum | SLE vs healthy controls | • Stimulates B cell proliferation and immunoglobulin class switching, resulting in increased antibody secretion• Antagonistic to regulatory T cells | 43,61–63 |
| Equal | Serum | SLE vs healthy controls | 65–68 | ||
| Elevated | Urine | SLE vs healthy controls | 68 | ||
| IFN-γ | Elevated | Plasma | Active and inactive SLE vs healthy controls | • Activates macrophages at the site of inflammation• Promote the production of BAFF• Induces the differentiation of naive T cells into Th1 cells | 76 |
| Elevated | Plasma | Severe SLE vs healthy controls | 77 | ||
| Decreased | Plasma | SLE vs healthy controls | 63 | ||
| IL-17 | Elevated | Serum | SLE vs healthy controls | • Induce and mediate proinflammatory responses• Amplify the immune response by increasing the production of autoantibodies through the stimulation of B lymphocytes | 7,25,43,61,91,93,94 |
| BAFF | Elevated | Serum | SLE with NL vs SLE | • Regulates expression of B-cell-surface proteins• Promote T cell activation, proliferation and differentiation• Increased autoantibody production | 82,84 |
| Elevated | Serum | Neuropsychiatric SLE vs SLE | 98 | ||
| Elevated | Serum | SLE vs healthy controls | 100–104 | ||
| Elevated | Urine | SLE with NL vs SLE | 105 | ||
| APRIL | Decreased | Serum | SLE with NL vs SLE | • Long-term survival of plasma cells• Induce apoptosis• B cell activation | 98 |
| Equal | Serum | Neuropsychiatric SLE vs SLE | 98 | ||
| Elevated | Serum | SLE vs healthy controls | 103 | ||
| Elevated | Urine | SLE with NL vs SLE | 105 | ||
| IL-2 | Decreased | Serum | SLE vs healthy controls | • Differentiation of TH cell subsets (including TH1, TH2 and TH17 cells)• Homeostasis of TReg cells | 108–111 |
| IL-10 | Elevated | Serum | SLE vs healthy controls | • Downregulates the expression of Th1 cytokines, MHC class II antigens, and co-stimulatory molecules on macrophages• Enhances B cell survival, proliferation, and antibody production | 7,43,122–125,62,93,94,116–119,121 |
TNF-α has two active forms, a membrane-bound and a soluble form.7 This cytokine is produced by different cells of the immune system, including activated T lymphocytes, natural killer cells (NK), mast cells, B lymphocytes, however, its main sources are monocytes, macrophages, and activated dendritic cells.21 TNF-α has pro-apoptotic functions and is linked to responses related to acute inflammation.22
In patients with SLE, contradictory results have been reported. Some studies report elevated levels of TNF-α compared to those of healthy controls,23,24 while others describe that there are no differences between patients and controls. Recently, Pacheco et al. reported a statistically significant increase in TNF-α in the plasma of Colombian SLE patients with lupus nephritis in comparison with patients without lupus nephritis. These results suggest the use of TNF-α as a predictor of renal involvement in SLE,25 however, Gómez et al. reported that TNF-α levels were higher in patients with inactive disease, compared to patients with highly active disease and healthy controls, suggesting that overexpression of TNF-α could be a protective factor in patients with SLE.26 On the other hand, Silva et al. reported that there is no statistically significant difference between SLE patients and controls, the authors stated that these results could be due to the fact that the study subjects had inactive or mild active disease.27 Therefore, the reported studies suggest that the role of this cytokine is not completely clear (see Table 1).
IL-8Interleukin-8 (IL-8) is one of the main mediators of the inflammatory response. It is secreted by various types of cells, mainly by macrophages.28 It functions as a chemoattractant and as a potent angiogenic factor.29,30 IL-8 mediates neutrophil chemotaxis, degranulation, increases intracellular free calcium concentration in the neutrophil, inducing a neutrophil activation characterized by a cytokine profiling. IL-8 also is implicated in the pathogenesis of several chronic inflammatory diseases.31–33 IL-8 is a powerful pro-inflammatory chemotactic cytokine acts on a variety of cells, the most important of which is to attract and activate neutrophil aggregation in response sites to release inflammatory mediators. Neutrophil plays a significant role in the pathogenesis of SLE and neutrophils function and phenotype disorder in SLE patients may be benefited for the pathological mechanism and complications of SLE.34
Multiple studies have found elevated IL-8 levels in SLE patients in comparison to healthy controls24,35–41 and decreased levels after the administration of rituximab (see Table 1).35 Elevated levels of IL-8 in cerebrospinal fluid, CSF, have also been detected in patients with neuropsychiatric lupus, compared to those who did not present it, additionally it was elevated during the outbreak of the disease, and it decreased significantly after treatment.7,42–44 Eilertsen et al., showed that IL-8 increased significantly compared to patients with a diagnosis of rheumatoid arthritis.23 The serum level of IL-8 in patients with SLE and pulmonary involvement was also significantly higher than in patients without this type of compromise.45 IL-8 has been evaluated in other fluids such as urine and CSF, where it has also been increased and associated with lupus activity.41 Urine IL-8 levels are associated with SLE activity and lupus nephritis.46 Sekikawa et al.47 observed a significant positive correlation between the number of glomerular neutrophils and IL-8 expression in renal biopsy samples obtained from lupus nephritis patients. Urine IL-8 levels in the lupus nephritis group were higher than in the non-lupus nephritis group and the healthy control group, suggesting that IL-8 may reflect the degree of kidney inflammation in nephritis patients,48 When compared with SLE patients without organ damage, SLE patients with organ damage have significantly higher concentrations of IL-8.49
IL-8 has been found to induce neutrophil recruitment and the formation of neutrophilic extracellular traps (NETs), increasing the risk of antinuclear autoantibody production, suggesting that it plays an important role in the early stages of SLE.7,50 The formation of NETs in patients can produce neoepitopes that lead to loss of immune tolerance, and NETs can contribute to vasculopathy by impairing the function of endothelial cells, promoting the formation of atherosclerotic plaque.51 The disorder of apoptotic cell clearance and NETs lead to the formation of immune complexes, and then trigger a series of immune responses against their own antigens in SLE,34 therefore, IL-8, as a potent preformation factor of NETs, can be involved in the pathogenesis of SLE by increasing their formation.52,53 IL-8 attracts neutrophils to inflammation sites, and stimulates them to secrete a series of anti-infective agents such as a wide variety of degradative enzymes (proteases, hydrolases, nucleases), plus reactive oxygen species (ROS) via an activated NADPH oxidase in combination with myeloperoxidase34 to protect the body from infection. However, the excessive effect of IL-8 on neutrophils can also cause tissue damage secreting molecules that are normally retained in phagocytic vesicles following phagocytosis of pathogens, these secreted molecules can attack host tissues if they overwhelm endogenous tissue levels of anti-proteinases or anti-oxidants altering the properties of the plasma membrane.50
The levels of IL-8 in CSF in patients with neuropsychiatric involvement were higher than in patients with SLE without neurological alteration, which suggested that IL-8 could change the permeability of the blood-brain barrier, and then attract B and T cells to the inflammatory site.49,51,54 IL-8 regulated the permeability by down-regulation of tight junction proteins by the activation of vascular endothelial growth factor receptor-2 (VEGFR2) and up-regulation of nerve growth factor (NGF).55,56
IL-8 may mediate the pathogenesis of SLE through leukocyte harvesting to organs and tissues for autoimmune damage, suggesting that IL-8 may be used as a biomarker in organ-damaged SLE patients, and a local inflammation level monitoring index. IL-8 could be considered a target for intervention and a plausible therapeutic target in SLE.7
IL-6IL-6 is a pleiotropic cytokine produced in response to tissue damage. This interleukin is produced by various cell types including fibroblasts, keratinocytes, mesangial cells, vascular endothelial cells, mast cells, macrophages, dendritic cells, T and B cells.39 It has both pro-inflammatory and anti-inflammatory actions, and its effects on immunity depend on the context and its local concentration.7,57 IL-6 exerts different hematological, immunological, endocrine and metabolic actions. This interleukin is the main stimulator of the production of most acute phase proteins. In the immune system, it promotes the differentiation and maturation of T and B lymphocytes, stimulates the production of immunoglobulins by B cells, and inhibits the secretion of pro-inflammatory cytokines such as TNF-α and IL-1. In this sense, IL-6 has anti-inflammatory actions and, together with the increase in cortisol production helps to control the inflammatory response.58,59 This expression is strictly controlled by transcriptional and post-transcriptional mechanisms. The continuous deregulated synthesis of IL-6 has a pathological effect on chronic inflammation and autoimmunity.39
In our search regarding IL-6 levels in lupus, we found heterogeneous results. Multiple studies showed significantly higher IL-6 levels in SLE patients compared to healthy controls (Table 1).40,43,60–64 Thanadetsuntorn et al., stated that the combination of circulating immune complexes and IL-6 strongly predicts active clinical SLE, and that it can be used to monitor lupus activity.64 Talaat et al., showed higher levels of IL-6 in SLE patients than in controls, and IL-6 was associated with disease activity, since IL-6 levels were correlated with high levels of anti-dsDNA antibodies.63 However, other studies conclude that there was no significant correlation between serum IL-6 levels and disease activity or flare-ups.65–67 Dima et al., showed that urinary, but not serum IL-6, was related to SLE activity.68 IL-6 does not consistently correlate with SLE disease activity, this may be due in part to a relatively short half-life and circadian rhythm of IL-6.41 IL-6 and IL-8 predicted non-renal flare, the performance of these cytokines to predict active clinical SLE is superior to complement and anti-dsDNA.69
An important role of IL-6 among the various events involved in the pathogenesis of lupus is that it promotes the differentiation of B cells into plasma cells, with the consequent secretion of immunoglobulins. Evidence suggests an important role of IL-6 in B lymphocyte hyperactivity; B lymphocytes from lupus patients spontaneously express IL-6 receptor on the cell surface.70,71 Reactive T cell clones from SLE patients also produce large amounts of IL-6 and thus promote B cell activation and autoantibody production.4 Some authors conclude that the association of IL-6 with the disease activity is not too strong to suggest it as routine measurement, and the problems with circadian variation should be overcome before being used as a biomarker,41 because IL-6 shows diurnal variation, some studies conclude the most marked effect in early morning, others studies evidence a peak in the evening, these variation should be taken into account in order to avoid confounding by time of day in studies of IL-6 in plasma or serum.66 However, others propose it as a sensitive biomarker to assess disease activity, as well as a predictor of remission in lupus nephritis.72,73
IFN-γIFN-γ is the only cytokine belonging to the family of type II interferons. It is secreted by macrophages, NK cells, and T lymphocytes, especially CD4 and CD8 T lymphocytes. IFN-γ activates macrophages at the site of inflammation, contributes to the cytotoxic activity of T cells, it has antiviral capabilities, and is strongly associated with Th1 responses. IFN-γ induces the differentiation of naive T cells into Th1 cells, and triggers Th1 differentiation in an autocrine manner.7,74 Type I and type II IFNs binding to their respective receptors activates multiple signaling pathways, especially janus kinases (JAKs) and STAT pathways, to activate the transcription of hundreds of genes within target cells. Type I and type II IFNs have a high degree of overlap in the genes they control, inducing common biological pathways.75
As described before for TNF-α, serum IFN-γ levels also present contradictory results (see Table 1). Some studies report a significant increase in patients with active SLE compared to inactive SLE and healthy controls,76,77 while other studies report decreased levels in lupus patients, or similar levels compared to controls.63,78,79 Talaat et al., propose that this decrease can be attributed to a previously observed reduction in the frequency and number of Th1 cells in patients with SLE.63
IFN-γ is a characteristic Th1 cytokine, it participates in the pathogenesis of lupus by promoting the production of BAFF, a B-cell activating factor.80 Patients with lupus nephritis show a dominant Th1 phenotype but a decrease in the Th281 response in peripheral blood and the glomerulus. This phenotype is related with the severity of the renal damage. The production of autoantibodies and the incidence of glomerulonephritis decrease by blocking the IFN-y receptor, however, it has been reported that IFN-y can be detected in the kidney of patients with renal manifestations.82 Several studies on lupus models suggest that an imbalance toward the Th1 response plays a role in accelerating the disease.83 In patients with SLE, an imbalance was observed in the mechanisms that regulate Th1 and Th17 cells, with an increased frequency of Th17 cells.84 The complex role of IFN-γ in SLE is underlined by conflicting clinical studies, which find a correlation between serum IFN-γ levels and disease activity, and a correlation between IFN-γ expression and the severity of lupus nephritis, while others show reduced levels of IFN-γ in patients with lupus nephritis.82,85
IL-17IL-17 is a type I transmembrane protein, spans the entirety of the cell membrane and may function as gateways to permit the transport of specific substances across the membrane (membrane bound form). Also exist in its soluble form.86,87 It is a potent pro-inflammatory cytokine produced by activated T lymphocytes, being Th17 the most important producers. These Th17 cells are a subset of CD4 T lymphocytes.88 IL-17 recruits monocytes and neutrophils, facilitates T cell infiltration, and positively regulates adhesion molecule expressions.89 Although naive CD4 T cells can differentiate into effector subsets, the characteristic cytokine environment of SLE patients (poor in IL-2 but rich in IL-6 and IL-21) favors Th17 expansion. This set of cytokines can stimulate B cells and trigger local inflammation and tissue injury, which are related to various phenomena in the pathophysiology of SLE.90 IL-17 can amplify the immune response by increasing the production of autoantibodies through the stimulation of B lymphocytes.IL-17-producing cells play a crucial role in disease pathogenesis and represent an attractive therapeutic target.69
Several studies have shown significantly elevated levels of IL-17 in patients with SLE, compared to healthy controls, showing a correlation with lupus activity which is even higher in patients with nephropathy (Table 1).7,25,39,43,60,61,91–94
The ability of IL-17 to induce local inflammation (target organs: kidney, skin), and a direct response of B lymphocytes allowed to postulate its participation in SLE physiopathology. Specifically, 17A and 17F have the ability to induce tissue inflammation, through the secretion of chemokines such as monocyte chemoattractant protein-1, MCP-1, growth-related oncogenic alpha protein, IL-8, IL-9, responsible for the proliferation, maturation and recruitment of neutrophils and monocytes.62 IL-17 induces tissue damage by regulating positively the expression of matrix metalloproteinases, and by stimulating the dendritic cells and macrophages to increase the production of IL-1, IL-6 and TNF-α.93 IL-17, in addition to acting as an inflammation mediator, it also acts as a direct regulator of B lymphocyte function; specifically, this cytokine promotes B lymphocyte survival through NF-κB and BAFF, it also alters the deletion of autoreactive B lymphocyte clones, breaks the programmed cell death of the B lymphocyte, and favors the differentiation of the B lymphocytes into plasmatic cells. All of the above effects increase autoantibody production, formation of germinal centers, and retention of autoreactive B lymphocytes in target organs.95 With its main role in the pathogenesis of SLE, basal serum levels of IL-17 can be used as a sensitive biomarker for disease activity, and also as predictor in remission of lupus nephritis72,96 Saber et al., propose it as a valuable target for future therapeutic applications.97
Complex APRIL/BAFFThe BAFF complex is composed of the B cell activation factor (BAFF) and a proliferation induction ligand (APRIL), which are cytokines produced by macrophages, neutrophils, dendritic cells, and B lymphocytes.98 Both cytokines bind with different affinity to three receptors expressed on B cells: BAFF receptor, transmembrane activator and cyclophilin ligand interactor (TACI), and B cells maturation antigen (BCMA). BAFF and APRIL binds to TACI as well as BCMA which is expressed on plasmablasts and plasma cells, while BAFF-R is exclusive of BAFF.99
Multiple studies reported an increase in the serum and urine levels of BAFF and APRIL in patients with SLE compared with the healthy controls in response to the activation of Toll-like receptors on B lymphocytes, especially trough TLR9100–105; on the other hand, in SLE patients with manifestations in the central nervous system there is evidence of an increase of BAFF without a difference in APRIL; other studies with patients with renal failure found an increase of these two cytokines in urine samples but an increase in BAFF and a decrease in APRIL in the blood, these results suggest that this decrease in APRIL could due to its renal excretion when glomerulonephritis occurs. The role of the BAFF complex in SLE is based on the importance of this cytokine in the maturation, selection, and survival of B lymphocytes and self-reactive plasma cells, and the change of immunoglobulin class isotype.106 This has been tested within animal models that overexpress BAFF where a high number of B cells and autoantibodies leads to autoimmune diseases similar to lupus.107
IL-2IL-2 is a cytokine that exhibits an impressive number of different functions, it is pivotal for cellular activation, important for primary T-cell responses and essential for secondary T-cell responses.108 In addition, IL-2 has the key function of downregulating immune responses. The IL-2 production by T cells is part of a complex network in which a discrete alteration is capable of disrupting the whole system.109
In the 1980s, studies showed that expression of the IL-2 receptor was increased in B cells from patients with active SLE compared with those from healthy controls, and expression of the receptor correlated with disease activity, suggesting a pathogenetic role for the IL-2 pathway in SLE. The IL-2 pathway was also discovered to be deficient in the T cells of patients with SLE, often resulting in fewer regulatory T cells compared with healthy controls.110
It has been reported that production of IL-2 is decreased in patients with SLE and this defect affects multiple aspects of host immunity.111 Why do SLE T cells produce less IL-2? The decreased IL-2 production observed in SLE patients is based on the relationship between the transcription factors CRE (cAMP response element)-binding protein (CREB) and CRE-modulator (CREM). These two transcription factors share a binding of the IL-2 promoter and are responsible for activating or repressing IL-2 production. CREB occupies the binding site in resting T cells and upon activation, it is phosphorylated (pCREB) thereby promoting IL-2 transcription. IL-2 transcription is repressed by the replacement of pCREB by phosphorylated CREM (pCREM). SLE patients have higher levels of CREM than CREB, resulting in decreased IL-2 production.
In some strains of mice that develop an SLE-like disease, treatment with low-dose IL-2 prolonged survival, resolved nephritis, and reduced lymphadenopathy.110
In vivo low-dose IL-2 administration in humans has been confirmed to be safe and effective in expanding Treg,110 it is likely that it may be considered for the treatment of several autoimmune diseases including SLE. Low-dose IL-2 significantly expands regulatory T cells, expansion of regulatory T cells while suppressing inflammation is an attractive approach to management of patients with SLE. Several clinical trials have explored the use of low-dose IL-2 in patients with SLE,69,112 showing that low-dose IL-2 therapy is safe and well tolerated and selectively promotes the expansion of functional regulatory T cells in patients with moderate-to-severe systemic lupus erythematosus.
IL-10Interleukin-10 is a homodimeric protein produced by macrophages, dendritic cells, and helper T cells in response to multiple stimuli.73 It decreases the activation of antigen-presenting cells, negatively regulates the expression of costimulatory molecules, and reduces the activation of T cells and the secretion of TNF-α.113 IL-10 plays a crucial role in inflammatory and immune reactions. It has potent anti-inflammatory and immunosuppressive activities on myeloid cell functions which forms a solid basis for its use in acute and chronic inflammatory diseases.114 The anti-inflammatory and tolerogenic cytokine IL-10 appears to play a paradoxical pathogenic role in SLE and is therefore currently therapeutically targeted in clinical trials. It is generally assumed that the pathogenic effect of IL-10 in SLE is due to its growth and differentiation factor activity on autoreactive B-cells, but effects on other cells might also play a role.115
Multiple studies have shown that serum IL-10 is increased in SLE patients compared to controls (see Table 1).25,39,43,62,92–94,116–126 IL-10 levels were positively correlated with disease activity and anti-dsDNA antibody presence. The association observed between IL-10 and disease activity measured by SLEDAI index is supported by the correlations seen between IL-10 and other markers of disease severity included to measure the SLE activity, such as active renal disease and patients’ ESR increase, C3 and C4 decrease.73
IL-10 stimulates B cell proliferation and immunoglobulin class switching, resulting in increased antibody secretion with the ability to enter the extravascular compartments and promote inflammation in SLE. Various stimuli, including anti-dsDNA antibodies altering the mononuclear cell function and immune complexes by a dependent FcγIIR mechanism are potent triggers for IL-10.127,128
The ability of IL-10 to enhance B cell survival, proliferation, differentiation, and antibody production, as well as to inhibit apoptosis of autoreactive B cells, may contribute to elevated anti-dsDNA titers in SLE patients.128 It has been shown that circulating immune complexes increase the synthesis of IL-10, and this IL-10 can facilitate the production of autoantibodies; it could be suggested that IL-10 acts pathogenetically in SLE, amplifying and perpetuating the inflammatory cycle.129–131 Several studies suggest that high concentrations of IL-10 could be used as a new biomarker to evaluate clinical activity in SLE.73,88,132
ConclusionCurrently, the most important problem in the treatment of SLE is preventing and treating organ damage. Alterations in the balance between inflammatory mediators and regulators may be targets of new immunotherapeutic agents for the management of autoimmune diseases.43 Cytokines play a preponderant role in pathophysiology, and may be useful to monitor the activity and severity of the disease, since many of them have been associated with this condition (Fig. 1). The use of cytokines as biomarkers is a current challenge, and perhaps the most successful strategy for their use as a biomarker is the combination of several of them, and not just one. A cytokine profile including IFN-α, IL-10, IL-8, BAFF and IL-17 could be considered as a group of cytokines to be evaluated simultaneously due to the consistent reported results across reviewed studies. The contradictory results described seem to be given by the different measurement techniques, the type of sample where the measurements are made, and the specific characteristics of the population of SLE patients studied like disease activity score. Further studies are required to deepen the understanding of the cellular and molecular mechanisms that trigger cytokines in SLE, to widen the range of therapeutic targets as potential treatments in the disease.
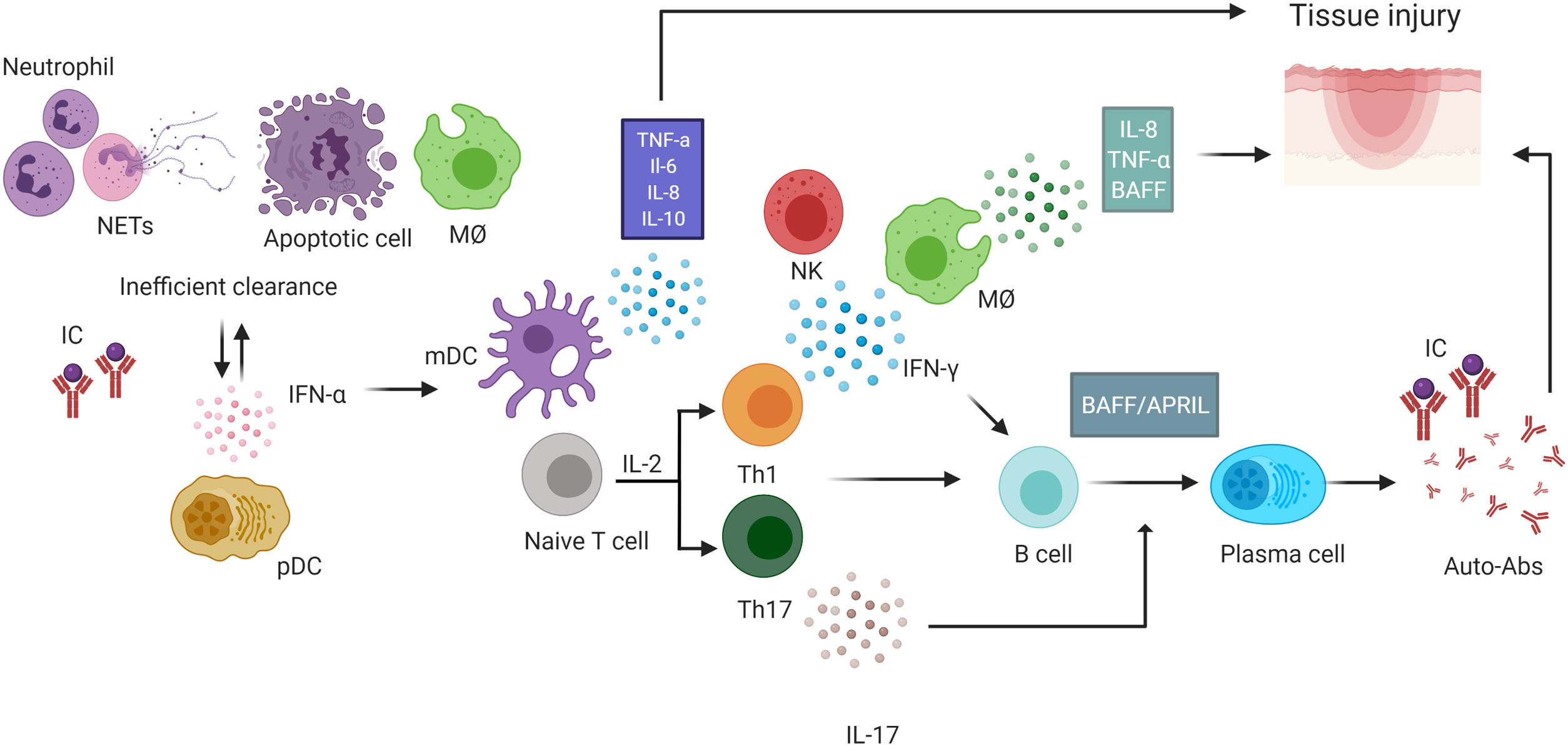
Role of cytokines in the pathophysiology of systemic lupus erythematosus (SLE). SLE is characterized by a global loss of tolerance with activation of cells of innate and adaptive immunity. In SLE, failure to effectively remove apoptotic cells by the macrophages leads an inflammatory environment and neutrophils that form NETs and undergo the NETosis, a process that also contributes to the inflammatory environment. Both processes also increase the availability of autoantigens. The antigen-presenting cell after capture autoantigens and process them to induce the activation and polarization of naïve T cells toward a Th1 profile, the predominant producer of IFN-γ that contributes to the production of BAFF and Th17 T cells, producers of IL-17. In addition to the presence of T cells, autoreactive B cells activated with the help of BAFF and APRIL produce cytokines such as IL-6, IL-8 and IL-10 and differentiate into autoantibody-producing plasma cells with the help of these cytokines and IL-17. Autoantibodies promotes tissue damage by the formation of IC. Activation of pDC by these ICs increases IFN-α production. Both mDC and macrophages produce cytokines such as TNF-α, IL-8, IL-6, and IL-10 among others. IL-8 contributes to the recruitment of neutrophils that form NETs and leads to perpetuation of the inflammatory environment. IC: immune complexes, NK: natural killer, BAFF: B-cell activation factor, pDC: plasma dendritic cell, NETs: extracellular neutrophil traps, MØ: macrophage. mDC: myeloid dendritic cells. This figure was created with Biorender.com.
The authors declare that they have no conflict of interest.
The authors thank the Young Researchers program of the Ministry of Sciences, call 850-2019 and 886-2019 for the funding of Catherin Tovar-Sánchez, the Pontificia Universidad Javeriana for the administrative management and the Ministry of Sciences for the financial support, ID PRY 120389666081.